Introduction
The intestinal microenvironment is a highly complex
and dynamic system, wherein the maintenance of the intestinal
barrier serves a pivotal role in preserving the structural
integrity of the intestines (1).
Consisting of the mucous layer, intercellular tight junction (TJ)
proteins and epithelial cells, the intestinal epithelial barrier
acts as a robust defense mechanism, protecting against tissue
damage and the onset of various diseases, such as inflammatory
bowel disease and colitis (2).
Perturbations in the microbial ecosystem of the intestines
frequently increase intestinal permeability and induce intestinal
epithelial dysfunction (3).
Lipopolysaccharide (LPS), a constituent of the outer membrane of
gram-negative bacteria, has been shown to exacerbate inflammatory
responses by increasing the production of nitric oxide and
pro-inflammatory cytokines in intestinal epithelial cells,
specifically in NCM460 cells (4).
Notably, higher levels of LPS are implicated in TJ disruption,
compromised barrier integrity and perturbed epithelial cell
turnover (5,6). Therefore, there is a crucial need to
explore targeted therapeutic strategies for LPS-induced intestinal
epithelial dysfunction.
A previous study indicated that indole possesses
anti-inflammatory properties, and positively affects
gastrointestinal tract and liver homeostasis (7). Of the intestinal microorganisms,
Clostridium sporogenes is responsible for the production of
indole-3-propionic acid (IPA) (8,9). IPA
has been shown to serve as a biomarker of disease remission for
active colitis, since a gradual restoration of serum IPA level has
been detected during the recovery phase (10). Notably, IPA serves a crucial role
in strengthening mucus and mechanical barriers by promoting TJ
expression, thereby enhancing barrier function (11,12).
While the role of IPA in preserving the intestinal barrier has been
established, a comprehensive understanding of its mechanisms of
action on intestinal epithelial cells is still lacking.
Toll-like receptor 4 (TLR4) dependent on myeloid
differentiation factor 88 (MyD88) and the downstream NF-κB
signaling pathway is essential for the induction of inflammation
(13). YIt has been observed that
gut microbial diversity affects the TLR4/NF-κB signaling pathway
during an inflammatory response. LPS rapidly increases cytokine
levels, impairing intestinal integrity in intestinal epithelial
cells (14). Furthermore,
activation of TLR4 by LPS initiates signaling via either MyD88 or
TRIF, resulting in the translocation of nuclear transcription
factors NF-κB, AP-1 and IRF3 (15). Nuclear stimulation by LPS activates
the MyD88-dependent and downstream NF-κB signaling pathway,
compromising intestinal barrier function (16). Previous studies have suggested that
IPA exhibits beneficial effects in regulating immune responses
within the intestines via activation of the aryl hydrocarbon
receptor and the pregnane X receptor ligands (17,18).
Furthermore, IPA effectively has been shown to protect against
LPS-induced C2C12 cell inflammation (19). However, the precise mechanisms
underlying the protective effects of IPA on LPS-induced epithelial
intestinal cell injury are currently unclear.
The effects of IPA on intestinal barrier function
have been reported in few studies, most of which used the Caco-2
cell line (11,20), whereas the effects on the NCM460
cell line have rarely been reported. Furthermore, the NCM460 cell
line has been used in cutting-edge research, such as that
associated with infectious diseases, cell signaling and cytokine
production (4,21). Therefore, in the present study, the
human NCM460 colonic epithelial cell line was used to explore the
mechanisms by which IPA may protect against LPS-induced intestinal
epithelial cell injury using three concentrations of IPA. The
findings of the present study highlight novel avenues for the
potential therapeutic role of IPA in intestinal inflammatory
diseases characterized by LPS-induced intestinal epithelial cell
injury.
Materials and methods
Reagents
IPA (>98%) was purchased from Shanghai Aladdin
Biochemical Technology Co., Ltd. LPS was purchased from
MilliporeSigma. Fetal bovine serum (FBS; cat. no. 16000-044) was
purchased from Gibco (Thermo Fisher Scientific, Inc.) and DMEM
(cat. no. BL304A) was purchased from Biosharp Life Sciences. The
penicillin-streptomycin solution (100X; cat. no. P1400-100) and
trypsin-EDTA (0.25%; cat. no. T1300-100) were obtained from Beijing
Solarbio Science & Technology Co., Ltd. The Cell Counting Kit-8
(CCK-8) assay was purchased from Beyotime Institute of
Biotechnology. Nuclease-Free Water (cat. no. 10601ES76),
Diethylpyrocarbonate (DEPC; cat. no. 10602ES25),
Hifair®II 1st Strand cDNA Synthesis Kit (cat. no.
11119ES60) and Hieff® quantitative (q)PCR SYBR Green
MasterMix (cat. no. 11203ES03) were purchased from Shanghai Yeasen
Biotechnology Co., Ltd. The BCA Protein Quantification Kit (cat.
no. 23223) was purchased from Thermo Fisher Scientific, Inc. Alexa
Fluor 488-labeled goat anti-rabbit IgG (H+L) (cat. no. A0423) and
Annexin V-FITC Apoptosis Detection Kit (cat. no. C1062) were
obtained from Beyotime Institute of Biotechnology The
Dual-Luciferase Reporter Gene Assay Kit (cat. no. E1910) was
purchased from Promega Corporation.
Cell culture
The NCM460 human colonic epithelial cell line was
purchased from Shanghai Jinyuan Biotechnology Co., Ltd. The cells
were cultured in DMEM supplemented with 10% FBS and 1%
penicillin-streptomycin solution in an incubator at 37°C supplied
with 5% CO2. NCM460 cells were treated with various
concentrations of LPS with or without IPA for 3 or 24 h at 37°C.
The cells were grouped as follows: Untreated cells served as a
control; LPS 10 µg/ml; LPS 10 µg/ml + IPA 0.05 mM; LPS 10 µg/ml +
IPA 0.5 mM; and LPS 10 µg/ml + IPA 5 mM.
Measurement of cell viability
Cell viability was measured using the CCK-8 assay
according to the manufacturer's protocol. Briefly, 5×103
cells/well were plated in 96-well plates and treated with 0, 0.5,
1, 5 or 10 µg/ml LPS for 3 or 24 h at 37°C. The concentration of
LPS that best attenuated cell viability was used as the inducing
concentration for the follow-up experiments. Subsequently, NCM460
cells were co-treated with LPS and 0.05, 0.5 or 5 mM IPA for 72 h
at 37°C. For the CCK-8 assay, 10 µl CCK-8 reagent was added to each
well and incubated for 1 h at 37°C. A microplate reader (cat. no.
9602G; Perlong Medical Equipment Co., Ltd.) was then used to
measure the absorbance at a wavelength of 450 nm. Each experiment
was repeated five times and the cell survival rate was calculated
using the following formula: Cell viability (%)=(mean absorbance of
test wells-mean absorbance of blank wells)/(mean absorbance of
control wells-mean absorbance of blank wells) ×100.
Detection of apoptosis
Apoptosis was detected using the Annexin V-FITC
Apoptosis Detection Kit according to the manufacturer's protocol.
Adherent cells seeded in a 6-well plate at a density of
5×105/well were washed once with PBS and then digested
using trypsin containing EDTA. After centrifugation at 1,000 × g
for 5 min at 37°C, the cells were collected and incubated with
Annexin V-FITC (5 µl) for 15 min, followed by staining with
propidium iodide (PI, 5 µl) staining solution for 5 min at 4°C in
the dark. A sample without Annexin V-FITC and PI staining was used
as a negative control. Subsequently, cell apoptosis was detected
using a flow cytometer (CytoFLEX; Beckman Coulter, Inc.) and data
were analyzed using FlowJo software version 10.6.2 (Tree Star,
Inc.) to determine the percentage of apoptotic cells, with Annexin
V-FITC fluorescing green and PI fluorescing red. The total
apoptotic rate was calculated as the sum of early apoptosis and
late apoptosis. Each experiment was repeated three times and the
mean apoptotic rate was calculated.
