Introduction
Due to the prevalence of hypertension, diabetes,
obesity and other diseases that cause kidney damage, the incidence
of chronic kidney disease (CKD) is also on the rise year by year
and has been one of the most serious illnesses that jeopardize
human health (1). CKD is a
progressive disease in which irreversible damage to the function
and structure of the kidney occurs gradually over months or years.
Renal fibrosis is an important pathological alteration in the
progression of CKD to end-stage renal disease. Fibrosis is a repair
of impairment; however, pathological fibrosis can cause organ
dysfunction (2). The degree of
renal fibrosis is also considered to be closely related to the
prognosis (3). Thus, it was deemed
extremely important to investigate effective treatment for renal
fibrosis.
Renal fibrosis is mainly divided into two parts:
glomerular fibrosis and interstitial fibrosis (4) and the present study only focused on
renal interstitial fibrosis, which is characterized by infiltration
of inflammatory cells and deposition of collagen fiber with various
degrees of renal insufficiency. The mechanisms of renal
interstitial fibrosis are generally thought to be related to
inflammatory responses, activation and proliferation of
fibroblasts, accumulation of extracellular matrix (ECM),
epithelial-mesenchymal transition (EMT) (5), agonism of the renin-angiotensin
system (RAS) and metabolic abnormalities, for instance, both an
excess of parathyroid hormone and a deficiency of vitamin D can
contribute to the progression of renal fibrosis in the patients
with CKD (2,3,6). In
recent years, the correlation between inflammatory response and
renal fibrosis has received increasing attention. The inflammatory
response is a critical part of renal interstitial fibrosis, which
is mainly accomplished by two types of inflammatory cells,
macrophages and lymphocytes (4,7,8). In
addition to secreting inflammatory mediators that exacerbate tissue
injury, they also encourage fibroblast activation and accumulation
of ECM (3). In unilateral ureteral
obstruction (UUO) mice, knocking out the recombinant activator 1
protein hinders lymphocyte maturation attenuated renal fibrosis
injury. Conversely, renal fibrosis is appreciably worse after
lymphocyte importation (9). During
the progression of acute kidney injury to CKD, inhibition of
macrophage secretion significantly attenuated renal fibrosis
(10). Li et al (5) demonstrate that renal fibrosis injury
is alleviated following the suppression of the inflammatory
response. These previous studies confirmed that inhibition of the
inflammatory response reduced renal interstitial fibrosis.
Dioscin (DIS) is an active ingredient of
Dioscoreaceae herbs with a number of biological activities
(11). Its pharmacological effects
are extensive, mainly involving the heart, liver, lung, kidney and
other organs, with anti-inflammatory, anti-fibrotic, anti-tumor,
anti-atherosclerotic, immunomodulatory and modulating oxidative
stress responses (11–15). The regulation of the NF-κB pathway
can trigger inflammatory responses (16). Studies have found that DIS can
diminish the expression of inflammatory factors by inhibiting the
phosphorylation of NF-κB p65 and thus suppress the inflammatory
response (13,14). In addition, it has been found that
DIS could reduce renal fibrosis by upregulating the Sirt3 signal
(17). However, it is unclear
whether DIS alleviates renal fibrosis by reducing NF-κB signaling
pathway-mediated inflammatory response. The present study analyzed
the targets of DIS action on renal fibrosis by network pharmacology
(as shown in the Fig. S1), which
included the NF-κB signaling pathway. The purpose of the current
study is to determine how DIS affects renal fibrosis and
inflammation, as well as potential underlying mechanisms.
Materials and methods
Identification of therapeutic targets
for DIS in renal interstitial fibrosis
To investigate the effect of DIS on renal
interstitial fibrosis, the present study used network pharmacology
to predict the targets of DIS. PubChem (https://pubchem.ncbi.nlm.nih.gov/) was used to get the
structured and SMILE files of DIS. The SMILE files were imported
into SwissTargetPrediction (http://www.swisstargetprediction.ch/) and TargetNet
(http://targetnet.scbdd.com/) to search
the potential targets. The keywords ‘renal interstitial fibrosis’
were entered into DrugBank (https://go.drugbank.com), CTD (http://ctdbase.org), Genecards (http://www.genecards.org), DisGeNET (https://www.disgenet.org) and OMIM databases
(http://omim.org) to collect the targets of disease.
Then, the process of DIS on renal fibrosis was analyzed using Kyoto
Encyclopedia of Genes and Genomes pathway analysis in Metascape
database (http://www.metascape.org/).
Significant pathways with P<0.05 were chosen.
UUO mice models and DIS treatment
To determine the sample size for each group of mice,
the present study reviewed studies reporting on UUO mice and noted
the high mortality rate of mice when UUO surgery was performed on
the mice. The kidneys of the mice were intended for western
blotting, reverse transcription-quantitative (RT-q) PCR and
histopathological staining. Due to the amount of mouse kidney
tissue required for these analyses, it decided to set the sample
size at 10 mice per group, based on the research paper by Cao et
al (18) and Gu et al
(19).
Male C57BL/6 mice from Beijing Weitong Lihua
Laboratory Animal Technology Co., Ltd. weighing 17–22 g. The mice
were kept in a cage with a 12-h light/dark cycle, a standard feed
and unlimited access to water at a temperature of 22±2°C and
humidity of 40±5%. The bedding of the cages was changed daily and
the condition of the mice was checked daily. The animal study was
reviewed and approved by the Ethics Committee of the China-Japan
Friendship Institute of Clinical Medical Sciences (approval no.
zryhyy21-22-01-09; affiliated with the China-Japan Friendship
Hospital, Beijing, China).
