Introduction
Inflammation is a protective response to external
stimuli such as pathogens, physical damage, and chemicals. It can
inactivate or destroy invading organisms to maintain normal tissues
(1). The inflammatory reaction
begins with the release of arachidonic acid from cell membrane
phospholipids. Through lipoxygenase or cyclooxygenase (COX) action,
various mediators of inflammatory reactions such as leukotriene,
thromboxane, and prostaglandin are produced (2). These mediators also include nitric
oxide (NO), prostaglandin E2 (PGE2), and inflammatory
cytokines (3). Moderate amounts of
NO can kill bacteria or tumors. However, excessive NO production by
iNOS can aggravate inflammatory responses, causing tissue damage,
genetic mutation, and nerve damage (4). PGE2, one of major mediators
of inflammatory responses, contributes to tumor formation by
inhibiting apoptosis and inducing angiogenesis (5). Cyclooxygenase (COX) is an enzyme that
can promotes the transformation of arachidonic acid into
prostaglandin. In particular, COX-2 is induced by various
stimulations of inflammation, growth factors, and cytokines caused
by cancer cells (6). Tumor necrosis
factor (TNF-α), interleukin (IL-6), and IL-1β are typical
pro-inflammatory cytokines whose expression levels are increased
during inflammatory reactions. Macrophages secrete them to mediate
various inflammatory reactions and induce NO and PGE2
production (7). Normal inflammatory
reaction protects the body. However, when inflammation continues
and progresses to chronic inflammation, cancer can develop due to
excessive secretion of inflammatory mediators. Insulin resistance
can also increase, thereby acting as a mediator of diabetes and
various diseases (8,9). Therefore, effective methods for
controlling inflammation are needed. Nuclear factor kappa-B (NF-κB)
and mitogen-activated protein kinase (MAPK) are important signaling
molecules in the Toll-like receptor (TLR) pathway. Studies are
actively underway to control inflammation by inhibiting these
molecules (10).
Chrysanthemum zawadskii is a perennial herb
of the Asteraceae family. It is cultivated in China, Russia,
Mongolia, and Japan. It has been reported that C. zawadskii
possess antioxidant, antifungal, and anti-inflammatory effects
(11). Peppermint (Mentha
piperita L.), one of the most popular herbal tea, has been used
as a folk remedy and alternative medicine to relieve gastritis,
enteritis, biliary disorders, indigestion, and flatulence (12). Licorice (Glycyrrhiza glabra),
one of the most commonly used herbal medicine, has been found to
possess anti-inflammatory, antiviral, and immunomodulatory
activities (13). The purpose of
this study was to investigate the anti-inflammatory effect of a
herbal mix of C. zawadskii, peppermint, and G. glabra
(CPG) and its mechanism of action in mouse macrophages.
Materials and methods
Materials
Chrysanthemum zawadskii, Peppermint
(Mentha piperita) and Glycyrrhiza glabra were
purchased from Kyeondong market. Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS), and penicillin/streptomycin
antibiotics were purchased from Gibco; Thermo Fisher Scientific,
Inc. Griess reagent and LPS (L2630) were procured Sigma-Aldrich;
Merck KGaA. Quanti-Max™ WST-8 Cell Viability Assay Kit has gotten
from Biomax. Goat anti-mouse IgG (H+L) Alexa Fluor plus 488
conjugated secondary antibodies were purchased from Invitrogen;
Thermo Fisher Scientific, Inc. Enzyme-linked immunosorbent assay
(ELISA) kit PGE2, TNF-α, IL-6, and IL-1β were procured
from R&D System. Mouse IFN Beta ELISA Kit (TCM, Serum) was
supplied by BL Assay Science. HO-1 activity kit was purchased from
Cusabio. Radio-immunoprecipitation assay buffer (RIPA buffer) came
from Thermo Fisher Scientific, Inc. Bradford's assay reagent was
purchased from Bio-Rad Laboratories, Inc. 5X SDS-PAGE loading
buffer was purchased from Biosesang. Antibodies against iNOS,
COX-2, p-NF-κB, p-IκB, p-Akt, p-STAT1, STAT1, and β-actin were
purchased from Santa Cruz Biotechnology, Inc. HO-1 antibody was
procured from Abcam. Horseradish peroxidase (HRP)-IgG secondary
antibodies and diamidino-2-phenylindole (DAPI) were purchased from
Cell Signaling Technology, Inc.
