Introduction
Targeted non-invasive treatment of tumors is the
most promising area of medical research worldwide. The application
of pulsed electric fields (PEF) is emerging as a new technique for
tumor therapy. According to the pulse duration, PEF can be
classified into millisecond (msec), microsecond (μsec), nanosecond
(nsec) and picosecond (psec). Most researchers have focused on the
millisecond, microsecond and nanosecond pulse range for a more
in-depth study.
Weaver noted that the lipid bilayer of cells is
temporarily rearranged, followed by the formation of aqueous
channels in the cell membrane when exposed to long pulses (msec to
μsec), called electroporation (1).
These pulses cause reversible electrical breakdown (REB),
accompanied with a tremendous increase in molecular transportation
across the cell membrane; thus, many electroporation techniques are
applied in cell transfection for gene expression and drug delivery.
Okino et al (2) first
originated the concept of electrical chemotherapy (ECT) on the
basis of electroporation. Hofmann et al (3) and Dev et al (4) applied ECT together with
administration of bleomycin for the treatment of tumors. The drug
was able to kill the cancer cells effectively at a relatively low
concentration with minimal systematic side effects. Although ECT
may enhance the delivery of drugs, it is still not able to directly
kill tumor cells and negate their side effects. Yet, if the
electric field strength continues to increase, the pores in the
cell membrane enlarge, causing a loss of membrane intactness and
the direct killing of cancer cells (5). This phenomenon is termed irreversible
electrical breakdown (IREB). Miller et al (6) and Rubinsky et al (7) demonstrated that with proper
parameters, IREB could completely ablate human hepatocarcinoma
cells (HepG2) and prostate cancer cells in vitro without
inducing thermal damage. As the pulse duration decreases to
nanoseconds, this leads to intracellular electromanipulations such
as apoptosis, intracellular calcium burst, cytoskeleton, nuclear
membrane, DNA and telomere damage, with the outer membrane
remaining intact. Thus, this technique may be used in tumor
treatment and gene therapy (8–14).
Most recently, it has been shown that such PEF caused shrinkage and
even complete elimination of melanoma tumors (15).
However, the application of millisecond, microsecond
or nanosecond PEF requires the use of an invasive or minimally
invasive needle or plate electrodes, to guide the puncture of tumor
tissue, which to some extent limits the clinical application of
this method. Picosecond PEF (psPEF) has a wealth of ultra-broadband
spectrum, with extended time and spatial resolution, and low signal
distortion. It could be transferred to target deep tissue
non-invasively and precisely with wideband antennas (16,17).
Yet, research on the biological effect of psPEF on cells is
limited. Electric theory predicts that intense psPEF will target
mitochondria and lead to changes in transmembrane potential,
therefore it is hypothesized that it may induce cell apoptosis
through the mitochondrial pathway.
Our group has dedicated its study of the antitumor
effects of μsPEF or nsPEF for many years. In this study, we tested
the hypothesis that intense psPEF induces cell death through
mitochondrial apoptosis. HeLa cells were exposed to psPEF. Our
study included three steps: to investigate i) the dose-effect of
psPEF on cells, ii) the morphology of apoptosis and iii) the
mechanisms of mitochondrial apoptosis.
Materials and methods
Cell culture
HeLa, a human cervical carcinoma cell line was
obtained from the Institute of Ultrasound Engineering in Medicine
of Chongqing Medical University. Cells were cultured in RPMI-1640
medium (Hyclone, USA) supplemented with 10% fetal calf serum
(Amresco, USA), streptomycin (100 IU/ml) and penicillin (100 IU/ml)
at 37°C in a 5% humidified CO2 incubator. The cells were
fed until reaching 50–75% confluence, expanded by 0.25% trypsin
(Hyclone, USA) and subcultured at lower numbers in new culture
flasks.
Picosecond pulsed electric field (psPEF)
treatment
Cells were harvested with trypsin and re-suspended
in fresh RPMI-1640 medium to a concentration of 2x106
cells/ml. Cells loaded into cuvettes and merely placed into the
circuit without being pulsed were used as the normal controls. A
total amount of 100 μl of cell suspension was placed in cuvettes
and exposed to 800 psec pulses with an electric field amplitude of
250 kV/cm. In the MTT assay, the quantities of pulse numbers were
from 100 to 5000, and in other tests, the groups were divided by
the quantities of pulse numbers (group A, normal control; group B,
1000 pulses; group C, 3000 pulses; and group D, 5000 pulses).