A total of 80 mice were randomly assigned to one of
four groups (n=20/group), which included the sham-operated group,
the UUO group, the UUO + 50 mg/ kg DIS group and the UUO + 100
mg/kg DIS group. After three days of adaptive feeding, a model of
interstitial renal fibrosis was prepared in mice by using UUO
surgery. Pentobarbital (1%; 40 mg/kg) was used 1% to anesthetize
mice by intraperitoneal injection before the surgery. The
unilateral ureteral obstruction surgery was described previously
(20). The sham-operated group
underwent a sham operation. The mice were fasted for 12 h but were
given free access to drinking water before the operation. The DIS
group was administered from the first day after surgery and gavaged
with DIS 50 mg/ kg or 100 mg/kg every 24 h for 7 days. DIS was
purchased from Herbpurify company (CAS: 19057-60-4, purity
>98.0%, ID: S-048, Chengdu Herbpurify Co., Ltd.). The
sham-operated and the UUO groups were orally administered
physiological saline of equal volumes every 24 h for 7 days. Half
of the mice were sacrificed on the 3rd and 7th day of treatment to
obtain kidney tissue, respectively. The mice were fasted for 12 h
before sacrifice. Pentobarbital sodium (1%; 50 mg/kg) by
intraperitoneal injection was used, followed by intracardiac
exsanguination. Animal mortality was verified by the absence of a
heartbeat and respiration for >3 min.
Determination of urine protein and
urine creatinine in mice
At the end of the experiment, urine in mice was
collected. Urine protein was detected by mouse albumin ELISA kit
(Bethyl Laboratories, Inc.). Urine creatinine was detected by an
automatic biochemical analyzer. Then, the ratio of urine protein to
urine creatinine was calculated.
Cell culture and pharmaceutical
treatment
Human renal tubular epithelial cells (HK-2) were
obtained from Professor HY Lan (Chinese University of Hong Kong)
and placed in a cell incubator at 37°C and 5% CO2. The
cells were cultured with DMEM/F-12 medium (Corning, Inc.), 10%
fetal bovine serum (Thermo Fisher Scientific, Inc.) and 1X double
antibiotics (penicillin and streptomycin). TGF-β1 (2 ng/ml) was
used to induce renal fibrosis model in HK-2 cells. Then, the cells
were respectively treated with DIS (3.125, 6.25, 12.5 µM) and
Bay11-7082 (1 µM; cat. no. B5556; MilliporeSigma) for another 24 h.
DIS (CAS: 19057-60-4; purity >98.0%; cat. no. SD8350) was
purchased from Beijing Solarbio Science & Technology Co.,
Ltd.
Cell viability analysis
CCK-8 assay (Mei5 Biotechnology, Co., Ltd.) was used
to evaluate DIS on HK-2 viability. HK-2 cells were seeded and grown
in a 96-well plate for 24 h at 37°C. Subsequently, the cells were
treated with DIS for indicated periods (24, 48 and 72 h),
respectively. Then the cells were incubated for 1 h with 10 µl of
CCK-8 solution (5 mg/ml). An enzyme marker was used for analyzing
cell absorbance at 450 nm.
Histopathological and
immunohistochemistry staining
Kidney tissue specimens from mice were fixed with
10% formalin solution overnight at 4°C and were dehydrated by
ethanol. These specimens were then embedded in paraffin and were
cut into 2–3-µm sections by a routine procedure (21). The sections were stained with
hematoxylin and eosin (HE) and Masson's trichrome as described
previously (22). Briefly, for HE
staining, the sections were stained with hematoxylin for 5 min
followed by eosin for 3 min, both at room temperature. For Masson's
trichrome staining, the sections were stained with hematoxylin for
5 min, lichon red acidic magenta solution for 10 min, 1%
phosphomolybdic acid solution for 5 min and aniline blue solution
for 5 min. All Masson's trichrome staining steps were conducted at
room temperature. All stained sections were viewed by a light
microscope (Olympus BX53; Olympus Corporation).
The sections were routinely dewaxed and hydrated
with 3% H2O2 solution for 10 min and rinsed
three times with distilled water. Subsequently, the antigen was
retrieved using a microwave oven. The sections were heated in a
microwave oven on high with 0.01 M sodium citrate buffer for 10
min, cooled and rinsed once with PBS buffer. The blocking solution
was added dropwise for 20 min. Primary antibodies against TNF-α
(1:50; cat. no. sc-52746; Santa Cruz Biotechnology, Inc.),
alpha-smooth muscle actin (α-SMA; 1:50; cat. no. 19245; Cell
Signaling Technology, Inc.) and fibronectin (FN; 1:50; cat. no.
ab2413; Abcam) were added dropwise, incubated for 2 h at room
temperature and then rinsed 5 times for 2 min each using PBS
buffer. The secondary bio-goat anti-mouse IgG and bio-goat
anti-rabbit IgG antibodies (1:50 dilution; both from the
immunohistochemistry staining kit; cat. no. MF501-01; Mei5
Biotechnology Co., Ltd.) were added dropwise, incubated for 30 min
at room temperature and then rinsed 5 times with PBS buffer.
Streptavidin-POD working solution was added according to the
immunohistochemistry kit instructions, kept for 30 min and rinsed 5
times with PBS buffer. The reaction was terminated by dropwise
addition of ready-to-use DAB, color development for 30 min and
rinsing with distilled water. Finally, after light re-staining with
hematoxylin and dehydration, neutral gum was used to seal the
sections.
Immunofluorescence staining
The HK-2 cells on coverslips were fixed with 4%
paraformaldehyde for 10 min at 4°C. The cells were stained with
primary antibodies against NOD-like receptor thermal protein domain
associated protein 3 (NLRP3; 1:100; cat. no. ab260017; Abcam),
p-NF-κB p65 (1:100; cat. no. sc-136548; Santa Cruz Biotechnology,
Inc.) and secondary antibody goat anti-mouse IgG/Alexa Fluor 488
(1:1,000; cat. no. K0031G-AF488; Solarbio Technology Company,
Beijing, China), respectively. Subsequently, the cells were
incubated with DAPI (1 µg/ml; cat. no. C0060; Beijing Solarbio
Science & Technology Co., Ltd.) for 5 min protected from light
and sealed with 80% glycerol. A fluorescent inverted microscope was
used to capture final fluorescence images.