Preparation of CPG (FHH-CZ)
Chrysanthemum zawadskii was extracted with
distilled water at 85°C. Peppermint was extracted with 50% ethanol
at 50°C. Glycyrrhiza glabra was extracted with distilled
water at 50°C. All plant materials were extracted for 6 h. These
extracts were filtered twice, concentrated, and freeze-dried.
Glycyrrhiza glabra extract was mixed with 40% dextrin before
freeze-drying. Freeze-dried samples were stored at 4°C before
experiments. The CPG mixing ratio was Chrysanthemum
zawadskii 15: Peppermint 75: Glycyrrhiza glabra 10. The
mixture was named FHH-CZ.
Cell culture
RAW264.7 cells (ATCC, SC-6003) were cultured in DMEM
supplemented with 10% FBS and 1% penicillin/streptomycin
antibiotics at 37°C with 5% CO2 in an incubator. These
cells were not serum-starved for all experiments.
Cell viability
RAW264.7 cells (2×105 cells/ml) were
cultured in 96-well plates for 24 h. CPG was used to treat cells at
various concentrations (0 µg/ml ~ 500 µg/ml). After incubating for
20 h, 10 µl of Quanti-MaxTM was added. After 4 h of further
incubation, the absorbance of each well was measured using a
spectrophotometer (Tecan) at 450 nm. The absorbance of each well
conformed to RAW264.7 cell viability.
Nitric oxide assay
RAW264.7 cells (2×105 cells/ml) were
seeded into a 96-well plate and incubated for 24 h. These cells
were then treated with CPG (0, 50, 100, 200 µg/ml) for 1 h and then
stimulated with LPS (1 µg/ml) for 16 h. After stimulation, 100 µl
of Griess reagent was mixed with 100 µl of supernatant and
incubated at room temperature (RT) for 15 min. The absorbance at
540 nm was measured using a spectrophotometer (Tecan).
NaNO2 standards were diluted and used to determine NO
concentration.
Assay of cytokine production
RAW264.7 cells (2×105 cells/ml) were
cultured in 60 mm cell culture dishes for 24 h, treated with CPG
(0, 50, 100, 200 µg/ml), and then incubated for 1 h in an
incubator. After that, cells were stimulated with LPS (1 µg/ml) for
16 h. Cell culture supernatant was then obtained to measure levels
of PGE2 (R&D KGE004B), TNF-α (R&D MTA00B), IL-6
(M6000B) and IL-1β (MLB00C). Using ELISA kits following the
manufacturer's protocol without modification.
Protein extraction and western
blot
Raw264.7 cells (2×105 cells/ml) were
cultured in 60 mm cell culture dishes for 24 h, treated with CPG
(0, 50, 100, 200 µg/ml), and incubated for 1 h in an incubator.
After that, cells were stimulated with LPS (1 µg/ml) for 16 h.
Whole proteins were then extracted from each sample using protease
and phosphatase inhibitors treated-RIPA buffer. After
quantification using Bradford protein analysis, proteins (30 µg)
present in each sample were separated for 1 h at 100 V on 12%
polyacrylamide gels. After separation, proteins were transferred
onto PVDF membranes for 1 h at 100 V. After blocking with 5% bovine
serum albumin (BSA) for 1 h, membranes were washed three times with
TBST solution (10 min each wash) and then incubated with each
antibody (iNOS, COX-2, p-NF-κB, p-IκB, p-Akt, p-STAT1, STAT1, HO-1,
β-actin) at 4°C overnight. Membranes were washed three times with
TBST solution for 10 min and then incubated with mouse or rabbit
HRP-conjugated secondary antibodies containing BSA for 2 h at RT.
Subsequently, membranes were washed three times with TBST solution
for 10 min and visualized with an imaging system (Alliance version
15.11; UVITEC) using EZ-western Lumi Pico Alpha chemiluminescent
reagent. Band densities were analyzed using ImageJ (developed by
the National Institutes of Health) and converted into a graph.
Immunofluorescence
RAW264.7 cells (2×105 cells/ml) were
cultured in 4-well cell culture slides for 24 h. These cells were
then treated with CPG (0, 50, 100, 200 µg/ml), incubated for 1 h in
an incubator, and then stimulated with LPS (1 µg/ml) for 16 h.
Next, cells were fixed in ice cold methanol for 10 min at RT.