Assessment of barrier integrity
The Millicell®-ERS Voltohmmeter
(MilliporeSigma) was used to assess barrier integrity via measuring
transmembrane electrical resistance (TEER). NCM460 cells were
seeded in the upper chambers of a 24-well Transwell plate
(diameter, 6.5 mm; pore size, 8.0 µm; cat. no. 3422; Costar;
Corning Inc.) at a density of 2×105
cells/cm2, and were cultured in a 5% CO2
incubator at 37°C. The medium (DMEM; cat. no. SH30243.01; Hyclone;
Cytiva) added in the lower chambers was changed every 3 days until
the cells grew in a monolayer on the upper side of the polyester
membrane, after which, the transmembrane resistance was measured.
The resistance per unit area (TEER) was calculated using the
following formula: TEER (resistance per unit area,
Ω·cm2)=(Rexperiment-Rblank)
(resistance measurement, Ω) × effective membrane area
(cm2). The effective area of the Millicell membrane used
was 0.6 cm2. Rexperiment was the resistance
of the membrane with a cell layer in each experimental group while
Rblank was the resistance of the membrane without a cell
layer. The TEER value was measured three consecutive times and the
mean TEER value in each group was calculated.
Immunofluorescence staining
NCM460 cells were cultured and treated with the
control medium, or medium containing 10 µg/ml LPS alone or
alongside various concentrations of IPA (0.05, 0.5 or 5 mM). Cells
were seeded onto 24-well round glass coverslips (14 mm; cat. no.
WHB-24-CS; Beijing Solarbio Science & Technology Co., Ltd.) at
a density of 3×104 cells/well were washed with 0.02 M
PBS to remove the media, fixed with 4% formaldehyde for 30 min and
permeabilized with 0.5% Triton X-100 for 10 min at 37°C. Slides
were then blocked with 1% bovine serum albumin (cat. no. A8010;
Beijing Solarbio Science & Technology Co., Ltd.) for 1 h at
37°C and washed three times using 0.02 M PBS. Subsequently, the
cells were incubated with the recombinant anti-ZO1 TJ protein
antibody (rabbit monoclonal; 1:100; cat. no. ab221547; Abcam)
overnight at 4°C, followed by incubation with the
fluorescent-tagged secondary antibody (1:200; cat. no. A0423;
Beyotime Institute of Biotechnology). After washing with PBS, an
anti-quenching solution containing DAPI (1:500 dilution) was used
to seal the slides. Finally, images of three random fields of view
were captured using a fluorescence microscope (magnification,
×200). Semi-quantitative assessment of fluorescence intensity was
performed using ImageJ software version 1.53a (National Institutes
of Health). Mean fluorescence intensity was calculated using the
following formula: Mean fluorescence intensity (AU)=Integrated
density/area.
Reverse transcription (RT)-qPCR
Total RNA was extracted from cells using
TRleasy™ Total RNA Extraction Reagent (cat. no.
10606ES60; Shanghai Yeasen Biotechnology Co., Ltd.), and was then
isolated using chloroform, precipitated with isopropanol, washed
with 75% ethanol and dissolved in DEPC water. The RNA was reverse
transcribed into cDNA using Hifair® II 1st Strand cDNA
Synthesis Kit (cat. no. 11119ES60; Shanghai Yeasen Biotechnology
Co., Ltd.) according to the manufacturer's instructions. qPCR was
performed using Hieff® qPCR SYBR Green Master Mix (cat.
no. 11203ES03; Shanghai Yeasen Biotechnology Co., Ltd.) on a
fluorescence qPCR instrument (cat. no. CG-05; Hangzhou Lattice
Scientific Instrument Co., Ltd.). The qPCR conditions were set as
follows: Pre-denaturation at 95°C for 5 min, followed by 40 cycles
of denaturation at 95°C for 10 sec, annealing at 55–60°C for 20 sec
and extension at 72°C for 20 sec. Fluorescence was detected and
melting curve analysis was performed at the end of each PCR cycle
to assess amplification specificity. The relative expression levels
of the target genes were calculated using the 2−ΔΔCq
method (22), and β-actin was used
as an internal reference. The mRNA expression levels of IL-1β,
IL-6, TNF-α, TLR4, MyD88, TRIF and p65-NF-κB were detected in
triplicate, and the sequences of the primers used are listed in
Table SI.
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of proinflammatory cytokines
IL-1β, IL-6 and TNF-α were quantified using ELISA kits (cat. nos.
SDH0014-48T, SDH0021-48T, SDH0001-48T; Shanghai Siding
Biotechnology Co., Ltd.) according to the manufacturer's
instructions. The OD was detected using a microplate reader (Tecan
Infinite F50; Tecan Group, Ltd.) at 450 nm. Concentrations were
calculated by standard curve plotting.
Dual-luciferase reporter gene
assay
Dual-luciferase reporter gene detection was
performed using the Dual-Luciferase Reporter Gene Detection Kit
(cat. no. E1910; Promega Corporation) according to the
manufacturer's protocol. Cells in the logarithmic growth phase were
digested with trypsin, and 5×105 cells/well were plated
in a 6-well plate and cultured for 24 h. The GV238 (pGL3 basic)
vector plasmid and p65-NF-κB gene sequence were digested with
Kpnl/Xhol restriction enzymes (Shanghai GeneChem Co.,
Ltd.). The p65-NF-κB gene promotor was obtained from the cDNA
library of Shanghai GeneChem Co., Ltd. The primer sequences used to
amplify the sequence were as follows: Forward,
5′-TTTCTCTATCGATAGGTACCGGGAATTTCCGGGGACTTTC-3′ and reverse,
5′-CTTAGATCGCAGATCTCGAGCTGGAAGTCGAGCTTCCATTATATAC-3′. Subsequently,
the p65-NF-κB gene promotor was cloned into the GV238 vector to
construct an overexpression vector, which was then transfected into
NCM460 cells. NCM460 cells transfected with 1.5 µg
luciferase-p65-NF-κB reporter plasmid and 1.5 µg Renilla
control plasmid (cat. no. E1910; Promega Corporation). The
transfection solutions were prepared according to the instructions
of the transfection reagent Lipofectamine® 3000 (cat.
no. L3000001; Invitrogen; Thermo Fisher Scientific, Inc.). After 48
h of transfection, the cells were washed once with PBS and cultured
in 500 µl passive lysis buffer for 15 min. Subsequently, cells were
treated with 10 µg/ml LPS alone, or alongside various
concentrations of IPA (0.05, 0.5 or 5 mM) for 72 h at 37°C.
Afterwards the cells were collected and transferred to a 96-well
microplate, and 100 µl luciferase assay reagent II and 20 µl
passive lysis buffer were then added to detect firefly luciferase
activity. The luciferase activity was detected by adding 100 µl
Stop&Glo reagent and the ratio of the luciferase activity to
Renilla luciferase activity was calculated.
Western blotting
Total proteins were extracted from cells lysed using
RIPA lysis buffer (cat. no. P0038; Beyotime Institute of
Biotechnology) and the supernatant was centrifuged at 16,000 × g
for 15 min at 4°C. Protein concentrations were measured using the
BCA Protein Quantification Kit according to the manufacturer's
instructions. Subsequently, ~20 µg total protein was separated by
SDS-PAGE on 10% gels and transferred to PVDF membranes at 25 V for
30 min. The membranes were then blocked with 5% non-fat milk
solution at 4°C overnight and incubated with the following
antibodies at 4°C overnight: β-actin (1:5,000; cat. no. 81115-1-R;
Proteintech Group, Inc.), zonula occludens (ZO)-1 (1:1,000; cat.
no. ab276131; Abcam), occludin (1:1,000; cat. no. ab216327; Abcam),
Claudin-1 (1:2,000; cat. no. ab211737; Abcam) p65-NF-κB (1:1,000;
cat. no. ab32536; Abcam) and phosphorylated-p65-NF-κB (1:1,000;
cat. no. ab76302; Abcam). After washing, the membranes were
incubated with HRP-labeled secondary antibodies (1:10,000; cat. no.