Western blotting
Radioimmunoprecipitation lysis buffer was used to
extract proteins from the kidney tissue and cultured cells. The
concentration of protein was determined using a BCA Protein Assay
Kit (cat. no. MF071-01; Mei5 Biotechnology, Co., Ltd.). Each lane
was loaded with 100–120 µg of protein. Electrophoresis was
performed with 10 or 15% SDS-PAGE gels. The proteins were
transferred from the gels onto PVDF membranes (cat. no. IPFL00010;
Merck KGaA) and blocked with a BSA-based western blot blocking
solution (cat. no. MF432-01; Mei5 Biotechnology, Co., Ltd.) for 1 h
at room temperature. After blocking, the PVDF membranes were
incubated with primary antibodies for 2 h at room temperature and
secondary antibodies for 1 h at room temperature (23). In the present study, primary
antibodies against β-actin (1:10,000; cat. no. sc-47778; Santa Cruz
Biotechnology, Inc.), IL-1β (1:1,000; cat. no. sc-52012; Santa Cruz
Biotechnology, Inc.), IL-6 (1:1,000; cat. no. sc-130326; Santa Cruz
Biotechnology, Inc.), TNF-α (1:1,000; cat. no. sc-52746; Santa Cruz
Biotechnology, Inc.), NF-κB p65 (1:1,000; cat. no. sc-8008; Santa
Cruz Biotechnology, Inc.), phosphorylated (p-)NF-κB p65 (1:1,000;
cat. no. sc-136548; Santa Cruz Biotechnology, Inc.), NLRP3
(1:1,000; cat. no. ab260017; Abcam) and monocyte chemotactic
protein 1 (MCP-1; 1:1,000; cat. no. sc-52701; Santa Cruz
Biotechnology, Inc.) and secondary antibodies against goat
anti-rabbit IgG (1:10,000; cat. no. MF094-01; Mei5 Biotechnology,
Co., Ltd.), goat anti-mouse IgG (1:10,000; cat. no. MF093-01; Mei5
Biotechnology, Co., Ltd.) and goat anti-rat IgG (1:10,000; cat. no.
MF756-01; Mei5 Biotechnology, Co., Ltd.) were used for incubation.
An iBright CL1000 gel imaging system program (Thermo Fisher
Scientific, Inc.) was used for capturing signals, which were
quantified by ImageJ software (ImageJ bundled with 64-bit Java;
version 1.8.0_172; National Institutes of Health).
RNA extraction and RT-qPCR
To extract RNA, HK-2 cells were seeded in 6-well
culture plates at a density of 2×106 cells/well. The
total RNA from HK-2 and renal tissues was isolated (Universal RNA
Mini kit; Mei5 Biotechnology, Co., Ltd.) and reversed-transcribed
into first strand cDNA (RevertAid First Strand cDNA Synthesis kit;
Mei5 Biotechnology, Co., Ltd.), both according to the
manufacturer's protocol. Subsequently, the UltraSYBR Green Mixture
qPCR kit (Mei5 Biotechnology, Co., Ltd.) was used to perform
RT-qPCR according to the manufacturer's protocol. The thermocycler
conditions were as follows: 95°C for 10 min, followed by 40 cycles
of 95°C for 15 sec, 65°C for 15 sec and 72°C for 45 sec, ending
with 1 cycle of 72°C for 10 min. All experiments were repeated
three times. The results were analyzed by the 2−ΔΔCq
method and normalized to the expression levels of the internal
control gene, β-actin (24). All
primer sequences used in this study are shown in Table I.
 | Table I.Specific primers for reverse
transcription-quantitative PCR. |
Table I.
Specific primers for reverse
transcription-quantitative PCR.
| Animal | GenePrimer | Sequence
(5′-3′) |
|---|
| Mouse | NLRP3 | F:
ATTACCCGCCCGAGAAAGG |
|
|
| R:
TCGCAGCAAAGATCCACACAG |
|
| TNF-α | F:
CATGAGCACAGAAAGCATGATCCG |
|
|
| R:
AAGCAGGAATGAGAAGAGGCTGAG |
|
| IL-6 | F:
CTGCAAGAGACTTCCATCCAG |
|
|
| R:
AGTGGTATAGACAGGTCTGTTGG |
|
| IL-1β | F:
CTTCAGGCAGGCAGTATCACTCAT |
|
|
| R:
TCTAATGGGAACGTCACACACCAG |
|
| MCP-1 | F:
TAAAAACCTGGATCGGAACCAAA |
|
|
| R:
GCATTAGCTTCAGATTTACGGGT |
|
| β-actin | F:
ACCCTAAGGCCAACCGTGAAAAG |
|
|
| R:
CATGAGGTAGTCTGTCAGGT |
| Human | NLRP3 | F:
CGTGAGTCCCATTAAGATGGAGT |
|
|
| R:
CCCGACAGTGGATATAGAACAGA |
|
| TNF-α | F:
GAGGCCAAGCCCTGGTATG |
|
|
| R:
CGGGCCGATTGATCTCAGC |
|
| IL-6 | F:
ACTCACCTCTTCAGAACGAATTG |
|
|
| R:
CCATCTTTGGAAGGTTCAGGTTG |
|
| IL-1β | F:
ATGATGGCTTATTACAGTGGCAA |
|
|
| R:
GTCGGAGATTCGTAGCTGGA |
|
| MCP-1 | F:
CAGCCAGATGCAATCAATGCC |
|
|
| R:
TGGAATCCTGAACCCACTTCT |
|
| β-actin | F:
AGGCATCCTCACCCTGAAGTA |
|
|
| R:
CACACGCAGCTCATTGTAGA |
ELISA
A total of 100 µl each of cell culture medium and
kidney protein suspension after drug treatment was added to a
96-well plate. The concentrations of IL-1β (HiPer Human IL-1β ELISA
Kit; cat. no. MFH-02-01; Mei5 Biotechnology Co., Ltd.) and IL-18
(HiPer Human IL-18 ELISA Kit; cat. no. MFH-22-01; Mei5
Biotechnology Co., Ltd.) were detected with Elisa kit, according to
the manufacturer's instructions. Then, the absorbance was measured
in an enzyme marker instrument (Bioteke Corporation). The
concentrations were calculated using a standard curve.