Slides were washed three times with PBS. After that, slides were
incubated with PBS containing 1% BSA for 1 h to prevent
non-specific antibody binding. After that, slides were incubated at
4°C overnight with antibodies (NF-κB, p65) in 1% BSA. Incubated
slides were washed three times with PBS. After that, slides were
incubated with Alexa Fluor 488-conjugated goat anti-mouse or rabbit
IgG antibody in 1% BSA at RT for 1 h. Subsequently, slides were
washed with PBS, mounted with DAPI, and photographed under a ZEISS
Fluorescence Microscope.
NF-κB DNA-binding activities
NF-κB DNA-binding activities were measured in the
nuclear protein extracts using the NF-κB transcription factor assay
kits. Nuclear extracts (10 µg) and complete binding buffer (0.03
ml) were added into wells pre-fixed with NF-κB p65 DNA target and
incubated for 1 h at room temperature with mild agitation.
Following the incubation, the wells were washed three times with
wash buffers, and 0.1 ml of diluted NF-κB antibodies, which
recognize only p65 epitopes that are bound to DNA in the wells, was
added to each well and incubated for 1 h at room temperature. Then,
the wells were washed as above, and HRP-conjugated antibodies (0.1
ml) were added to each well and incubated for 1 h at room
temperature. Following incubation with the HRP-conjugated
antibodies, the wells were washed. 0.1 ml of developing solution
was added into the wells for 5 min and then, the reaction was
stopped by adding 0.1 ml of stopping solution. The absorbance,
which corresponded with the DNA binding activities, was read at 450
nm using a spectrophotometer (Tecan).
HO-1 activity
Raw264.7 cells (2×105 cells/ml) were
cultured in 60 mm cell culture dish for 24 h. the cells were
treated with CPG (50, 100, 200 µg/ml) or without CPG and incubated
for 1 h in the incubator. After that, the cells were stimulated by
LPS (1 µg/ml) for 16 h. After that, whole proteins were extracted
from each sample using protease and phosphatase inhibitors
treated-RIPA buffer. And enzyme activity was measured using mouse
HO-1 ELISA kit.
Statistical analysis
Data are presented as mean ± SD. Statistically
significant differences among groups were determined by one-way
analysis of variance (ANOVA) analysis followed by Tukey's post hoc
test. P<0.05 was considered statistically significant.
Results
Effect of CPG on NO level and DPPH
radical scavenging activity
Chrysanthemum zawadskii, peppermint and
Glycyrrhiza glabra were mixed in various ratios to determine
the optimal mixing ratio (Table
SI). NO assay and DPPH radical scavenging activity were
measured for each mixing ratio. As a result, the No. 5 mixture
(Chrysanthemum zawadskii 15: Peppermint 75: Glycyrrhiza
glabra 10) showed the highest NO generation inhibitory effect
and DPPH radical scavenging activity (Figs. S1 and S2). Therefore, subsequent experiments
were conducted using No. 5 Mixture.
CPG inhibits NO and PGE2
production in LPS-stimulated RAW264.7 macrophages
To investigate anti-inflammatory effects of CPG in
LPS-stimulated RAW264.7 cells, we determined cytotoxic effects of
CPG on RAW264.7 cells. After cells were treated with various
concentrations (0, 15.6, 31.2, 62.5, 125, 250, and 500 µg/ml) of
CPG for 24 h, cell cytotoxicity was investigated. CPG did not cause
cytotoxicity to RAW264.7 cells at concentration up to 500 µg/ml.
Based on such result, we selected concentrations of 50, 100, and
200 µg/ml to study the anti-inflammatory effect of CPG on
LPS-stimulated RAW264.7 cells (Fig.
1A). Next, we evaluated effects of CPG treatment on NO and
PGE2 production in LPS-stimulated RAW264.7 cells. We
found that LPS treatment significantly increased NO production in
RAW264.7 cells. However, cells pre-treated with CPG before
administration of LPS showed significant decreases of NO and
PGE2 production in a dose-dependent manner compared to
cells treated with LPS only (Fig. 1B
and C).