MTT assay
The cell viability was investigated using MTT [3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay.
This assay was performed in quintuplicate. After treatment, HeLa
cells were seeded in 96-well plates (5,000 cells/well-1200
μl−1) and routinely cultured in an incubator for 6, 12,
24 and 48 h. A normal control and a blank group (without cells)
were also included. After incubation, 20 μl MTT (5 mg/ml) (Amresco)
was added to each well, and the plates were incubated for another 4
h. Following incubation, the culture medium was removed by gentle
aspiration and replaced with 150 μl DMSO, and the plates were
agitated for 10 min to dissolve the formazan crystals. Then, the
optical density of the 96-well culture plates was measured using an
enzyme-linked immunosorbent assay (ELISA) reader at 490 nm.
Transmission electron microscopy (TEM)
analysis
After treatment with 5000 pulses, the cells were
harvested and grown in RPMI-1640 medium containing 10% SFS for 12
h. Floating cells were then harvested together with adherent cells
and centrifuged at 200 x g for 5 min. The cell pellets were fixed
overnight in a 0.2 M sodium cacodylate buffer solution (pH 7.4)
containing 2% glutaraldehyde at 4°C. Samples were then post-fixed
in cacodylate-buffered 1% osmium tetroxide, dehydrated, and
embedded in Epon 812 (Structure Probe, Inc., West Chester, PA, USA)
for ultra-thin sectioning. The ultra-thin sections were stained
with uranyl acetate and lead citrate and viewed with a Hitachi-7500
transmission electron microscope (Hitachi, Japan) at the Chongqing
Medical University Cell Imaging Facility.
Measurement of mitochondrial membrane
potential (Δψm)
Δψm was measured by laser scanning confocal
microscopy (LSCM) (Leica TCS-SP2, Germany) using the cationic
lipophilic green fluorochrome Rhodamine-123 (Rh123) (Molecular
Probes) (Sigma, USA). After treatment with pulses, the cells were
routinely cultured in an incubator for 6 and 12 h. After
incubation, they were harvested, washed, resuspended in medium
containing Rh123. Then the cells were incubated at 37°C in an
CO2 incubator for 15 min. The cells were washed again
and resuspended in medium. Finally, the cells were subjected to
LSCM analysis by detecting the green fluorescence signals.
Western blot analysis
After treatment with pulses, the cells were
harvested and lysed in ice-cold RIPA cell lysates (CST, USA).
Lysates were centrifuged at 12,000 x g for 20 min at 4°C, and the
protein content was measured with the BCA Protein Assay kit (Sangon
Biotech, China). Thirty micrograms of protein was mixed with sodium
dodecyl sulfate (SDS) (Sigma) sample buffer, denatured by boiling,
and separated on 12% SDS-polyacrylamide gels. Proteins were then
electroblotted to nitrocellulose membranes (Millipore, USA) and
blocked for 2 h at room temperature in PBS buffer containing 5%
non-fat milk. Membranes were then incubated overnight at 4°C with
the respective primary antibodies. Antibodies against cytochrome C
(Cyt c) and apoptosis-inducing factor (AIF) were purchased from
CST. Anti-mouse or anti-rabbit secondary antibody conjugated to
horseradish peroxidase (CST) was used to visualize the stained
bands with an enhanced chemiluminescence (ECL) visualization kit.
Equal loading of protein was confirmed by stripping the blots and
reprobing with β-actin antibody.