Statistical analysis
All results were evaluated using the GraphPad Prism
version 8.0 (Dotmatics) and quantitative data are expressed as the
mean ± standard error of the mean. One-way ANOVA and Dunnett's test
for multiple comparisons were used for analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Targets of DIS in renal interstitial
fibrosis
According to a network pharmacology analysis
(Fig. S1), it was demonstrated
that the NF-κB signaling pathway is an important pathway for DIS in
improving renal interstitial fibrosis.
DIS attenuates renal pathological
damage in UUO mice
The sham-operated group demonstrated no abnormal
pathological changes in morphological structure (Fig. 1). In the UUO group, the renal
tubular structure was disorganized, some of the tubular epithelial
cells were detached from the brush border, cellular debris was
occasionally seen in the tubular lumen and the renal interstitium
was infiltrated with inflammatory cells and collagen was deposited.
Although the ratio of urine protein to urine creatinine in mice did
not show a statistically significant difference (Fig. S2), the degree of kidney tissue
damage in the DIS group at each time point was less than that in
the UUO group. In brief, DIS attenuated renal injury in UUO
mice.
DIS attenuates the renal inflammatory
response and fibrosis in the UUO mice
To further clarify the mechanism of DIS, the kidney
tissue in mice was analyzed. Immunohistochemistry staining
(Fig. 2) showed that the
expression of TNF-α, fibronectin (FN) and α-SMA increased in the
UUO group. However, the expression of TNF-α, FN and α-SMA decreased
in the UUO + DIS group. Western blotting (Fig. 3A) and RT-qPCR (Fig. 3B) indicated that the expression of
MCP-1, TNF-α, the ratio of p-NF-κB p65 and NF-κB p65, NLRP3, IL-1β
and IL-6 increased in the UUO group compared to the sham-operated
group. After being treated with DIS, the expressions of NLRP3,
MCP-1, TNF-α, the ratio of p-NF-κB p65 and NF-κB p65, IL-1β and
IL-6 were significantly decreased in the DIS group. Therefore, it
was hypothesized that DIS alleviated renal injury in UUO mice by
inhibiting the secretion of inflammatory factors linked to the
NF-κB pathway.
 | Figure 3.The effect of DIS on renal
inflammation and fibrosis in mice. (A) Western blotting showed the
expression of NLRP3, IL-6, IL-1β, MCP-1, TNF-α, NF-κB p65 and
p-NF-κB p65 after 7 days of DIS treatment. (B) The mRNA level of
NLRP3, IL-6, IL-1β, MCP-1 and TNF-α after 7 days of DIS treatment.
Date represented the mean ± standard error of the mean (n=3).
*P<0.05, **P<0.01 and ****P<0.0001 vs. the UUO group. DIS,
dioscin; NLRP3, NOD-like receptor thermal protein domain associated
protein 3; MCP-1, monocyte chemotactic protein 1; p-,
phosphorylated; UUO, unilateral ureteral obstruction. |
Effects of DIS and Bay11-7082 on
TGF-β1-induced HK-2 cells
To further clarify the mechanism of DIS, TGF-β1 was
used to induce a renal fibrosis model in vitro. First, the
cells were treated with NF-κB inhibitor Bay11-7802. The expressions
of NLRP3, IL-6, TNF-α, the ratio of p-NF-κB p65 and NF-κB p65,
MCP-1, IL-1β and IL-18 were reduced (Fig. 4), which indicated that the
inflammatory response was attenuated and the NF-κB pathway was
inhibited. Subsequently, the cells were treated with DIS. The
expressions of NLRP3, MCP-1, IL-6, TNF-α, IL-1β, IL-18 and the
ratio of p-NF-κB p65 and NF-κB p65 were also reduced (Fig. 5), showing a similar effect to
Bay11-7082, which indicated that DIS attenuated the inflammatory
response by suppressing the NF-κB pathway.
 | Figure 4.The effect of Bay11-7082 on renal
inflammation in TGF-β induced HK-2 cells. (A) Immunofluorescence
staining reflected the expressions of NLRP3 and p-NF-κB p65