 | Figure 1.Effects of CPG on cell viability, NO
levels, PGE2 levels, iNOS and COX-2 expression in
LPS-stimulated RAW264.7 macrophages. (A) RAW264.7 cells were
treated with CPG at the indicated concentrations for 24 h and
relative cell viability was assessed using a WST-1 assay. RAW264.7
cells were pretreated with CPG at the indicated concentrations and
stimulated with LPS for 16 h. Levels of (B) NO and (C)
PGE2 in the culture supernatants were analyzed using a
Griess assay and ELISA, respectively. (D) iNOS and COX-2 expression
was determined by western blot analysis. (E) Relative density of
iNOS was calculated using ImageJ. (F) Relative density of COX-2 was
calculated using ImageJ. Data are presented as the mean ± SD. All
groups labelled with the same lower case letter (a-e) were not
significantly different from each other (P>0.05), whereas groups
labelled with different lower case letters were significantly
different (P<0.05). CPG, mixture of Chrysanthemum
zawadskii, peppermint and Glycyrrhiza glabra; LPS,
lipopolysaccharide; NO, nitric oxide; PGE2,
prostaglandin E2; iNOS, inducible nitric oxide synthase; COX-2,
cyclooxygenase-2. |
CPG inhibits iNOS and COX-2 expression
levels in LPS-stimulated RAW264.7 macrophages
To investigate inhibitory effects of CPG on NO and
PGE2 production, we examined iNOS (enzyme generating
nitric oxide) and COX-2 (enzyme producing PGE2)
expression in LPS-stimulated RAW264.7 cells. Results showed that
LPS-only treated cells had elevated expression levels of iNOS and
COX-2 while cells pre-treated with CPG before administration of LPS
showed significant decreases of iNOS and COX-2 expression compared
to cells treated with LPS only (Fig.
1D-F).
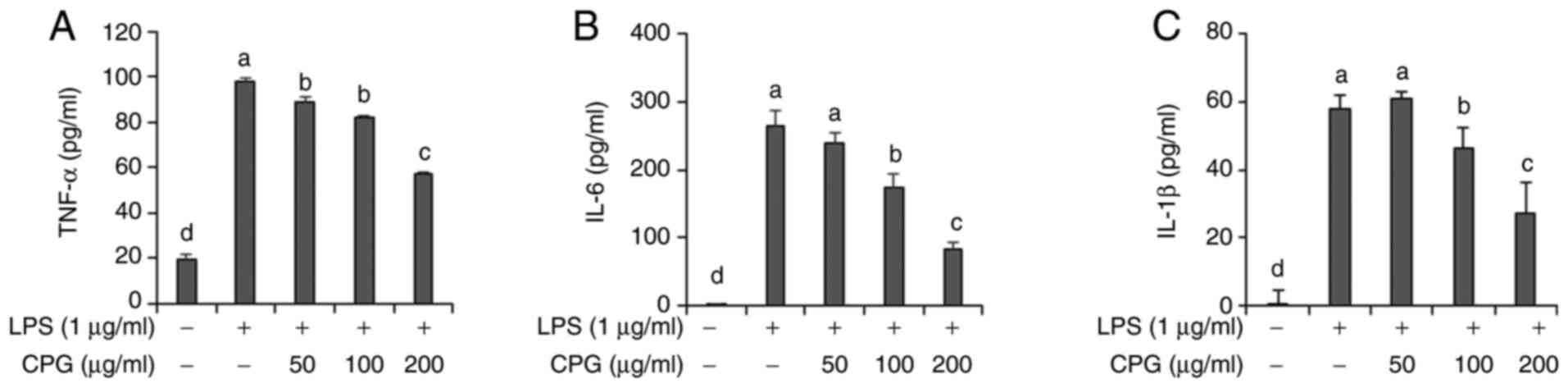
CPG inhibits TNF-α, IL-6 and IL-1β
production in LPS-stimulated RAW264.7 macrophages
Next, we evaluated effects of CPG on the production
of pro-inflammatory mediators such as IL-6, IL-1β, and TNF-α in
LPS-stimulated RAW264.7 cells. Results demonstrated that cells
treated with LPS only had significantly increased production of
IL-6, IL-1β, and TNF-α cytokines in culture media of cells compared
to untreated cells (Fig. 2).
However, production levels of TNF-α, IL-6 and IL-1β were found to
be decreased in cells pre-treated with 100 or 200 µg/ml of CPG
before the administration of LPS (Fig.
2).