Real-time polymerase chain reaction
(PCR)
Total RNA was extracted from treated cells by using
TRIzol reagent (Invitrogen, USA) following the manufacturer's
protocol. Reverse transcription of 2 μg of RNA was performed using
the reverse transcription kit, M-MLV1 (Promega, China). The PCR
reactions were carried out using intercalation of SYBR Green master
mix following the manufacturer's protocol (Applied Biosystems, USA
7900HT Fast Real-Time PCR System). Equal amounts of cDNA, as
determined by detection of the fluorescence signals, were used to
quantify the expression of caspase-3, Bax, Bcl-2 and p53 genes. The
following primers were used: caspase-3 forward,
5′-CGTGTATTGTGTCCATGCTCAC-3′ and reverse,
5′-CCATCATTGACAGTTACTTGCTCC-3′; Bax forward,
5′-GACGAACTGGACAGTAACATG-3′ and reverse,
5′-AGGAAGTCCAATGTCCAGCC-3′; Bcl-2 forward, 5′-CGGGAGAACAGGGTATGA-3′
and reverse, 5′-CAGGCTGGAAGGAGAAGAT-3′; p53 forward,
5′-CAGTCTACCTCCCGCCATA-3′ and reverse, 5′-GCA AGCAAGGGTTCAAAG-3′.
Reactions were performed in duplicate from three separate RNA
preparations.
Statistical analysis
Statistical analyses were performed using SPSS for
Windows 13.0. Data are presented as the mean ± SD, and were
subjected to analysis using one-way ANOVA, followed by LSD t-test
for multiple comparisons among groups. A probability value of
P<0.05 was defined as statistically significant.
Results
Growth inhibition of HeLa cells
To determine cell viability after psPEF treatment,
HeLa cells were exposed to 0–5000 pulses of 250 kV/cm amplitude.
Growth inhibition was determined by MTT assay. The cell survival
rate in normal control cells was taken as 100% viability. The
percentage of cell inhibition was determined as the (absorbance of
normal control cells - absorbance of treated cells/absorbance of
normal control cells - absorbance of blank group) x 100%. MTT assay
demonstrated that psPEF inhibited the growth of HeLa cells in a
dose-dependent manner (Fig. 1).
For each line, the cell inhibition rate increased in parallel with
the number of pulses, while it significantly increased from 500
pulses (P<0.05). We evaluated the growth inhibition at 6, 12, 24
and 48 h post-pulses, and the result showed that at a given number
of pulses, psPEF achieved a plateau of maximum cell inhibition at
12-h post-pulses.
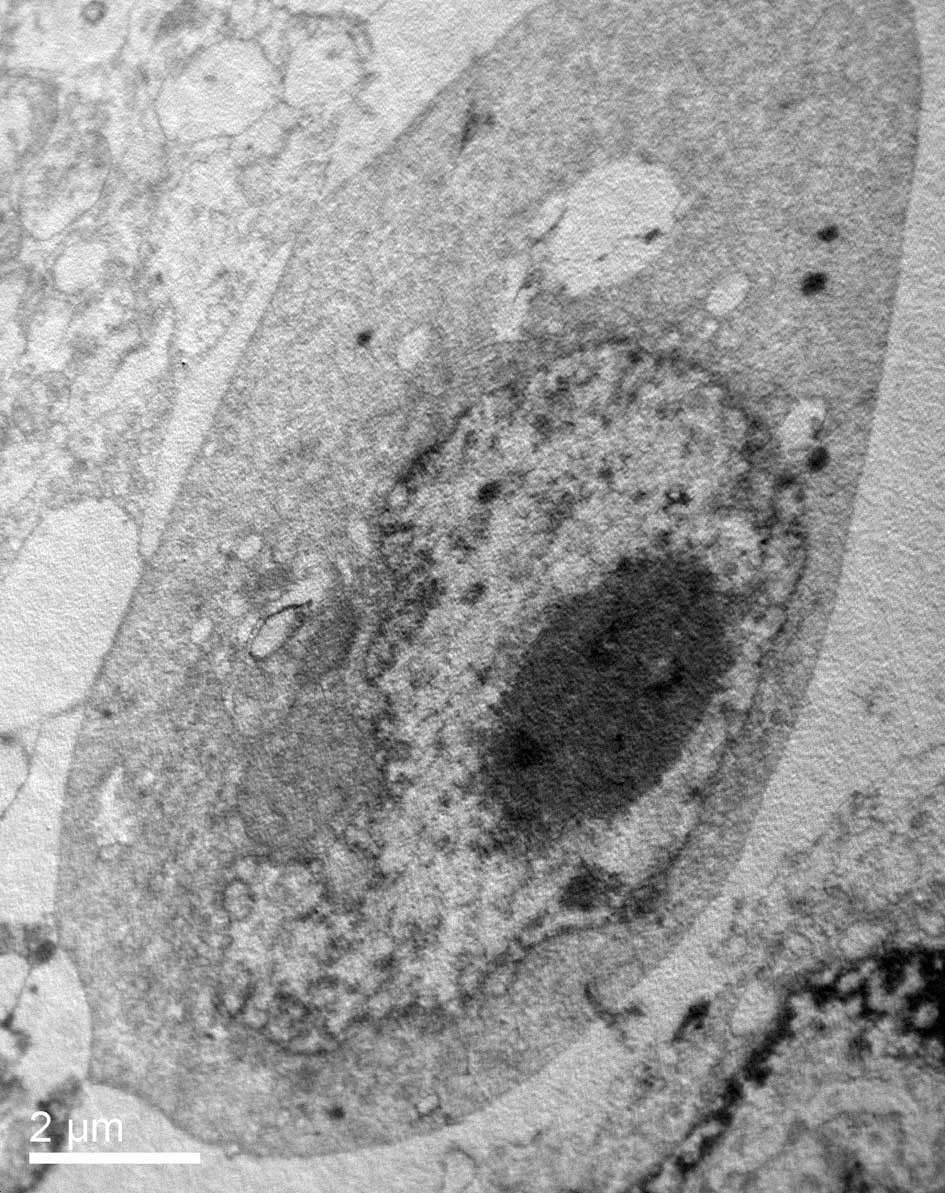
Ultrastructural observation
TEM was used to characterize the ultrastructural
changes in the HeLa cells in response to psPEF exposure. TEM