ZB-2301; OriGene Technologies, Inc.) for 1 h at 37°C. The membranes
were washed three times in Tris-buffered saline-0.1% Tween 20 (5
min/wash) between each incubation step. For visualization, ECL
solution (cat. no. WBKLS0100; MilliporeSigma) was prepared
according to the manufacturer's protocol and added to the membranes
in a dark room for 5 min. The solution was subsequently removed,
the blots were covered with a flat layer of cellophane and were
placed into the imaging system (Tanon 5200; Tanon Science and
Technology Co., Ltd.) for scanning. Western blotting was performed
in triplicate. ImageJ software version 1.53a (National Institutes
of Health) was used to analyze the results and normalize all target
proteins to β-actin.
Statistical analysis
GraphPad Prism version 9.5.1 (Dotmatics) was used
for statistical analysis. Continuous variables are presented as the
mean ± the standard error of the mean. Differences between multiple
groups were analyzed using a one-way ANOVA followed by post hoc
Tukey test. P<0.05 was considered to indicate a statistically
significant difference.
Results
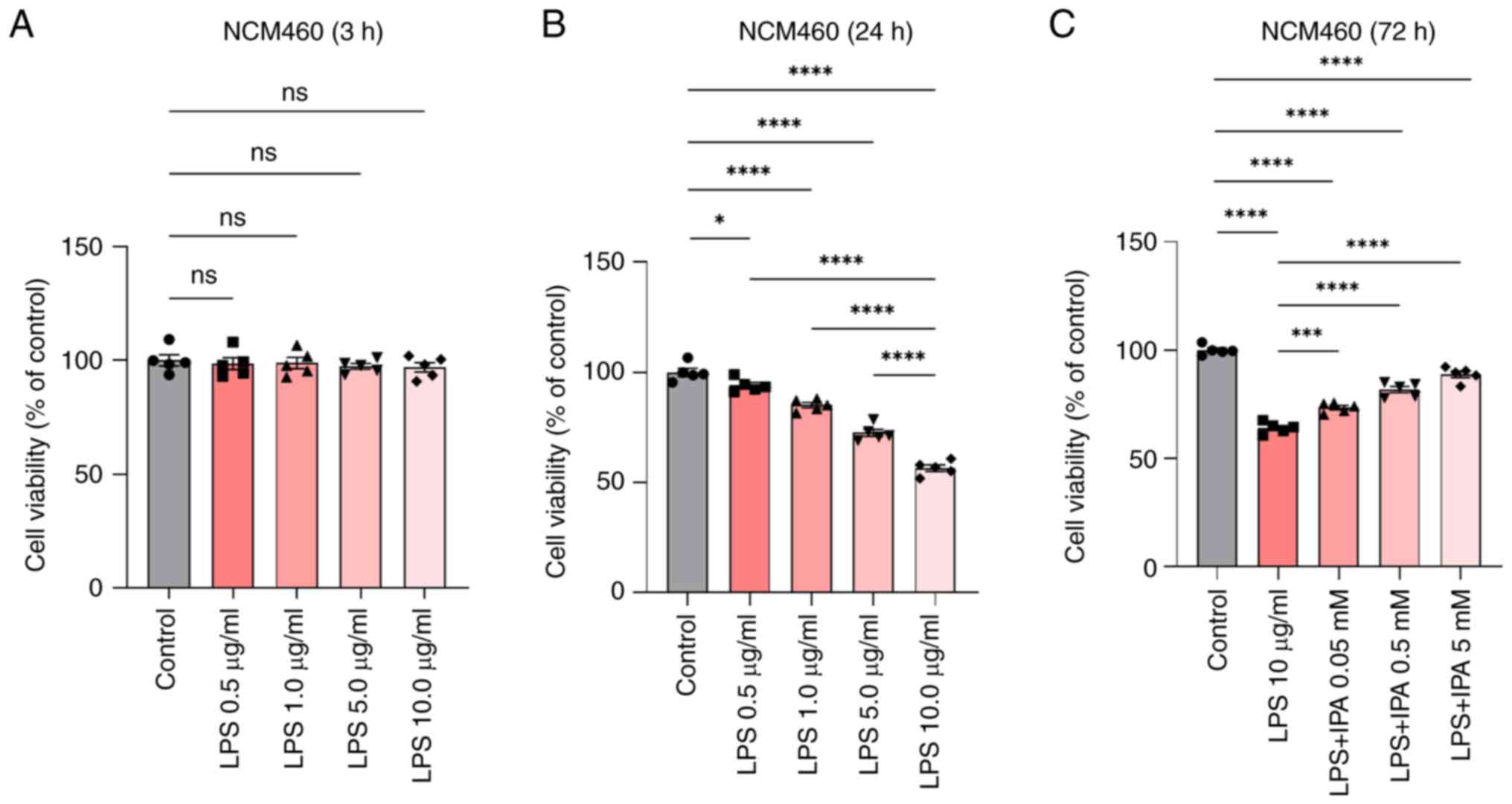
LPS induces intestinal epithelial cell
injury
To investigate the role of LPS in intestinal
epithelial cells, cell viability was assessed using the CCK-8 assay
after treatment of cells with different concentrations of LPS.
Based on previous literature, reference concentrations for LPS were
0.05–50 µg/ml for 8–48 h (19,23),
and for IPA were 0.01–10.0 mM for 24–72 h (24–26).
IPA was also observed to induce a dose-dependent suppression of the
aggregation of denatured proteins in cells experiencing endoplasmic
reticulum stress (25). Therefore,
in the present study, NCM460 cells were pre-stimulated with 0–10
µg/ml LPS for 3 and 24 h, followed by treatment with 0.05, 0.5 or 5
mM IPA for 72 h to explore its effect on LPS-induced intestinal
epithelial cell injury. The findings indicated that LPS
concentrations ranging between 0 and 10 µg/ml did not significantly
affect the viability of intestinal epithelial cells following a 3-h
treatment (P>0.05; Fig. 1A).
Notably, there was a sharp decrease in cell viability after 24 h of
exposure. The decrease in cell viability was particularly
significant when the LPS concentration was >1.0 µg/ml, and the
decline was most prominent at 10 µg/ml. These results indicated
that specific LPS concentrations may reduce the viability of
intestinal epithelial cells, and treatment with 10 µg/ml LPS for 24
h was determined to be the appropriate concentration of LPS that
was used for further experiments (P<0.0001; Fig. 1B). Subsequently, NCM460 cells were
pre-stimulated with LPS, and then treated with different
concentrations of IPA for 72 h and a CCK-8 assay was performed. The
results showed that LPS-induced NCM460 cells treated with IPA
resulted in a dose-dependent increase in cell viability compared
with that in the LPS group (P<0.001; Fig. 1C).