CPG inhibits NF-κB and Akt activation
in LPS-stimulated RAW264.7 macrophages
To determine molecular mechanisms underlying the
anti-inflammatory effects of CPG, we evaluated NF-κB pathways in
LPS-stimulated RAW264.7 macrophages. As shown in Fig. 3A, NF-κB p65 subunit was translocated
into the nucleus in LPS-stimulated RAW264.7 cells. However, such
translocation induced by LPS was markedly suppressed in CPG
pretreated RAW264.7 cells. DNA binding activity of NF-κB was also
significantly increased in LPS-stimulated RAW264.7 cells. However,
when cells were pre-treated with CPG before administration of LPS,
the DNA binding activity of NF-κB was significantly reduced in a
dose-dependent manner (Fig. 3B). We
further investigated whether CPG affected the phosphorylation of
NF-κB and IκB in LPS-stimulated RAW264.7 cells. Results revealed
that cells treated with LPS alone had significantly increased
phosphorylation of NF-κB and IκB. However, phosphorylated NF-κB and
IκB were reversed when cells were pre-treated with 50 to 200 µg/ml
of CPG before administration of LPS (Fig. 3C-E). We also determined effects of
CPG on Akt activation in LPS-stimulated RAW264.7 cells. Akt
phosphorylation was significantly increased in cells treated with
LPS alone. However, when cells were pre-treated with 50 to 200
µg/ml of CPG before administration of LPS, the phosphorylation of
Akt was significantly reduced in a dose-dependent manner (Fig. 3F).
CPG inhibits IFN-β production and
STAT1 activation in LPS-stimulated RAW264.7 macrophages
Next, we analyzed the effect of CPG on IFN-β
production in LPS-stimulated RAW264.7 cells. As shown in Fig. 4A, the production of IFN-β was
significantly increased in cells treated with LPS only. However,
cells pre-treated with 50 to 200 µg/ml of CPG before administration
of LPS showed a significant and dose-dependent decrease in IFN-β
production compared to cells treated with LPS only. To further
analyze the mechanism of action involved in the effect of CPG on
IFN-β production, we determined STAT1 activation in LPS-stimulated
RAW264.7 cells. As shown in Fig. 4B and
C, when cells were treated with LPS alone, the phosphorylation
of STAT1 was significantly increased in RAW264.7 cells. However,
the phosphorylation of STAT1 was significantly decreased when cells
were pre-treated with 50 to 200 µg/ml of CPG before administration
of LPS.
CPG induces HO-1 expression in
LPS-stimulated RAW264.7 macrophages
To further determine anti-inflammatory effects of
CPG, we investigated HO-1 expression in LPS-stimulated RAW264.7
cells. As shown in Fig. 5A, cells
treated with LPS alone showed slightly reduced HO-1 expression than
non-treated cells. However, HO-1 expression was significantly
increased when cells were pretreated with 50 to 200 µg/ml of CPG
before the administration of LPS. We also investigated HO-1
activity in LPS-stimulated RAW264.7 cells (Fig. 5B). Results showed that HO-1 activity
was increased in cells pre-treated with 50 to 200 µg/ml of CPG
before the administration of LPS.
Discussion
As a result of measuring antioxidant and
anti-inflammatory effects of C. zawadskii, peppermint, and
Glycyrrhiza glabra in our preliminary study, it was
confirmed that there was a synergistic effect when these herb were
combined than a single substance. Therefore, in the present study,
we investigated anti-inflammatory effects of CPG in LPS-treated
RAW264.7 cells. LPS can cause inflammation in the human body via
excessive production of pro-inflammatory mediators such as NO,
PGE2, iNOS, COX-2, TNF-α, IL-6, and IL-1β (14,15).
In the present study, we found that CPG significantly decreased
LPS-elevated NO, PGE2, iNOS, COX-2 production in
RAW264.7 cells. The production of TNF-α, IL-6 and IL-1β in
stimulated cells was also suppressed by CPG. Several studies have
found that herbal mixture can suppress NO, PGE2, iNOS,
COX-2, TNF-α, IL-6, and IL-1β in macrophages (16,17).
Thus, CPG might be useful for suppressing inflammatory
mediators.
The production of pro-inflammatory cytokines by LPS
is implicated in the NF-κB signaling pathway. Therefore, NF-κB has
been studied as a key target in the treatment of inflammation
(18). NF-κB activation was
investigated in this study to elucidate the precise mechanism
involved in the effect of CPG on LPS-induced inflammation in
RAW264.7 cells. When LPS is recognized by Toll-like receptor 4
(TLR4), NF-κB is activated through the activation of MyD88 with
phosphorylation and decomposition of IκBα (19). IκBα, an inhibitor of NF-κB, is
regulated by IκB kinase (IKK). When activated, NF-κB subunit p65
separates from IκBα and migrates from the cytoplasm to the nucleus,
causing transcription of pro-inflammatory cytokine genes (20). Previous studies have reported the
anti-inflammatory effect of a herbal mixture is due to inhibition
of NF-κB signaling pathway (21,22).