observation showed that the normal HeLa cells remained intact with
well-distributed chromatin and a clear nuclear membrane (Fig. 2A). However, in response to psPEF
exposure (5000 pulses), the cells changed to a shrunken state with
an intact membrane, aggregated chromatin and pseudopodia-like
protrusions which suggested apoptosis (Fig. 2B).
psPEF induce depolarization of
mitochondrial membrane potential (Δψm)
Depolarization of Δψm is associated with a lack of
Rh123 retention and a decrease in fluorescence. The value was
measured by LSCM using Rh123. Fig.
3 shows the fluorescence images of the control and treated
groups 6 h after the pulses. Obviously, the green fluorescence
intensity thinned down after the pulses, which indicates that Δψm
decreased. Fluorescence images 12 h after the pulses were also
obtained (data not shown). Fluorescence intensity is shown in
Table I. Fluorescence intensity in
the treated groups was significantly lower compared to that in the
control (all P<0.01). The minimum fluorescence intensity was
observed after 6 h, and no further decreases were observed
thereafter.
 | Table IFluorescence intensity of HeLa cells
by LSCM. |
Table I
Fluorescence intensity of HeLa cells
by LSCM.
| A | B | C | D |
|---|
| 6-h | 36.24±7.23 | 50.32±7.43a | 74.31±10.28a | 94.13±12.39a |
| 12-h | 39.14±5.75 | 52.40±8.67a,b | 75.90±4.97ab | 91.72±10.78a,b |
psPEF induce cytochrome C (Cyt c) and
apoptosis-inducing factor (AIF) release
Under physiological conditions, these two proteins
are located in the mitochondrial intermembrane space. In the
process of mitochondrial apoptosis, they are released from
mitochondria to the cytoplasm. Western blot analysis was used to
quantify the expression of Cyt c and AIF in the cytoplasm. β-actin
served as a control for sample loading. As a result, we found that
Cyt c and AIF accumulated 6 h after treatment with psPEF. The
protein levels were significantly higher after pulsing (all
P<0.05) (Fig. 4).
psPEF generate activation of caspase-3
and caspase-9
Caspase-3 and caspase-9 are involved in the process
of mitochondrial pathway-mediated apoptosis. In response to
apoptotic stimuli, they become activated. Real-time PCR was
performed to quantify the altered expression of caspase-3 and
caspase-9. Activation of caspase-3 and caspase-9 was observed. Each
treated group showed significant difference in comparison to the
control group (P<0.05) (Fig.