(Magnification of ×200; scale bar, 50 µm). (B) Western blotting
showed the expressions of NLRP3, IL-6, IL-1β, MCP-1, TNF-α, NF-κB
p65 and p-NF-κB p65. (C) The mRNA level of NLRP3, IL-6, IL-1β,
MCP-1 and TNF-α. (D) Elisa assay reflected the concentration of
IL-1β and IL-18. Date represented the mean ± standard error of the
mean (n=3). *P<0.05, **P<0.01 and ***P<0.001 compared with
the TGF-β1 group. NLRP3, NOD-like receptor thermal protein domain
associated protein 3; MCP-1, monocyte chemotactic protein 1; p-,
phosphorylated; UUO, unilateral ureteral obstruction. |
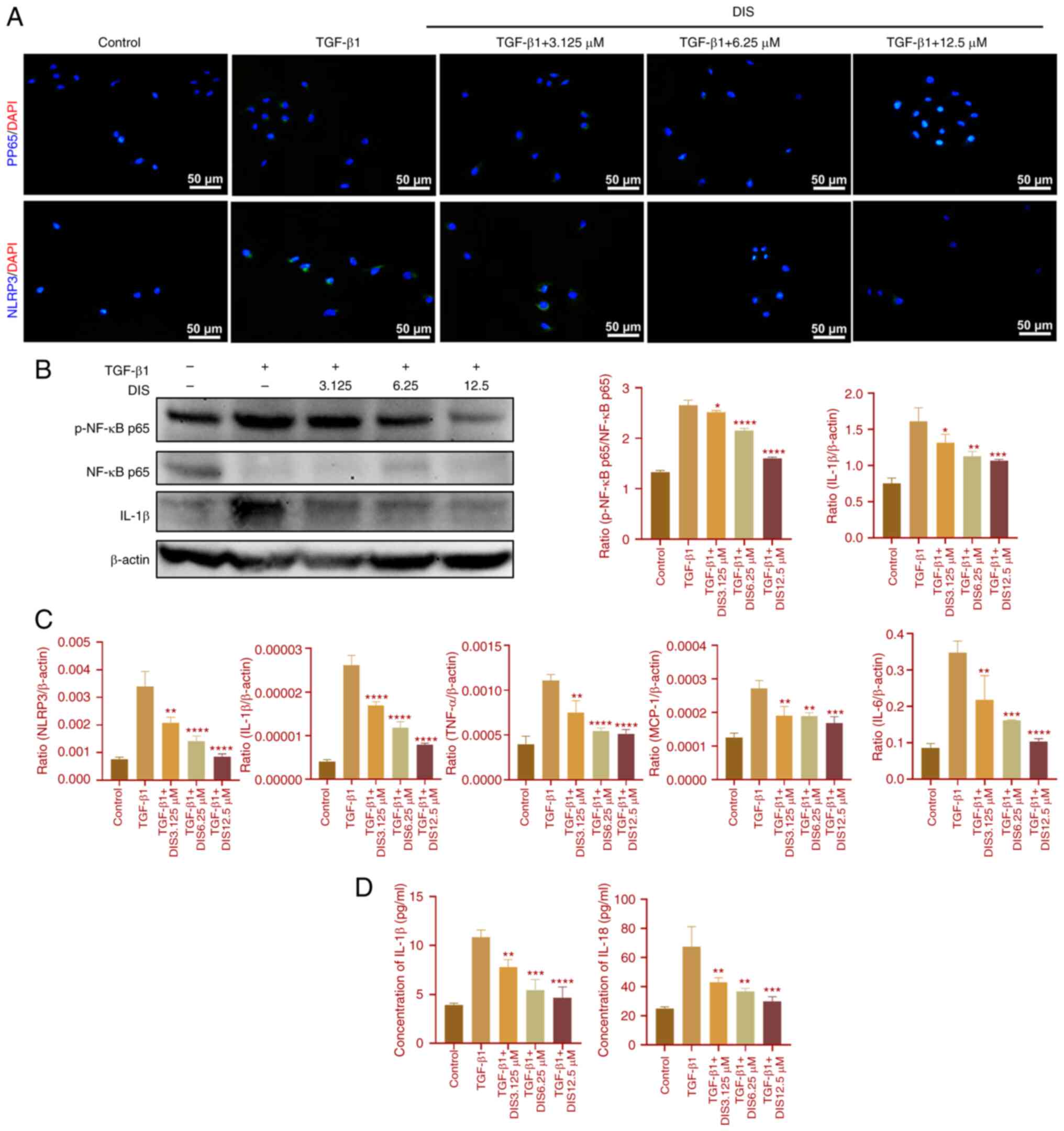 | Figure 5.The effect of DIS on renal
inflammation in TGF-β induced HK-2 cells. (A) Immunofluorescence
staining reflected the expression of NLRP3 and p-NF-κB p65
(Magnification of ×200; scale bar, 50 µm). (B) Western blotting
showed the expression of IL-1β, NF-κB p65 and p-NF-κB p65. (C) The
mRNA level of NLRP3, IL-1β, MCP-1, TNF-α and IL-6. (D) Elisa assay
reflected the concentration of IL-1β and IL-18. Date represented
the mean ± standard error of the mean (n=3). *P<0.05,
**P<0.01, ***P<0.001 and ****P<0.0001 compared with the
TGF-β1 group. DIS, dioscin; NLRP3, NOD-like receptor thermal
protein domain associated protein 3; MCP-1, monocyte chemotactic
protein 1; p-, phosphorylated. |
Safety of DIS
To clarify the impact of DIS on renal interstitial
fibrosis, the toxicity of DIS on cells was tested by CCK-8 assay.
The result showed no significant toxicity to cells when the
concentration of DIS was in the range of 0–20 µM and significant
toxicity to cells when its concentration was in the range of 40–80
µM (Fig. 6A). The concentrations
of 3.125, 6.25 and 12.5 µM were selected for further experiments
and did not find that DIS trigger inflammation (Fig. 6B).
Discussion
CKD has developed into a global public health issue
that imposes a heavy economic burden on society and individual
patients. The hallmark pathological features of CKD include
interstitial fibrosis, tubular atrophy and interstitial
inflammation (25). The close link
between inflammation and fibrosis has become increasingly
recognized (26). The inflammatory
response plays a core position in the pathological mechanisms of
renal fibrosis. In the early stages of CKD, various injuries induce
an inflammatory response in the kidney. Persistent inflammation and
cytokine release can activate fibroblasts. Activated fibroblasts
overexpress α-SMA and FN, which are subsequently deposited in the
kidney and trigger renal fibrosis (27). Previous studies have shown that a
number of herbal extracts can reduce renal fibrosis by inhibiting
the inflammatory response. Li et al (5) demonstrated that salidroside treatment
markedly decreases the secretion of inflammatory cytokines and
ameliorates renal fibrosis by the inhibition of the TLR4/NF-κB and
MAPK pathway in renal interstitial fibrosis in vivo and
in vitro. Similarly, Liao et al (28) found that isoliquiritigenin reduces
renal inflammation and fibrosis in UUO-induced chronic kidney
damage via inhibiting the inflammatory response and fibrosis
transformation in macrophages in vitro. DIS is a natural
herbal extract with a wide range of biological activities (11). Available studies have demonstrated
that DIS has a protective effect against renal injury induced by
diabetic and nephrotoxic drugs, mainly through the regulation of
oxidative stress, inflammatory response, lipid metabolism and
cellular autophagy (17,29–32).
However, no studies have been reported, to the best of the authors'
knowledge, on the ability of DIS to attenuate renal fibrosis by
inhibiting NF-κB signaling pathway-mediated inflammatory
responses.