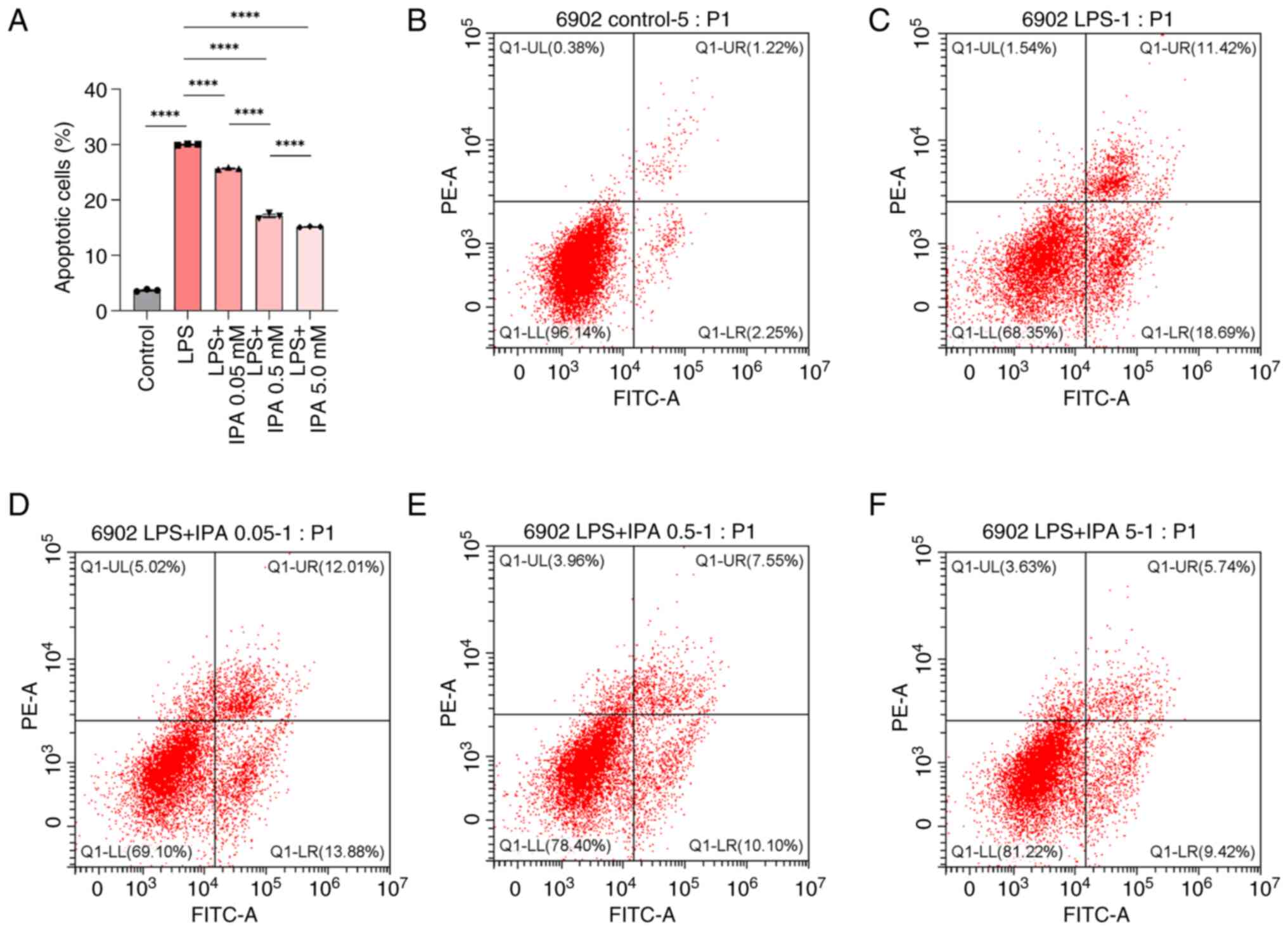
IPA inhibits the LPS-induced apoptosis
of intestinal epithelial cells
To evaluate the effects of IPA on LPS-induced cell
injury, the apoptosis of intestinal epithelial cells treated with
LPS alone or in combination with various concentrations of IPA was
subsequently analyzed. Compared with in the control group, the LPS
group exhibited a significant increase in the proportion of
apoptotic intestinal epithelial cells (P<0.0001; Fig. 2A-C). By contrast, the LPS + IPA
groups exhibited a marked reduction in the number of apoptotic
cells in a concentration-dependent manner compared with that in the
LPS group (P<0.0001; Fig. 2A and
C-F). These findings demonstrated the effective inhibition of
LPS-induced apoptosis in intestinal epithelial cells by IPA. IPA
exerted a protective effect on injured intestinal epithelial cells
by preventing LPS-induced apoptosis.
IPA improves intestinal epithelial
barrier function
To assess the effects of IPA on intestinal barrier
function, TEER values were measured. The results showed that LPS
significantly reduced TEER as compared to control group
(P<0.0001), indicating impairment of the barrier function in
intestinal epithelial cells (Fig.
3A). By contrast, treatment with IPA alleviated LPS-induced
impairment (P<0.05). Treatment with low (0.05 mM), medium (0.5
mM) and high (5 mM) concentrations of IPA all significantly
increased TEER values in a concentration-dependent manner compared
with in the LPS group (P<0.05).
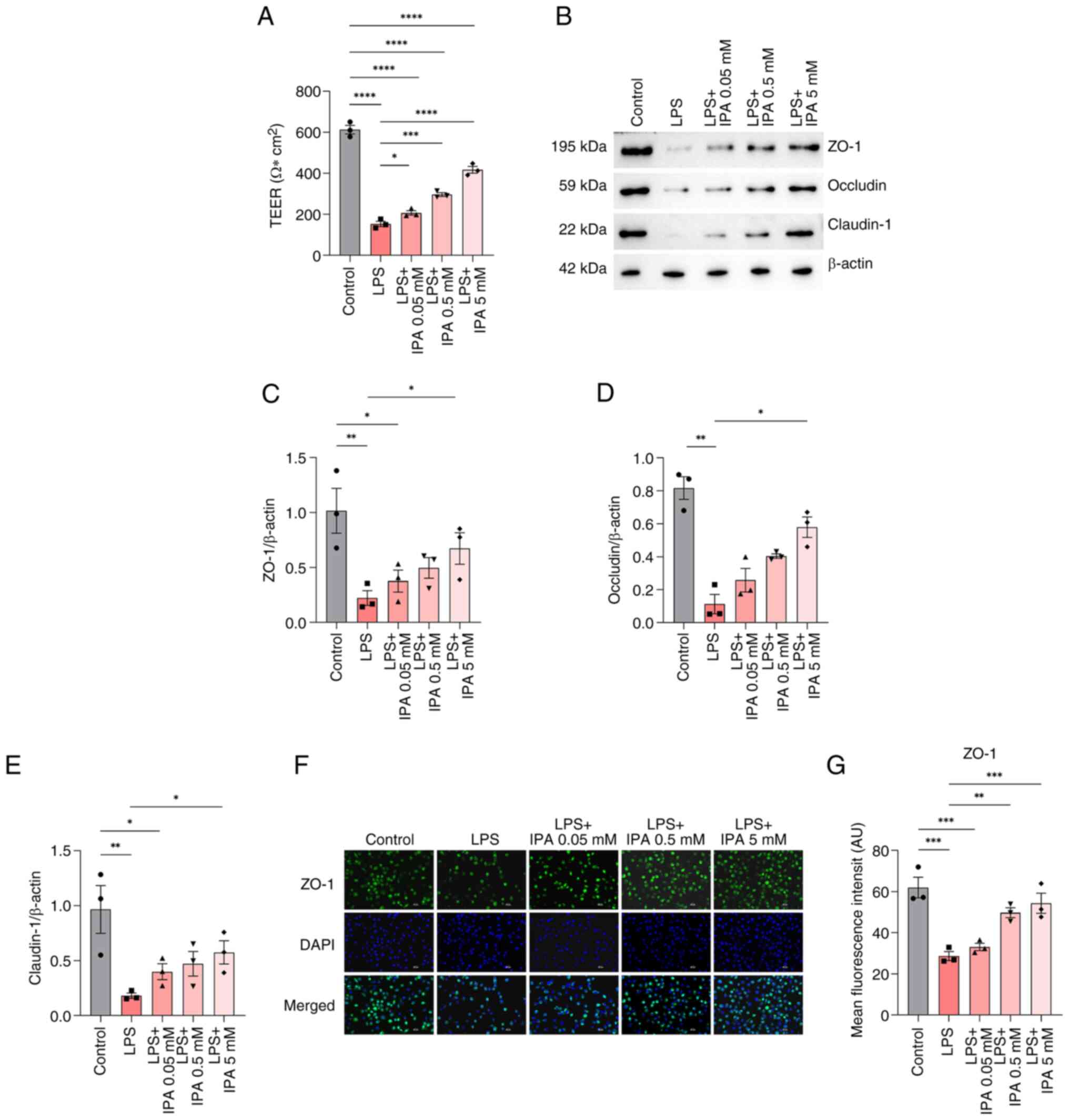 | Figure 3.Effects of IPA on the intestinal
barrier function in cells treated with LPS. (A) TEER values of the
human-derived NCM460 colonic epithelial cells in the different
groups. (B) Western blotting, and relative levels of (C) ZO-1, (D)
occludin and (E) claudin-1 proteins. (F) Immunofluorescence
staining of ZO-1 (green) and nuclei (blue). Scale bars, 50 µm.