5).
psPEF induce upregulation of Bax, p53 and
downregulation of Bcl-2
The Bcl-2 protein family and p53 gene play an
important role in the mitochondrial apoptotic pathway.
Anti-apoptotic protein, Bcl-2, and pro-apoptotic protein, Bax, were
detected by real-time PCR. Elevated expression levels of Bax, p53
and reduced levels of Bcl-2 were observed compared to the normal
group. Each treated group showed significant difference in
comparison to the control group (all P<0.05) (Fig. 6).
Discussion
Cervical cancer is one of the most common
gynecological malignancies. Its incidence in young women has
increased in recent years (18).
The traditional surgical treatment often leads to severe damage of
the genital tract and affects the sexual function and fertility of
patients. Despite advances in surgical techniques, including
conservative treatment such as radical trachelectomy, the fertility
of patients is still highly effected (19). Non-invasive treatment with
preserved fertility is the expectation for both doctors and
patients.
Pulsed-electric field is a new biomedical
engineering technique which can be used as electrochemotherapy,
tumor ablation and intracellular electromanipulation. Whereas
studies on the effects of millisecond, microsecond or nanosecond
PEF have already led to medical applications, to our knowledge
there are few experimental studies on the biological effects of
psPEF.
The electric field possesses parameters related to
different biophysical effects, that is, the impact of electric
field pulses on cells has a certain window effect (20,21).
When the pulse duration is used as a reference point, the
corresponding changes in the biological effects caused by different
parameters of pulses are shown in Fig.
7. Millisecond or microsecond PEF mainly target the outer
membrane, and there is little influence to the cell nucleus,
mitochondria and other organelles; thus, it causes electroporation
to the outer membrane. As the pulse duration decreases, the
electroporation effect changes gradually from the outer membrane to
the intracellular organelle membrane. Submicrosecond PEF is capable
of inducing significant voltages across both the inner and outer
membranes, therefore, causing damage to both the inner and outer
membranes.
While these effects of PEF continue to be explored,
a new domain of pulsed electric field interactions with cell
structures and functions unfolds when the pulse duration is reduced
to values such that membrane charging becomes negligible. For
mammalian cells, this holds for a pulse duration of one nanosecond
or less (22). We dare to assume
from the rules above, that when the electrical pulse duration is
shorter than one nanosecond, PEF are able to induce larger voltage
across the inner membrane and to act mostly on intracellular
substructures. According to cell biology and electromagnetic
theory, the mitochondrial membrane charging time constant (a few
hundreds of picoseconds) is much shorter than the nuclear membrane
and the cell membrane charging time constant (tens of nanoseconds,
and hundreds of nanoseconds, respectively) (23,24).
Under the action of intense psPEF, the mitochondrial membrane will
charge rapidly, at a time when the nuclear membrane and the cell
membrane have no chance to respond. Thus, the mitochondrial
transmembrane potential will be altered. We speculate that intense
psPEF target the mitochondria and lead to changes in transmembrane
potential, release of Cyt c and AIF, activation of caspase 9 and
caspase 3, and finally apoptosis.
Schoenbach et al applied subnanosecond pulses
to B16 (mouse melanoma) cells. Initial experiments at a 800-psec
pulse width and an extremely high electric field of 950 kV/cm
showed that with a relatively small number of pulses, a
considerable increase in caspase activation, externalization of
phosphatidyl serine and programmed cell death was initiated
(22). For pulse amplitudes of 550
kV/cm, approximately 50% of the cells absorbed trypan blue
immediately after pulsing, whereas only 20% absorbed it after 1 h.
This indicated that the plasma membrane in a majority of the cells
affected by the pulses recovered with a time constant of
approximately 1 h. The cells that exhibited trypan blue uptake
after this time suffered cell death through apoptosis (25). Experiments where platelets were
exposed to 150-psec pulses with an electric field of 150 kV/cm
indicated a pulse-number-dependent uptake of calcium (26).