The NF-κB family has important roles in inflammatory
responses, immune-related diseases and tumorigenesis (33). In mammals, the NF-κB family
contains five-member proteins, including p65/RelA, RelB, cRel, p50
and p52 (34). Among them, NF-κB
p65 is a central regulator of the inflammatory response (16). NF-κB p65 is activated by
phosphorylation into p-NF-κB p65. The p-NF-κB p65 enters the
nucleus and is involved in the regulation of cytokine expression,
resulting in increased expressions of IL-6 (35), TNF-α (36), MCP-1, NLRP3, cytokine IL-18 and
IL-1β (37). Subsequently, these
cytokines participate in the inflammatory response and fibrotic
processes (38).
MCP-1 is a member of the chemotactic cytokine family
that recruits inflammatory cells to reach the site of injury.
Reducing expression of MCP-1 reduces the renal inflammatory
response in mice (39). Du et
al (40) showed that CD38
deficiency increases the phosphorylation of NF-κB p65 and the
expression of its downstream target protein MCP-1 also increases in
CD38-deficient wild mice. Wada et al (27) found that MCP-1 recruited macrophage
through CCR2 signaling, which resulted in renal fibrosis. NLRP3
inflammasome is a multimeric protein complex formed by the
interaction of NLRP3 and apoptosis-associated speck-like protein
containing (41), which enables
caspase-1 activation and thus promotes the maturation and secretion
of IL-18 and IL-1β (37). It has
been demonstrated that the conversion of tubular epithelial cells
to renal fibrosis is modulated by NLRP3 via the TGF-β/Smad pathway
in mice (42). IL-1β, a key signal
in promoting fibril formation, plays a part in facilitating fibril
formation by binding to IL-1βRs (43). In addition, IL-1β promotes
fibrogenesis not only through c-Jun, TGF-β, PI3K/Akt and ERK1/2 but
also by promoting the expression of platelet-derived growth factor
receptor (43), which then binds
to platelet-derived growth factor to stimulate fibrosis. This in
turn promotes the proliferation and migration of renal stromal
cells, leading to the deposition of ECM and the formation of renal
interstitial fibrosis (44). IL-18
also has a pro-fibrotic effect by promoting IFNγ and IL-13
production in Th1 cells (45) and
inducing macrophage differentiation into myofibroblast (46). In addition, Zhao et al
(47) found that the activation of
NLRP3 inflammasome could be regulated by phosphorylation of NF-κB
p65. IL-6 is a classical cytokine that plays an important part in
inflammation, autoimmune diseases, fibrotic diseases and cancer.
IL-6 gene expression is regulated by the NF-κB pathway (35). A previous study found that TNF-α,
mainly in macrophages, renal tubular cells and thylakoid cells, can
trigger an inflammatory response and renal fibrosis (48). This study also found that renal
fibrosis injury in mice was significantly reduced after treatment
with a TNF receptor-1 inhibitor.
The present study (Fig.
7) established a renal fibrosis model in mice by UUO. In the
hematoxylin and eosin and Masson's trichrome staining, it was
observed that the interstitial structure of the kidney in the UUO
group was disturbed, accompanied by massive fibrotic deposits and
inflammatory cell infiltration. However, in the DIS group,
inflammatory cell infiltration in renal tissue was alleviated and
collagen fiber deposition was reduced. In addition, the expression
of TNF-α, FN and α-SMA were reduced. Therefore, it can be concluded
that DIS has a protective effect on the kidney. In addition, in
western blotting and RT-qPCR, the expressions of IL-1β, IL-6,
NLRP3, TNF-α and MCP-1 and the phosphorylation NF-κB p65 were
downregulated in the DIS group. In summary, the present study found
that DIS inhibited the inflammatory response in mice and also
attenuated renal fibrosis, so it was hypothesized that this is
related to the inhibition of the NF-κB signaling pathway. To
further clarify the mechanism of the renal protective effect of
DIS, a renal fibrosis model was established by using TGF-β-induced
HK-2 cells in vitro. First, the cells were treated with
Bay11-7082, a known inhibitor of NF-κB nuclear transcription factor
(49), which inhibits the
phosphorylation of nuclear transcription factor NF-κB p65 and
regulates the expression of downstream genes. It was shown that the
expressions of inflammatory mediators including NLRP3, TNF-α,
IL-1β, IL-6, MCP-1 and IL-18 were downregulated in cells treated
with Bay11-7082. In addition, the ratio of p-NF-κB p65 and NF-κB
p65 was also reduced. Similarly, the expressions of inflammatory
mediators as well as the ratio of p-NF-κB p65 and NF-κB p65 were
downregulated under the DIS treatment.