Magnification, ×200. (G) Semi-quantitative analysis of the
fluorescence intensity of ZO-1. Data are presented as mean ± SEM,
n=3. *P<0.05, **P<0.001, ***P<0.001, ****P<0.0001. IPA,
indole-3-propionic acid; LPS, lipopolysaccharide; TEER,
transepithelial electrical resistance; ZO-1, zonula
occludens-1. |
It is well established that compromised intestinal
barrier integrity can lead to the activation of local immunity and
alterations in the structure of TJ proteins (27). To explore the potential impact of
IPA on intercellular TJs, the expression of TJ-associated proteins
was examined using western blotting. The findings revealed a
significant decrease in the expression levels of claudin-1,
occludin and ZO-1 in the LPS group compared with those in the
control group (P<0.01; Fig.
3B-E). However, only treatment with a high concentration of IPA
significantly reversed the LPS-induced reduction in the expression
levels of these proteins (P<0.05) when compared with the LPS
group.
To further validate these findings,
immunofluorescence analysis of ZO-1 was performed. The results
showed that the expression of ZO-1 in the LPS-induced group was
significantly decreased compared with that in the control group
(P<0.001), and there was a substantial increase in ZO-1 protein
secretion in both the medium and high concentration IPA-treated
cells (P<0.01; Fig. 3F and G).
Collectively, these findings suggested that IPA may improve the
barrier function of intestinal epithelial cells injured by LPS by
increasing TEER values and upregulating the expression of TJ
proteins.
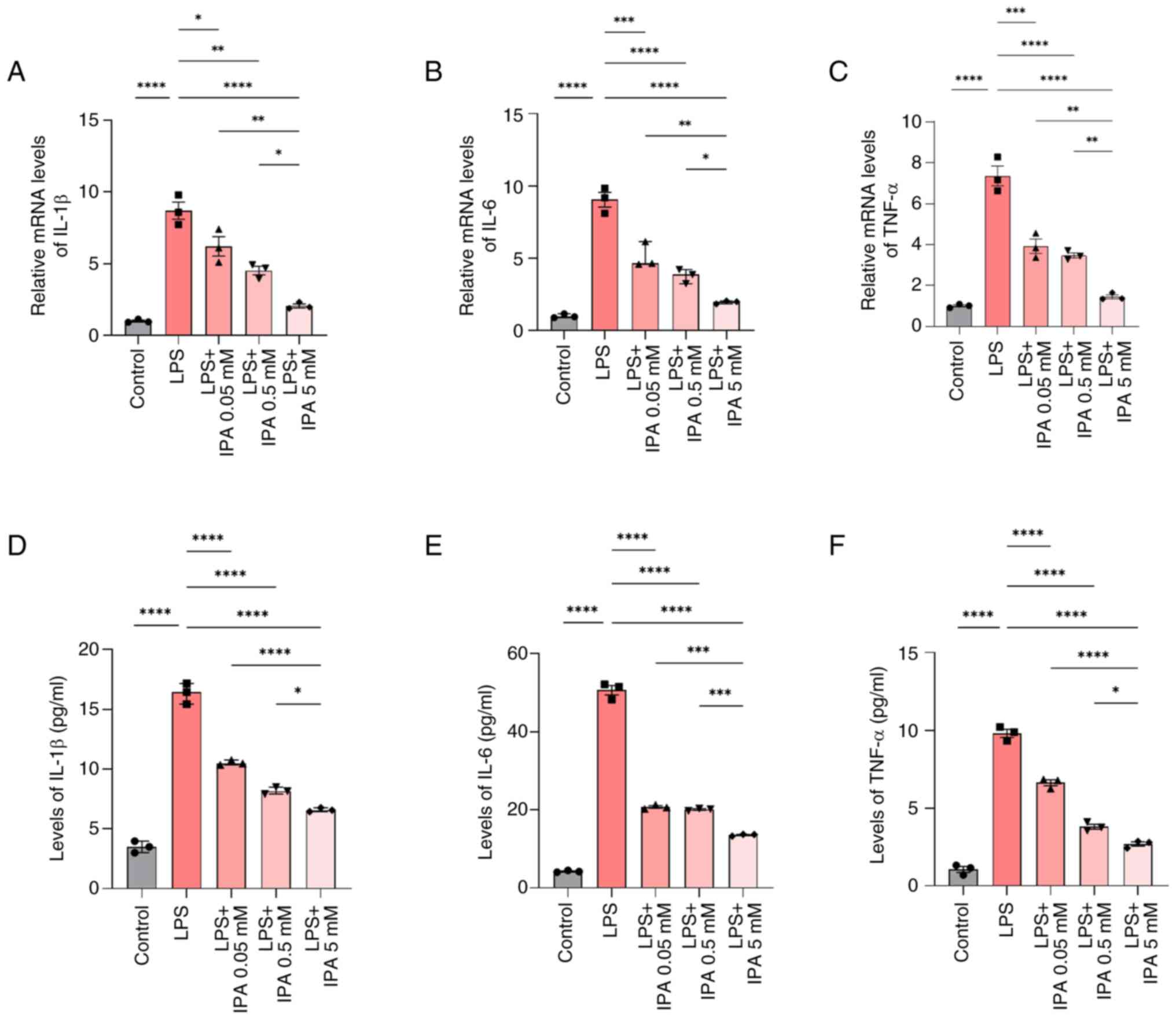
IPA inhibits the levels of LPS-induced
pro-inflammatory cytokines
RT-qPCR was used to investigate the expression
levels of pro-inflammatory cytokines in intestinal epithelial
cells. Following treatment with LPS, there was a significant
increase in the mRNA expression levels of IL-1β, IL-6 and TNF-α
relative to the control group (P<0.0001; Fig. 4A-C). However, IPA treatment
resulted in a concentration-dependent downregulation in IL-1β, IL-6
and TNF-α mRNA expression levels compared with those in the LPS
group (P<0.05). Furthermore, the mRNA expression levels of
IL-1β, IL-6 and TNF-α were significantly reduced in cells treated
with 5 mM IPA compared with in cells treated with 0.05 or 0.5 mM
IPA (P<0.05). In addition, the levels of IL-1β, IL-6 and TNF-α
secreted by NCM460 cells were analyzed by ELISA. It was similarly
observed that IL-1β, IL-6 and TNF-α levels were increased in
response to LPS stimulation and were downregulated when cells were
also treated with IPA (P<0.05; Fig.
4D-F). These findings collectively indicated that IPA could
mitigate LPS-induced pro-inflammatory cytokine expression.
IPA protects intestinal epithelial
cells from LPS-induced inflammatory injury via regulation of the
TLR4/NF-κB pathway
Activation of the NF-κB pathway has previously been
implicated in the maintenance of intestinal barrier integrity in
response to LPS-induced injury accompanied by the release of
pro-inflammatory cytokines (22).