In the present experiments we demonstrated the
response of HeLa cells to psPEF. Our results revealed that psPEF is
capable of inducing cell apoptosis through a mitochondrial-mediated
pathway. Firstly, MTT assay demonstrated that intense psPEF
significantly inhibited the proliferation of HeLa cells, and
typical characteristics of apoptosis in the HeLa cells were
observed under TEM. Proteins located in the inner mitochondrial
membrane, such as Cyt c and AIF, play an important role in
apoptosis. Once Cyt c enters the cytoplasm, it conjucts with
apoptotic peptidase activating factor 1 (Apaf-1) and facilitates
the activation of procaspase-9, which finally activates
procaspase-3. Thus, the release of Cyt c from the mitochondria into
the cytoplasm triggers apoptosis via the caspase-3-dependent
pathway. AIF is a key trigger of caspase-independent apoptosis.
Western blot analysis showed that Cyt c and AIF significantly
increased in the cytoplasm after exposure to psPEF compared to the
untreated groups, and loss of Δψm was observed by LSCM. This
suggests that both the caspase-dependent and -independent apoptotic
pathways are involved in psPEF-induced apoptosis in HeLa cells
through the mitochondria pathway. The Bcl-2 family members and the
p53 gene are key regulators of the mitochondrial pathway of
apoptosis. Bcl-2 and Bax are the best characterized proteins of the
Bcl-2 family, and the Bax/Bc1-2 ratio is a key factor to determine
cell death after exposure to death stimuli. Elevated expression
levels of caspase-3, caspase-9, Bax, p53 and reduced levels of
Bcl-2 in the treated groups were observed in comparison to the
normal group indicating apoptosis.
In summary, the present data provide evidence that
psPEF induce apoptosis in cultured human cervical cancer cells, and
the apoptotic effect is possibly through the mitochondrial-mediated
pathway. The use of picosecond pulses not only allows us to enter a
new field of field-cell interactions, but it may open the door to a
range of noninvasive therapeutic applications. Further studies are
needed to elucidate the cell responses to psPEF in detail.
Acknowledgements
This study was supported by two grants from the
National Natural Science Foundation of China (project nos. 81172123
and 50877083). The authors thank the members of our laboratory for
their helpful discussion.
References
|
1
|
JC WeaverElectroporation: a general
phenomenon for manipulating cells and tissuesJ Cell
Biochem51426435199310.1002/jcb.24005104078496245
|
|
2
|
M OkinoH TomieH KanesadaOptimal electric
conditions in electrical impulse chemotherapyJpn J Cancer
Res8310951101199210.1111/j.1349-7006.1992.tb02727.x1280635
|
|
3
|
GA HofmannSB DevS DimmerElectroporation
therapy: a new approach for the treatment of head and neck
cancerIEEE Trans Biomed
Eng46752759199910.1109/10.76495210356882
|
|
4
|
SB DevDP RabussayG WideraMedical
applications of electroporationIEEE Trans Plasma
Sci28206223200010.1109/27.842905
|
|
5
|
HT TienA OttovaThe bilayer lipid membrane
(BLM) under electrical fieldIEEE Trans Dielectr Electr
Insul10717727200310.1109/TDEI.2003.1237323
|
|
6
|
L MillerJ LeorB RubinskyCancer cell
ablation with irreversible electroporationTechnol Cancer Res
Treat4699705200510.1177/15330346050040061516292891
|
|
7
|
J RubinskyG OnikP MikusB RubinskyOptimal
parameters for the destruction of prostate cancer using
irreversible electroporationJ
Urol18026682674200810.1016/j.juro.2008.08.00318951581
|
|
8
|
M StaceyJ StickleyP FoxV StatlerK
SchoenbachSJ BeebeS BuescherDifferential effects in cells exposed
to ultra-short, high intensity electric fields: cell survival, DNA