 | Figure 7.An overview of the potential
mechanism by which dioscin (DIS) inhibits the NF-κB signaling
pathway to reduce renal inflammation and delay renal fibrosis. DIS
treatment inhibits the phosphorylation of NF-κB p65, downregulates
NLRP3 inflammasome expression and thereby reduces IL-1β secretion,
as well as IL-6, TNF-α, MCP-1 and IL-18 secretion, ultimately
reducing renal inflammation in UUO mice and TGF-β1-stimulated HK-2
cells. Meanwhile, Bay11-7082, an inhibitor of NF-κB p65
phosphorylation, was able to reduce renal inflammation by
inhibiting NF-κB p65 phosphorylation, down-regulating NLRP3
inflammasome expression and ultimately attenuating the secretion of
IL-1β, IL-6, TNF-α, MCP-1 and IL-18. DIS, dioscin; NLRP3, NOD-like
receptor thermal protein domain associated protein 3; MCP-1,
monocyte chemotactic protein 1; UUO, unilateral ureteral
obstruction. |
In conclusion, the present study found that DIS
attenuated the inflammatory response and renal interstitial
fibrosis and that the mechanism may be the suppression of NF-κB
pathway-mediated inflammatory response.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Research Projects of the
National Natural Science Foundation of China (grant nos. 81904174,
82274489 and 82174296) and Natural Science Foundation of
Heilongjiang Province (grant no. LH2019H036).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW, PeL, PiL and NL conceived and designed the
study. YW, GM, CW and WZ conducted experiments on the cells and the
data analysis, and wrote part of the original manuscript. PS, WL,
XY and YZ conducted experiments on the mice and the data analysis,
and wrote part of the original manuscript. YW, NL and PiL reviewed
and edited the manuscript. PeL, NL and PiL participated in the
funding acquisition and confirmed the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal study was reviewed and approved by the
Ethics Committee of the China-Japan Friendship Institute of
Clinical Medical Sciences (approval no. zryhyy21-22-01-09;
affiliated with the China-Japan Friendship hospital, Beijing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ruiz-Ortega M, Rayego-Mateos S, Lamas S,
Ortiz A and Rodrigues-Diez RR: Targeting the progression of chronic
kidney disease. Nat Rev Nephrol. 16:269–288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Panizo S, Martinez-Arias L, Alonso-Montes
C, Cannata P, Martín-Carro B, Fernández-Martín JL, Naves-Díaz M,
Carrillo-López N and Cannata-Andía JB: Fibrosis in chronic kidney
disease: Pathogenesis and consequences. Int J Mol Sci. 22:4082021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y: Cellular and molecular mechanisms
of renal fibrosis. Nat Rev Nephrol. 7:684–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan Q and Liu YH: Recent advances on
understanding of the cellular and molecular mechisms of renal
fibrosis. J Anhui Univ Nat Sci Ed. 42:115–124. 2018.(In
Chinese).
|
|
5
|
Li R, Guo Y, Zhang Y, Zhang X, Zhu L and
Yan T: Salidroside ameliorates renal interstitial fibrosis by
inhibiting the TLR4/NF-κB and MAPK signaling pathways. Int J Mol
Sci. 20:11032019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simões e Silva AC, Silveira KD, Ferreira
AJ and Teixeira MM: ACE2, angiotensin-(1–7) and Mas receptor axis
in inflammation and fibrosis. Br J Pharmacol. 169:477–492. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang M and Zhang S: T cells in fibrosis
and fibrotic diseases. Front Immunol. 11:11422020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang PMK, Nikolic-Paterson DJ and Lan HY:
Macrophages: Versatile players in renal inflammation and fibrosis.
Nat Rev Nephrol. 15:144–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu L, Kou P, Zeng Q, Pei G, Li Y, Liang
H, Xu G and Chen S: CD4+ T lymphocytes, especially Th2 cells,
contribute to the progress of renal fibrosis. Am J Nephrol.
36:386–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lech M, Gröbmayr R, Ryu M, Lorenz G,
Hartter I, Mulay SR, Susanti HE, Kobayashi KS, Flavell RA and
Anders HJ: Macrophage phenotype controls long-term AKI
outcomes-kidney regeneration versus atrophy. J Am Soc Nephrol.
25:292–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tao X, Yin L, Xu L and Peng J: Dioscin: A
diverse acting natural compound with therapeutic potential in
metabolic diseases, cancer, inflammation and infections. Pharmacol
Res. 137:259–269. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duan H, Zhang Q, Liu J, Li R, Wang D, Peng
W and Wu C: Suppression of apoptosis in vascular endothelial cell,
the promising way for natural medicines to treat atherosclerosis.
Pharmacol Res. 168:1055992021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang D and Wang X: Diosgenin and its
analogs: Potential protective agents against atherosclerosis. Drug
Des Devel Ther. 16:2305–2323. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Passos FRS, Araújo-Filho HG, Monteiro BS,
Shanmugam S, Araújo AAS, Almeida JRGDS, Thangaraj P, Júnior LJQ and
Quintans JSS: Anti-inflammatory and modulatory effects of steroidal
saponins and sapogenins on cytokines: A review of pre-clinical
research. Phytomedicine. 96:1538422022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu L, Tao X, Xu Y, Han X, Qi Y, Xu L, Yin
L and Peng J: Dioscin alleviates BDL- and DMN-induced hepatic
fibrosis via Sirt1/Nrf2-mediated inhibition of p38 MAPK pathway.
Toxicol Appl Pharmacol. 292:19–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu H, Lin L, Zhang Z, Zhang H and Hu H:
Targeting NF-κB pathway for the therapy of diseases: Mechanism and
clinical study. Signal Transduct Target Ther. 5:2092020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiao Y, Xu L, Tao X, Yin L, Qi Y, Xu Y,
Han X, Tang Z, Ma X, Liu K and Peng J: Protective effects of
dioscin against fructose-induced renal damage via adjusting
Sirt3-mediated oxidative stress, fibrosis, lipid metabolism and
inflammation. Toxicol Lett. 284:37–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao D, Wang Y, Zhang Y, Zhang Y, Huang Q,
Yin Z, Cai G, Chen X and Sun X: Regulation of connective tissue
growth factor expression by miR-133b for the treatment of renal
interstitial fibrosis in aged mice with unilateral ureteral
obstruction. Stem Cell Res Ther. 12:1712021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu L, Wang Y, Yang G, Tilyek A, Zhang C,
Li S, Yu B, Chai C and Cao Z: Ribes diacanthum Pall (RDP)
ameliorates UUO-induced renal fibrosis via both canonical and
non-canonical TGF-β signaling pathways in mice. J Ethnopharmacol.
231:302–310. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hosseinian S, Rad AK, Bideskan AE,
Soukhtanloo M, Sadeghnia H, Shafei MN, Motejadded F, Mohebbati R,
Shahraki S and Beheshti F: Thymoquinone ameliorates renal damage in
unilateral ureteral obstruction in rats. Pharmacol Rep. 69:648–657.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Abhar H, Abd El Fattah MA, Wadie W and
El-Tanbouly DM: Cilostazol disrupts TLR-4, Akt/GSK-3β/CREB, and
IL-6/JAK-2/STAT-3/SOCS-3 crosstalk in a rat model of Huntington's
disease. PLoS One. 13:e2038372018. View Article : Google Scholar
|
|
22
|
Liu S, Chen X, Zhang S, Wang X, Du X, Chen
J and Zhou G: miR-106b-5p targeting SIX1 inhibits TGF-β1-induced
pulmonary fibrosis and epithelial-mesenchymal transition in asthma
through regulation of E2F1. Int J Mol Med. 47:048552021. View Article : Google Scholar
|
|
23
|
Okarmus J, Bogetofte H, Schmidt SI, Ryding
M, García-López S, Ryan BJ, Martínez-Serrano A, Hyttel P and Meyer
M: Lysosomal perturbations in human dopaminergic neurons derived
from induced pluripotent stem cells with PARK2 mutation. Sci Rep.