LPS activates TLR4 and NF-κB signaling pathways sequentially,
ultimately resulting in the release of large quantities of
pro-inflammatory cytokines, including IL-6, IL-1β and TNF-α
(23). Western blotting was
performed to examine the effects of IPA on p65-NF-κB expression.
The results showed that phosphorylation of p65-NF-κB was
significantly increased in the LPS group compared with that in the
control group (P<0.0001), which was reversed by treatment with
IPA in a dose-dependent manner (P<0.001; Fig. 5A and B).
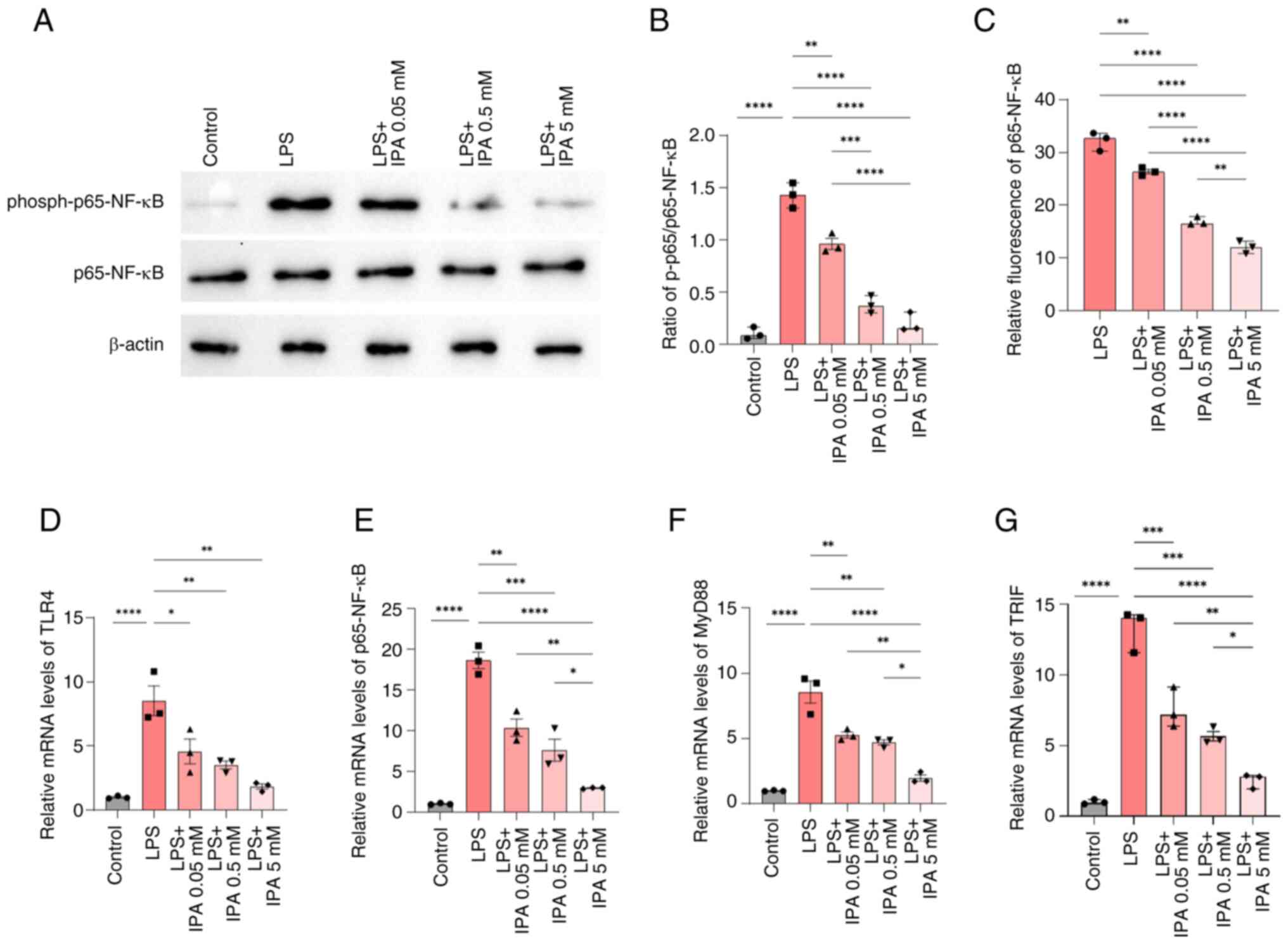 | Figure 5.IPA improves intestinal epithelial
barrier function via regulation of the NF-κB pathway. (A)
Representative blots of p65-NF-κB and phospho-p65-NF-κB expression.
(B) Ratio of phospho-p65/p65-NF-κB based on the results of western
blotting. (C) Effect of IPA on the bioactivity of p65-NF-κB using a
dual-luciferase reporter assay. (D) TLR4, (E) p65-NF-κB, (F) MyD88
and (G) TRIF mRNA expression levels were determined by reverse
transcription-quantitative PCR. Data are presented as the mean ±
SEM, n=3. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
IPA, indole-3-propionic acid; LPS, lipopolysaccharide; MyD88,
myeloid differentiation factor 88; phospho, phosphorylated; TLR4,
Toll-like receptor 4. |
The effect of IPA on the inhibition of p65-NF-κB was
validated using a dual-luciferase reporter gene assay system, and
the results were similar to those observed using western blotting.
Compared with in the LPS group, treatment with IPA effectively
inhibited the activation of p65-NF-κB in a concentration-gradient
dependent manner (P<0.0001), and high-concentration IPA showed
optimal inhibition in comparison with the low-concentration group
(P<0.0001; Fig. 5C).
To gain further insights into the mechanism
underlying IPA-mediated inhibition of p65-NF-κB, the mRNA
expression levels of key proteins in the NF-κB signaling pathway
were examined by RT-qPCR. The mRNA expression levels of TLR4,
NF-κB, MyD88 and TRIF were significantly higher in the LPS group
compared with those in the control group (P<0.0001); however, in
the presence of IPA, the expression levels of these genes were
reduced in a concentration-dependent manner compared with those in
the LPS group (P<0.05; Fig.
5D-G). These results indicated that IPA exerted a regulatory
effect on both the TLR4/MyD88/NF-κB signaling pathway and the
TLR4/TRIF/NF-κB signaling pathway, thereby inhibiting the release
of pro-inflammatory cytokines and protecting intestinal epithelial
cells against LPS-induced inflammatory injury. Taken together,
these results suggested that IPA may hold promise for mitigating
the detrimental effects of inflammation in the gut.
Discussion
Intestinal epithelial cells serve an important role
in maintaining a strong epithelial barrier and thus maintaining
good health (10). Previous
studies have demonstrated the toxic effects of high doses of LPS on
cells (23,28,29).
In the present study, treatment with 1 µg/ml LPS for 24 h reduced
cell viability, with the maximal effect observed in response to 10
µg/ml LPS, resulting in the significant induction of apoptosis of
intestinal epithelial cells. IPA exerted an inhibitory effect on
LPS-induced intestinal epithelial cell dysfunction, which is
consistent with previous research (29). The present study demonstrated that
LPS reduced TEER and the expression levels of TJ proteins in NCM460
cells; however, IPA treatment alleviated the effects of LPS,
highlighting the protective effects of IPA on LPS-induced
intestinal barrier dysfunction in vitro.
TJ proteins have a crucial role in forming
cell-to-cell interactions and serve as the primary defensive
barrier of the intestinal epithelium (30). Abrogating TJ protein structure can
lead to disruption of the barrier integrity, resulting in changes
in intestinal epithelial cell permeability, and thus the subsequent
development of various diseases associated with intestinal mucosal
inflammation (31). TJ proteins
consist of transmembrane proteins, such as occludin and claudin;
cytoplasmic proteins, such as ZOs and cingulin; and cytoskeletal
proteins, such as actin and myosin (32). Studies have shown that intestinal
injury is associated with the reduced expression and translocation
of TJ proteins (27,29,33,34).