Akt is activated by PI3K. It is involved in the activation of
transcription factors including NF-κB (23). Our results revealed that CPG
inhibited the translocation and DNA binding activity of NF-κB in
LPS-stimulated RAW264.7 cells. CPG also suppressed the
phosphorylation of Akt, IκB, and NF-κB p65 in LPS-stimulated
RAW264.7 cells. Results of the present study suggest that the
anti-inflammatory effect of CPG might be due to the inactivation of
NF-κB, at least in part.
LPS stimulation can induce IFN-β production in
macrophages through the TRIF/TBK1/IRF3 signaling pathway. IFN-β
production is related to NF-κB and MAPK activation (24). STAT1 activation is also required for
IFN-β production (25). Previous
studies have reported that a herbal mixture can attenuate
inflammatory responses by suppressing the inhibition of NF-κB and
IFN-β/STAT1 activation in macrophages (26). Consistent with previous studies, our
results demonstrated that CPG could suppress IFN-β production and
STAT1 phosphorylation increased by LPS stimulation in RAW264.7
cells. These findings suggest that CPG could suppress inflammatory
responses by regulating IFN-β/STAT1 activation and NF-κB
pathway.
In immune responses, HO-1 plays an important role in
the regulation of NO production. HO-1 induction is regulated by
Nrf2 transcription factor, a strong antioxidant/anti-inflammatory
regulator (27). Our results showed
that CPG increased levels of HO-1 expression induced by LPS
stimulation in RAW264.7 cells. Many studies have reported that
natural products and herbal mixture possess anti-inflammatory
activities by regulating Nrf2/HO-1 expression in macrophages
(28,29). Therefore, Nrf2 transcription factor
needs to be investigated in the future.
Herbal medicine is believed to have less side
effects compared to drugs such as chemical counterparts or
synthetic anti-inflammatory agents. Its effectiveness has been
proven empirically for a long time (30). Anti-inflammatory and antioxidant
functions of C. zawadskii, peppermint, and licorice have
been reported previously (11–13).
Linarin, the major bioactive substance of C. zawadskii, has
been reported to exhibit anti-inflammatory, antioxidant,
hepatoprotective, antibacterial, and anticancer activities
(31). Limonene, one of major
ingredients of peppermint, has been reported to have
anti-inflammatory, antioxidant, antibacterial, and anti-cancer
effects (32). A previous study has
shown that glycyrrhetinic acid exhibits anti-inflammatory effects
through NO scavenging in LPS-stimulated RAW264.7 cells (33). Our results also found that linarin,
limonene, and glycyrrhetinic acid could inhibit NO production
increased by LPS treatment in RAW264.7 cells (data not shown).
Therefore, the anti-inflammatory effect of CPG might be due to
bioactive substances contained in these three extracts used in the
present study.
In conclusion, CPG herbal mixture could suppress the
production of NO, iNOS, COX-2, and other pro-inflammatory mediators
including PGE2, TNF-α, IL-6, IL1-β, and IFN-β in
LPS-stimulated RAW264.7 macrophages. CPG also could suppress
LPS-induced inflammation through Akt/NF-κB and STAT1 signaling
pathways in RAW264.7 cells. Moreover, CPG could inhibit LPS-induced
inflammation through HO-1 induction in RAW264.7 cells. Results of
the present study demonstrates that CPG should be considered as
anti-inflammatory natural materials for the treatment of
inflammatory diseases. In addition, animal model studies are
required to verify whether the effect of CPG, which was shown at
the cellular level, appears in animals.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The Ministry of Trade, Industry, and Energy (MOTIE),
Korea, under the ‘Regional Specialized Industry Development Program
(grant no. S2913418)’ supervised by the Korea Institute for
Advancement of Technology (KIAT) financially supported this
research.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BOC and SIJ conceived the study and developed the
methodology. SIJ provided resources, reviewed and edited the
manuscript, and supervised the study. JYS, HJK, JHP, SH and FW
participated in acquisition, analysis and interpretation of data.