10:102782020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamaguchi J, Tanaka T and Nangaku M:
Recent advances in understanding of chronic kidney disease.
F1000Res. 4:12122015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vernon MA, Mylonas KJ and Hughes J:
Macrophages and renal fibrosis. Semin Nephrol. 30:302–317. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wada T, Furuichi K, Sakai N, Iwata Y,
Kitagawa K, Ishida Y, Kondo T, Hashimoto H, Ishiwata Y, Mukaida N,
et al: Gene therapy via blockade of monocyte chemoattractant
protein-1 for renal fibrosis. J Am Soc Nephrol. 15:940–948. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liao Y, Tan RZ, Li JC, Liu TT, Zhong X,
Yan Y, Yang JK, Lin X, Fan JM and Wang L: Isoliquiritigenin
attenuates UUO-induced renal inflammation and fibrosis by
inhibiting mincle/Syk/NF-kappa B signaling pathway. Drug Des Devel
Ther. 14:1455–1468. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhong Y, Liu J, Sun D, Guo T, Yao Y, Xia
X, Shi C and Peng X: Dioscin relieves diabetic nephropathy via
suppressing oxidative stress and apoptosis, and improving
mitochondrial quality and quantity control. Food Funct.
13:3660–3673. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai S, Chen J and Li Y: Dioscin protects
against diabetic nephropathy by inhibiting renal inflammation
through TLR4/NF-κB pathway in mice. Immunobiology. 225:1519412020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Xu Y, Qi Y, Xu L, Song S, Yin L,
Tao X, Zhen Y, Han X, Ma X, et al: Protective effects of dioscin
against doxorubicin-induced nephrotoxicity via adjusting
FXR-mediated oxidative stress and inflammation. Toxicology.
378:53–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Tao X, Yin L, Xu L, Xu Y, Qi Y,
Han X, Song S, Zhao Y, Lin Y, et al: Protective effects of dioscin
against cisplatin-induced nephrotoxicity via the
microRNA-34a/sirtuin 1 signalling pathway. Br J Pharmacol.
174:2512–2527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mankan AK, Lawless MW, Gray SG, Kelleher D
and McManus R: NF-kappaB regulation: The nuclear response. J Cell
Mol Med. 13:631–643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mitchell S, Vargas J and Hoffmann A:
Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med.
8:227–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hirano T: IL-6 in inflammation,
autoimmunity and cancer. Int Immunol. 33:127–148. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zusso M, Lunardi V, Franceschini D,
Pagetta A, Lo R, Stifani S, Frigo AC, Giusti P and Moro S:
Ciprofloxacin and levofloxacin attenuate microglia inflammatory
response via TLR4/NF-kB pathway. J Neuroinflammation. 16:1482019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20:33282019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Black LM, Lever JM and Agarwal A: Renal
inflammation and fibrosis: A double-edged sword. J Histochem
Cytochem. 67:663–681. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bianconi V, Sahebkar A, Atkin SL and Pirro
M: The regulation and importance of monocyte chemoattractant
protein-1. Curr Opin Hematol. 25:44–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Du Y, Zhang H, Guo Y, Song K, Zeng L, Chen
Y, Xie Z and Li R: CD38 deficiency up-regulated IL-1β and MCP-1
through TLR4/ERK/NF-κB pathway in sepsis pulmonary injury. Microbes
Infect. 23:1048452021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Afonina IS, Zhong Z, Karin M and Beyaert
R: Limiting inflammation-the negative regulation of NF-κB and the
NLRP3 inflammasome. Nat Immunol. 18:861–869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang WJ, Chen SJ, Zhou SC, Wu SZ and Wang
H: Inflammasomes and fibrosis. Front Immunol. 12:6431492021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Otto G: IL-1β switches on kidney fibrosis.
Nat Rev Nephrol. 14:4752018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Klinkhammer BM, Floege J and Boor P: PDGF
in organ fibrosis. Mol Aspects Med. 62:44–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hayashi N, Yoshimoto T, Izuhara K, Matsui
K, Tanaka T and Nakanishi K: T helper 1 cells stimulated with
ovalbumin and IL-18 induce airway hyperresponsiveness and lung
fibrosis by IFN-gamma and IL-13 production. Proc Natl Acad Sci USA.
104:14765–14770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liang H, Xu F, Zhang T, Huang J, Guan Q,
Wang H and Huang Q: Inhibition of IL-18 reduces renal fibrosis
after ischemia-reperfusion. Biomed Pharmacother. 106:879–889. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao W, Ma L, Cai C and Gong X: Caffeine
inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB
and A2aR signaling in LPS-induced THP-1 macrophages. Int J Biol
Sci. 15:1571–1581. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wen Y, Lu X, Ren J, Privratsky JR, Yang B,
Rudemiller NP, Zhang J, Griffiths R, Jain MK, Nedospasov SA, et al:
KLF4 in macrophages attenuates TNFα-mediated kidney injury and
fibrosis. J Am Soc Nephrol. 30:1925–1938. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang L, Xie J, Gan R, Wu Z, Luo H, Chen
X, Lu Y, Wu L and Zheng D: Synergistic inhibition of lung cancer
cells by EGCG and NF-κB inhibitor BAY11-7082. J Cancer.
10:6543–6556. 2019. View Article : Google Scholar : PubMed/NCBI
|





