Research on enterocyte cells has demonstrated that IPA can improve
barrier properties by increasing the expression of TJ proteins and
other junction proteins (11).
Additionally, in rats fed a high-fat diet, it was shown that IPA
treatment restored the height of villi in the ileum, and promoted
the expression of ZO-1, occludin and claudin-1 (29). In the present study, treatment with
a high concentration (5 mM) of IPA significantly reversed the
decrease in the expression levels of claudin-1, occludin and ZO-1
in NCM460 cells with LPS-induced intestinal epithelial injury,
indicating that higher concentrations of IPA improve intestinal
barrier function. Subsequent semi-quantitative immunofluorescence
analysis of ZO-1 protein expression demonstrated that not only a
high concentration (5 mM) of IPA, but also a medium concentration
(0.5 mM) of IPA, effectively reversed the LPS-induced decrease in
ZO-1 expression. The difference in the effects of the various
concentrations may be due to the assays being used, with
immunofluorescence staining focused more on localization, whereas
western blotting is used to assess total cellular protein. However,
both approaches indicated that the expression of the TJ protein
ZO-1 in the LPS-induced group was significantly decreased compared
with that in the control group, and that IPA up to a certain
concentration was effective in reversing the LPS-induced reduction
in the expression of ZO-1. Strategies targeting TJ proteins, such
as claudin-binding and angulin-binding agents, and their
application in drug development have been the focus of research
into novel therapeutics in previous years (27). However, the present study was
mainly limited to ZO-1, and future studies should focus on other TJ
proteins, including claudins, occludin, tricellulin, angulins and
junctional adhesion molecules to fully evaluate the mechanism of
the action of IPA on TJ proteins. Moreover, further refinement and
assessment of IPA concentrations should be investigated.
The disruption of intestinal barrier integrity can
lead to activation of local immunity and can result in an imbalance
of cytokines. However, there is evidence to suggest that IPA can
regulate intestinal permeability and barrier function during
inflammation by downregulating TNF-α in intestinal cells (29). Furthermore, another study reported
that IPA can activate the transcription factor aryl hydrocarbon
receptor in response to the production of byproducts from
commensals (35), thereby
maintaining intestinal homeostasis and regulating immunity
(36). Additionally, IPA has been
found to reduce the levels of proinflammatory factors and to
improve intestinal histopathology (10). Nonetheless, studies characterizing
the mechanisms of IPA in LPS-induced intestinal epithelial cell
injury are still lacking. It is well established that LPS can
trigger downstream MyD88/NF-κB signals via activation of TLR4,
leading to the production of proinflammatory cytokines (16,37).
Consistently, the results of the present study revealed that genes
related to the TLR4/NF-κB signaling pathway were significantly
upregulated following LPS intervention. By contrast, IPA reduced
the phosphorylation levels of p65-NF-κB in LPS-induced cells, as
well as the levels of pro-inflammatory cytokines, including IL-1β,
IL-6 and TNF-α. These findings suggested that IPA inhibited the
release of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in a
concentration-dependent manner via regulation of the
TLR4/MyD88/NF-κB and TLR4/TRIF/NF-κB pathways, and thereby
alleviated LPS-induced inflammatory injury in human colonic
epithelial cells. The present study provides valuable insights into
the therapeutic potential of IPA in alleviating LPS-induced
inflammatory injury.
The present study has some limitations. The
protective effects of IPA were only assessed in NCM460 cells; thus,
additional studies in other colonic epithelial cells are required.
Further experiments to detect additional TJ proteins, including
tricellulin and junctional adhesion molecules, may also better
reveal the interactive mechanism between IPA and intestinal barrier
integrity. Moreover, animal experiments were not performed to
determine if the mechanism identified was observed in vivo.
However, the results of the current in vitro study may
improve the understanding of the mechanism of action of IPA on
alleviating the inflammatory response induced by LPS and improving
intestinal barrier function. In future studies, additional in
vitro experiments using other cell lines and in vivo
experiments are required to validate the findings of the present
study.
In conclusion, the present study provides compelling
evidence for the protective effects of IPA on LPS-induced
intestinal epithelial cell injury and intestinal barrier function
in vitro. This was accompanied by the suppression of the
expression of TLR4, and downstream adaptor proteins MyD88 and TRIF,
as well as the inhibition of NF-κB. These findings shed light on
the potential therapeutic value of IPA in diseases characterized by
LPS-induced intestinal epithelial cell inflammatory injury and
intestinal barrier dysfunction. Nevertheless, further studies are
warranted to explore the precise mechanisms and optimal
concentration through which IPA exerts its protective effects and
to evaluate its efficacy in clinical settings.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Shanghai Minhang District
Natural Science Foundation (grant no. 2022MHZ028) to YC and the
Disciplinary Construction Project of Minhang Hospital (grant no.
YJXK-2021-08) to SC. The funders had no role in the study design,
data collection and analysis, decision to publish, or manuscript
preparation.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL, YC and WC carried out the experiments,
participated in collecting data and drafted the manuscript. XL and
QF performed the statistical analysis and participated in its
design. FL and SC participated in acquisition, analysis or
interpretation of data, and drafted the manuscript. YL and YC
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IPA
|
indole-3-propionic acid
|
|
TEER
|
transepithelial electrical
resistance
|
|
TJ
|
tight junction
|
|
LPS
|
lipopolysaccharide
|
|
TLR4
|
Toll-like receptor 4
|
|
MyD88
|
myeloid differentiation factor 88
|
References
|
1
|
Wlodarska M, Luo C, Kolde R, d'Hennezel E,
Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA, et
al: Indoleacrylic acid produced by commensal peptostreptococcus
species suppresses inflammation. Cell Host Microbe. 22:25–37.e6.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wan F, Wang M, Zhong R, Chen L, Han H, Liu
L, Zhao Y, Lv H, Hou F, Yi B and Zhang H: Supplementation With
chinese medicinal plant extracts from lonicera hypoglauca and
scutellaria baicalensis mitigates colonic inflammation by
regulating oxidative stress and gut microbiota in a colitis mouse
model. Front Cell Infect Microbiol. 11:7980522022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu QQ, Su ZR, Yang W, Zhong M, Xian YF and
Lin ZX: Patchouli alcohol attenuates the cognitive deficits in a
transgenic mouse model of Alzheimer's disease via modulating
neuropathology and gut microbiota through suppressing C/EBPβ/AEP
pathway. J Neuroinflammation. 20:192023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Cao Y, Zhang K, Guo Z, Liu Y, Zhou
P, Liu Z and Lu X: Gold nanoparticles alleviates the
lipopolysaccharide-induced intestinal epithelial barrier
dysfunction. Bioengineered. 12:6472–6483. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Lin J, Cheng Z, Wang T, Chen J and
Long M: Bacillus coagulans TL3 inhibits LPS-induced caecum damage
in rat by regulating the TLR4/MyD88/NF-κB and Nrf2 signal pathways
and modulating intestinal microflora. Oxid Med Cell Longev.
2022:54632902022.PubMed/NCBI
|
|
6
|
Hasain Z, Che Roos NA, Rahmat F, Mustapa
M, Raja Ali RA and Mokhtar NM: Diet and pre-intervention washout
modifies the effects of probiotics on gestational diabetes
mellitus: A comprehensive systematic review and meta-analysis of
randomized controlled trials. Nutrients. 13:30452021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Knudsen C, Neyrinck AM, Leyrolle Q, Baldin
P, Leclercq S, Rodriguez J, Beaumont M, Cani PD, Bindels LB,
Lanthier N and Delzenne N: Hepatoprotective effects of indole, a
gut microbial metabolite, in leptin-deficient obese mice. J Nutr.