damage, and cell cycle analysisMutat
Res5426575200310.1016/j.mrgentox.2003.08.00614644355
|
|
9
|
S KatsukiN NomuraH KogaBiological effects
of narrow band pulsed electric fieldsIEEE Trans Dielectr Electr
Insul14663668200710.1109/TDEI.2007.369529
|
|
10
|
N ChenAL GarnerG ChenNanosecond electric
pulses penetrate the nucleus and enhance speckle formationBiochem
Biophys Res
Commun364220225200710.1016/j.bbrc.2007.09.12517950251
|
|
11
|
GL CravisoP ChatterjeeG MaaloufNanosecond
electric pulse-induced increase in intracellular calcium in adrenal
chromaffin cells triggers calcium-dependent catecholamine
releaseIEEE Trans Dielectr Electr
Insul1612941301200910.1109/TDEI.2009.5293941
|
|
12
|
RP JoshiKH SchoenbachBioelectric effects
of intense ultrashort pulsesCrit Rev Biomed
Eng38255304201010.1615/CritRevBiomedEng.v38.i3.2021133836
|
|
13
|
WH BaldwinBW GregoryCJ OsgoodMembrane
permeability and cell survival after nanosecond pulsed electric
field exposure–significance of exposure media compositionIEEE Trans
Plasma Sci3829482953201022910297
|
|
14
|
M StaceyP FoxS BuescherJ KolbNanosecond
pulsed electric field induced cytoskeleton, nuclear membrane and
telomere damage adversely impact cell
survivalBioelectrochemistry82131134201110.1016/j.bioelechem.2011.06.00221719360
|
|
15
|
X ChenX ChenRJ SwansonKH SchoenbachS YinS
ZhengHistopathological follow-up by tissue micro-array in a
survival study after melanoma treated by nanosecond pulsed electric
fields (nsPEF)J Dermatolog
Treat22153161201110.3109/0954663090358508220666667
|
|
16
|
CE BaumAP StoneJS TyoUltra-Wideband,
Short-Pulse Electromagnetics 8Springer PressNew York, NY2007
|
|
17
|
C BajracharyaX ShuCE BaumKH
SchoenbachTarget detection with impulse radiating antennaIEEE
Antennas Wireless Propag
Lett10496499201110.1109/LAWP.2011.2157070
|
|
18
|
F BrayAH LoosP McCarronE WeiderpassM
ArbynH MøllerM HakamaDM ParkinTrends in cervical squamous cell
carcinoma incidence in 13 European countries: changing risk and the
effects of screeningCancer Epidemiol Biomarkers
Prev14677686200510.1158/1055-9965.EPI-04-056915767349
|
|
19
|
M Karimi ZarchiA MousaviM MalekzadehA
DehghaniZ BehnamfarA GodarziConservative treatment in young
patients with cervical cancer: a reviewAsian Pac J Cancer
Prev11589594201021039021
|
|
20
|
C YaoD MoC LiC SunY MiStudy of
transmembrane potentials of inner and outer membranes induced by
pulsed-electric-field model and simulationIEEE Trans Plasma
Sci3515411549200710.1109/TPS.2007.905110
|
|
21
|
C YaoY MiC LiStudy of transmembrane
potentials on cellular inner and outer membrane–frequency response
model and its filter characteristic simulationIEEE Trans Biomed
Eng55179217992008
|
|
22
|
KH SchoenbachS KatsukiH AkiyamaBiological
effects of intense subnanosecond electrical pulsesPower Modulator
Symposium, 2006. Conference Record of the 2006 Twenty-Seventh
InternationalMay 14–185735762006
|
|
23
|
I ErmolinaYL PolevayaYR FeldmanStudy of
normal and malignant white blood cells by time domain dielectric
spectroscopyIEEE Trans Dielectr Electr
Insul8253261200110.1109/94.919948
|
|
24
|
YR FeldmanI ErmolinaY HayashiTime domain
dielectric spectroscopy study of biological systemsIEEE Trans
Dielectr Electr Insul10728753200310.1109/TDEI.2003.1237324
|
|
25
|
KH SchoenbachX ShuRP JoshiJT CampT
HeerenJF KolbSJ BeebeThe effect of intense subnanosecond electrical
pulses on biological cellsIEEE Trans Plasma
Sci36414422200810.1109/TPS.2008.918786
|
|
26
|
JT CampX ShuSJ BeebePF BlackmoreKH
SchoenbachBioelectric studies with subnanosecond pulsed electric
fields2009 IEEE Pulsed Power ConferenceJune 28–July 28768792009
|