BOC and JYS wrote the original draft. BOC, JYS and SIJ confirmed
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheung RCF, Ng TB, Wong JH, Chen Y and
Chan WY: Marine natural products with anti-inflammatory activity.
Appl Microbiol Biotechnol. 100:1645–1666. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Funk CD: Prostaglandins and leukotrienes:
Advances in eicosanoid biology. Science. 294:1871–1875. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nathan C: Nitric oxide as a secretory
product of mammalian cells. FASEB J. 6:3051–3064. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weisz A, Cicatiello L and Esumi H:
Regulation of the mouse inducible-type nitric oxide synthase gene
promoter by interferon-gamma, bacterial lipopolysaccharide and
NG-monomethyl-L-arginine. Biochem J. 316:209–215. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bishop-Bailey D, Calatayud S, Warner TD,
Hla T and Mitchell JA: Prostaglandins and the regulation of tumor
growth. J Environ Pathol Toxicol Oncol. 21:93–101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seibert K, Zhang Y, Leahy K, Hauser S,
Masferrer J, Perkins W, Lee L and Isakson P: Pharmacological and
biochemical demonstration of the role of cyclooxygenase 2 in
inflammation and pain. Proc Natl Acad Sci USA. 91:12013–12017.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Islam SU, Lee JH, Shehzad A, Ahn EM, Lee
YM and Lee YS: Decursinol angelate inhibits LPS-induced macrophage
polarization through modulation of the NFκB and MAPK signaling
pathways. Molecules. 23:18802018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hofseth LJ and Ying L: Identifying and
defusing weapons of mass inflammation in carcinogenesis. Biochim
Biophys Acta. 1765:74–84. 2006.PubMed/NCBI
|
|
9
|
Nishida T, Yabe Y, Fu HY, Hayashi Y, Asahi
K, Eguchi H, Tsuji S, Tsujii M, Hayashi N and Kawano S:
Geranylgeranylacetone induces cyclooxygenase-2 expression in
cultured rat gastric epithelial cells through NF-kappaB. Dig Dis
Sci. 52:1890–1896. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsieh IN, Chang AS, Teng CM, Chen CC and
Yang CR: Aciculatin inhibits lipopolysaccharide-mediated inducible
nitric oxide synthase and cyclooxygenase-2 expression via
suppressing NF-κB and JNK/p38 MAPK activation pathways. J Biomed
Sci. 18:282011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim HS: Extracts of Chrysanthemum
zawadskii attenuate oxidative damage to vascular endothelial
cells caused by a highly reducing sugar. Cytotechnology.
69:915–924. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McKay DL and Blumberg JB: A review of the
bioactivity and potential health benefits of peppermint tea
(Mentha piperita L.). Phytother Res. 20:619–633. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu L, Fan Y, Fan C, Yu Y, Sun L, Jin Y,
Zhang Y and Ye RD: Licocoumarone isolated from Glycyrrhiza
uralensis selectively alters LPS-induced inflammatory responses
in RAW 264.7 macrophages. Eur J Pharmacol. 801:46–53. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee WS, Shin JS, Jang DS and Lee KT:
Cnidilide, an alkylphthalide isolated from the roots of Cnidium
officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and
TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7
macrophages. Int Immunopharmacol. 40:146–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seo S, Lee KG, Shin JS, Chung EK, Lee JY,
Kim HJ and Lee KT: 6′-O-Caffeoyldihydrosyringin isolated from
Aster glehni suppresses lipopolysaccharide-induced iNOS,
COX-2, TNF-α, IL-1β and IL-6 expression via NF-κB and AP-1
inactivation in RAW 264.7 macrophages. Bioorg Med Chem Lett.
26:4592–4598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang HJ, Hong SH, Kang KH, Park C and Choi
YH: Anti-inflammatory effects of Hwang-Heuk-San, a traditional
Korean herbal formulation, on lipopolysaccharide-stimulated murine
macrophages. BMC Complement Altern Med. 15:447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kao ST, Lin CS, Hsieh CC, Hsieh WT and Lin
JG: Effects of xiao-qing-long-tang (XQLT) on bronchoconstriction
and airway eosinophil infiltration in ovalbumin-sensitized guinea
pigs: In vivo and in vitro studies. Allergy. 56:1164–1171. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu XL, Liou CJ, Li ZY, Lai XY, Fang LW and
Huang WC: Sesamol suppresses the inflammatory response by
inhibiting NF-κB/MAPK activation and upregulating AMP kinase
signaling in RAW 264.7 macrophages. Inflamm Res. 64:577–588. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan H, Xu LH, Ouyang DY, Wang Y, Zha QB,
Hou XF and He XH: The second-generation mTOR kinase inhibitor
INK128 exhibits anti-inflammatory activity in
lipopolysaccharide-activated RAW 264.7 cells. Inflammation.