151:1507–1516. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Konopelski P and Ufnal M: Indoles-gut
bacteria metabolites of tryptophan with pharmacotherapeutic
potential. Curr Drug Metab. 19:883–890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Q, Yu Z, Tian F, Zhao J, Zhang H, Zhai
Q and Chen W: Surface components and metabolites of probiotics for
regulation of intestinal epithelial barrier. Microb Cell Fact.
19:232020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alexeev EE, Lanis JM, Kao DJ, Campbell EL,
Kelly CJ, Battista KD, Gerich ME, Jenkins BR, Walk ST, Kominsky DJ
and Colgan SP: Microbiota-derived indole metabolites promote human
and murine intestinal homeostasis through regulation of
interleukin-10 receptor. Am J Pathol. 188:1183–1194. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Zhang L, Wu T, Li Y, Zhou X and Ruan
Z: Indole-3-propionic acid improved the intestinal barrier by
enhancing epithelial barrier and mucus barrier. J Agric Food Chem.
69:1487–1495. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Y, Li J, Ding W, Ruan Z and Zhang L:
Enhanced intestinal barriers by puerarin in combination with
tryptophan. J Agric Food Chem. 69:15575–15584. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He S, Wang J, Huang Y, Kong F, Yang R,
Zhan Y, Li Z, Ye C, Meng L, Ren Y, et al: Intestinal fibrosis in
aganglionic segment of Hirschsprung's disease revealed by
single-cell RNA sequencing. Clin Transl Med. 13:e11932023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu YH, Lai YH, Hsiao FS and Cheng YH:
Effects of deoxynivalenol and mycotoxin adsorbent agents on
mitogen-activated protein kinase signaling pathways and
inflammation-associated gene expression in porcine intestinal
epithelial cells. Toxins (Basel). 13:3012021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Liu G, Yuan Y, Wu G, Wang S and
Yuan L: NEK7 interacts with NLRP3 to modulate the pyroptosis in
inflammatory bowel disease via NF-κB signaling. Cell Death Dis.
10:9062019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng S, Li W, Ouyang H, Xie Y, Feng X and
Huang L: A novel prognostic pyroptosis-related gene signature
correlates to oxidative stress and immune-related features in
gliomas. Oxid Med Cell Longev. 2023:42561162023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pulakazhi Venu VK, Saifeddine M, Mihara K,
Tsai YC, Nieves K, Alston L, Mani S, McCoy KD, Hollenberg MD and
Hirota SA: The pregnane X receptor and its microbiota-derived
ligand indole 3-propionic acid regulate endothelium-dependent
vasodilation. Am J Physiol Endocrinol Metab. 317:E350–E361. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Venkatesh M, Mukherjee S, Wang H, Li H,
Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, et al:
Symbiotic bacterial metabolites regulate gastrointestinal barrier
function via the xenobiotic sensor PXR and Toll-like receptor 4.
Immunity. 41:296–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Xi W, Zhang X, Bi X, Liu B, Zheng
X and Chi X: CTSB promotes sepsis-induced acute kidney injury
through activating mitochondrial apoptosis pathway. Front Immunol.
13:10537542023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ismael S, Rodrigues C, Santos GM, Castela
I, Mota IB, Barreiros-Mota I, Almeida MJ, Calhau C, Faria A and
Araújo JR: IPA and its precursors differently modulate the
proliferation, differentiation, and integrity of intestinal
epithelial cells. Nutr Res Pract. 17:616–630. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo C, Guo D, Fang L, Sang T, Wu J, Guo C,
Wang Y, Wang Y, Chen C, Chen J, et al: Ganoderma lucidum
polysaccharide modulates gut microbiota and immune cell function to
inhibit inflammation and tumorigenesis in colon. Carbohydr Polym.
267:1182312021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Y, Duan L, Zeng Y, Song X, Pan K, Niu
L, Pu Y, Li J, Khalique A, Fang J, et al: The panda-derived
lactiplantibacillus plantarum BSG201683 improves LPS-induced
intestinal inflammation and epithelial barrier disruption in vitro.
BMC Microbiol. 23:2492023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sehgal R, Ilha M, Vaittinen M, Kaminska D,
Männistö V, Kärjä V, Tuomainen M, Hanhineva K, Romeo S, Pajukanta
P, et al: Indole-3-propionic acid, a gut-derived tryptophan
metabolite, associates with hepatic fibrosis. Nutrients.
13:35092021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mimori S, Kawada K, Saito R, Takahashi M,
Mizoi K, Okuma Y, Hosokawa M and Kanzaki T: Indole-3-propionic acid
has chemical chaperone activity and suppresses endoplasmic
reticulum stress-induced neuronal cell death. Biochem Biophys Res
Commun. 517:623–628. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang B, Jiang M, Zhao J, Song Y, Du W and
Shi J: The mechanism underlying the influence of indole-3-propionic
acid: A relevance to metabolic disorders. Front Endocrinol
(Lausanne). 13:8417032022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hashimoto Y, Tachibana K, Krug SM,
Kunisawa J, Fromm M and Kondoh M: Potential for tight junction
protein-directed drug development using claudin binders and
angubindin-1. Int J Mol Sci. 20:40162019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su Y, Chen C, Guo L, Du J, Li X and Liu Y:
Ecological balance of oral microbiota is required to maintain oral
mesenchymal stem cell homeostasis. Stem Cells. 36:551–561. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao ZH, Xin FZ, Xue Y, Hu Z, Han Y, Ma F,
Zhou D, Liu XL, Cui A, Liu Z, et al: Indole-3-propionic acid
inhibits gut dysbiosis and endotoxin leakage to attenuate
steatohepatitis in rats. Exp Mol Med. 51:1–14. 2019. View Article : Google Scholar
|
|
30
|
Mu Q, Kirby J, Reilly CM and Luo XM: Leaky
gut as a danger signal for autoimmune diseases. Front Immunol.
8:5982017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi R, Yu F, Hu X, Liu Y, Jin Y, Ren H, Lu
S, Guo J, Chang J, Li Y, et al: Protective effect of
lactiplantibacillus plantarum subsp. Plantarum SC-5 on dextran
sulfate sodium-induced colitis in mice. Foods. 12:8972023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao X, Zeng H, Lei L, Tong X, Yang L,
Yang Y, Li S, Zhou Y, Luo L, Huang J, et al: Tight junctions and
their regulation by non-coding RNAs. Int J Biol Sci. 17:712–727.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He C, Deng J, Hu X, Zhou S, Wu J, Xiao D,
Darko KO, Huang Y, Tao T, Peng M, et al: Vitamin A inhibits the
action of LPS on the intestinal epithelial barrier function and
tight junction proteins. Food Funct. 10:1235–1242. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stephens M and von der Weid PY:
Lipopolysaccharides modulate intestinal epithelial permeability and
inflammation in a species-specific manner. Gut Microbes.
11:421–432. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rothhammer V, Mascanfroni ID, Bunse L,
Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M,
et al: Type I interferons and microbial metabolites of tryptophan
modulate astrocyte activity and central nervous system inflammation
via the aryl hydrocarbon receptor. Nat Med. 22:586–597. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hubbard TD, Murray IA and Perdew GH:
Indole and tryptophan metabolism: Endogenous and dietary routes to
Ah receptor activation. Drug Metab Dispos. 43:1522–1535. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang X, Zhu J, Jiang Y, Xu C, Lv Q, Yu D,
Shi K, Ruan Z and Wang Y: SU5416 attenuated
lipopolysaccharide-induced acute lung injury in mice by modulating
properties of vascular endothelial cells. Drug Des Devel Ther.
13:1763–1772. 2019. View Article : Google Scholar : PubMed/NCBI
|