37:756–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ni W, Zhang Q, Liu G, Wang F, Yuan H, Guo
Y, Zhang X, Xie F, Li Q and Tai G: Escherichia coli
maltose-binding protein activates mouse peritoneal macrophages and
induces M1 polarization via TLR2/4 in vivo and in vitro. Int
Immunopharmacol. 21:171–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang LH, Pan XP, Gong KR and Shao G:
Anti-inflammatory effects of three kinds of traditional Mongolian
medicine monomer and its combination on LPS-stimulated RAW264.7
macrophages. Eur Rev Med Pharmacol Sci. 20:950–958. 2016.PubMed/NCBI
|
|
22
|
Kalaiselvan S and Rasool MK: Triphala
herbal extract suppresses inflammatory responses in LPS-stimulated
RAW 264.7 macrophages and adjuvant-induced arthritic rats via
inhibition of NF-κB pathway. J Immunotoxicol. 13:509–525. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cianciulli A, Calvello R, Porro C, Trotta
T, Salvatore R and Panaro MA: PI3k/Akt signalling pathway plays a
crucial role in the anti-inflammatory effects of curcumin in
LPS-activated microglia. Int Immunopharmacol. 36:282–290. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawai T and Akira S: Signaling to
NF-kappaB by Toll-like receptors. Trends Mol Med. 13:460–469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim HY, Kim JH, So Y, Kang SY, Jeong HG
and Jin CH: Anti-inflammatory effect of lupinalbin A isolated from
Apios americana on lipopolysaccharide-treated RAW264.7
cells. Molecules. 23:5832018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han YK, Kim YS, Natarajan SB, Kim WS,
Hwang JW, Jeon NJ, Jeong JH, Moon SH, Jeon BT and Park PJ:
Antioxidant and anti-inflammatory effects of Chaenomeles
sinensis leaf extracts on LPS-stimulated RAW 264.7 cells.
Molecules. 21:4222016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shinkai Y, Yamanaka I, Duong HH, Quynh NT,
Kanaho Y and Kumagai Y: Garcinia vilersiana bark extract
activates the Nrf2/HO-1 signaling pathway in RAW264.7 cells. J
Toxicol Sci. 38:875–878. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong C, Cao J, Wu CF, Kadioglu O,
Schüffler A, Kauhl U, Klauck SM, Opatz T, Thines E, Paul NW and
Efferth T: The Chinese herbal formula Free and Easy Wanderer
ameliorates oxidative stress through KEAP1-NRF2/HO-1 pathway. Sci
Rep. 7:115512017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park JY, Kwon YW, Lee SC, Park SD and Lee
JH: Herbal formula SC-E1 suppresses lipopolysaccharide-stimulated
inflammatory responses through activation of Nrf2/HO-1 signaling
pathway in RAW 264.7 macrophages. BMC Complement Altern Med.
17:3742017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yatoo MI, Gopalakrishnan A, Saxena A,
Parray OR, Tufani NA, Chakraborty S, Tiwari R, Dhama K and Iqbal
HMN: Anti-inflammatory drugs and herbs with special emphasis on
herbal medicines for countering inflammatory diseases and
disorders-a review. Recent Pat Inflamm Allergy Drug Discov.
12:39–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim KY, Oh TW, Yang HJ, Kim YW, Ma JY and
Park KI: Ethanol extract of Chrysanthemum zawadskii Herbich
induces autophagy and apoptosis in mouse colon cancer cells through
the regulation of reactive oxygen species. BMC Complement Altern
Med. 19:2742019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vieira AJ, Beserra FP, Souza MC, Totti BM
and Rozza AL: Limonene: Aroma of innovation in health and disease.
Chem Biol Interact. 283:97–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou JX and Wink M: Evidence for
anti-inflammatory activity of isoliquiritigenin, 18β glycyrrhetinic
acid, ursolic acid, and the traditional chinese medicine plants
Glycyrrhiza glabra and Eriobotrya japonica, at the
molecular level. Medicines (Basel). 6:552019. View Article : Google Scholar : PubMed/NCBI
|