Introduction
Colorectal cancer (CRC) is the third most common
type of cancer in men and the second in women worldwide (1,2). It
is one of the leading causes of cancer-related mortality due to
therapy resistance (3). A better
understanding of the molecular mechanisms underlying CRC
progression is essential for the prevention and treatment of
advanced CRC.
A novel paradigm in tumor biology suggests that
cancer growth is driven by stem-like cells within a tumor. Growing
evidence suggests that human cancers are stem cell diseases. Recent
data support the hypothesis that cancer stem cells (CSCs) exist in
human cancers, including CRC (4).
CSCs are a subpopulation of cells within a tumor that can
self-renew, drive tumor growth and recurrence and are resistant to
many current anticancer treatments (5,6).
Epithelial-mesenchymal transition (EMT) plays a
crucial role in the differentiation of multiple tissues and organs,
and is a key developmental program that is often activated during
cancer invasion and metastasis (7–9).
Previous studies have provided morphological evidence that EMT
occurs at the invasive fronts of human tumors, including CRC, and
EMT occurring at the tumor-host interface is thought to enhance
metastasis (10,11).
During the process of EMT, epithelial cells undergo
a phenotypic switch, giving rise to a fibroblastoid phenotype.
However, since this ‘transformation’ is reversible and mesenchymal
cells revert to epithelial cells via mesenchymal-epithelial
transition (MET) (12), the
mesenchymal state is associated with the capacity of cells to
prevent apoptosis and senescence, and contributes to
immunosuppression, migration to distant organs and maintaining
stemness (7,8). The induction of EMT in normal and
cancer cell populations renders them more resistant to
chemotherapeutic drugs (5).
E-cadherin (encoded by CDH1) is a
transmembrane glycoprotein localized in the apical adherens
junction typically found in epithelial cells, and plays an
important role in maintaining the structural integrity of
epithelial sheets (12).
E-cadherin is an important molecule in cancer progression and EMT
process. Indeed, E-cadherin perturbation in mammalian cell systems
is sufficient to trigger EMT (13). The inhibition of E-cadherin
expression has been reported in several types of cancer, including
advanced colorectal cancer, oesophageal adenocarcinoma, gastric
cancer, vulvar squamous cell carcinoma and pancreatic cancer
(9,12,14).
The loss of E-cadherin is considered to augment cellular
dissemination and tumor metastasis (12).
In this study, we demonstrate that the knockdown of
E-cadherin in the SW480 colorectal cancer cell line leads to
significant EMT-like alterations and acquirement of most the
properties of CSCs. The aim of this study was to create a model of
CSC enrichment for cancer study, especially for screening anti-CSC
chemotherapeutic drugs.
Materials and methods
Cell culture
The SW480 human colorectal cancer cell line was
purchased from the cell bank of the China Academy of Medical
Science (China) and cultured in Leibovitz L15 medium (Gibco)
supplemented with 10% fetal bovine serum (FBS; Gibco). The cells
were then maintained at 37°C with 5% CO2.
Gene knockdown
Gene knockdown was achieved by transfecting SW480
cells with CDH1 shRNA-pSilencer 4.1-CMV using Lipofectamine
2000 (Invitrogen) in accordance with the manufacturer’s
instructions. After transfection, the cells were maintained under
G418 selection pressure at a concentration of 600 μg/ml for 14
days. Limited dilution assay was performed, and 10 clones with
>90% knockdown efficiency verified by qRT-PCR were selected
(Fig. 1A), expanded and then
cultured together. The cells transfected with CDH1
shRNA-pSilencer 4.1-CMV were annotated as SW480-shE. The cells
transfected with pSilencer 4.1-CMV were annotated as SW480-shC.
qRT-PCR analysis
Total RNA was extracted from the cultured cells
using the RNeasy mini kit (Qiagen) and treated with RNase-free
DNase I (Qiagen) according to the manufacturer’s instructions. The
RNA from SW480-shC and SW480-shE cells was reverse-transcribed into
cDNA with the Reverse Transcriptase M-MLV (Promega). The expression
of CDH1 was measured using the SYBR-Green PCR Master Mix
(Applied Biosystems), on the StepOnePlus system (Applied
Biosystems). The CDH1 levels were normalized to GAPDH
expression.
The primers for qRT-PCR analysis were the following:
CDH1 forward, 5′-GCTCACATTTCCCAACTC-3′ and reverse,
5′-GTCACCTTCAGCCATCC-3′; GAPDH forward, 5′-CTT
AGCACCCCTGGCCAAG-3′ and reverse, 5′-GATGTTCTGG AGAGCCCCG-3′.
Western blot analysis
SW480-shC and SW480-shE cells were lysed using M-PER
Mammalian Protein Extraction Reagent (Thermo) supplemented with
protease inhibitor cocktail (Sigma). After blocking with 5% non-fat
milk in TBST for 60 min, the membranes were incubated with primary
antibodies dissolved in 5% bovine serum albumin (BSA) in TBST
overnight at 4°C. The following primary antibodies were used:
anti-human-E-cadherin (Cell Signaling Technology),
anti-human-N-cadherin (Epitomics) and anti-human-Vimentin
(Epitomics) at a 1:2,000, 1:5,000 or 1:1,000 dilution,
respectively. The membranes were washed with TBST for 5 min 3 times
and then incubated for 1 h at room temperature with secondary
antibody (1:3,000, swine anti-rabbit IgG/HRP; Gene Tech) dissolved
in 5% non-fat milk in TBST. Human GAPDH (KangChen) was used as an
internal reference at a 1:5,000 dilution.
Proliferation assays
SW480-shC and SW480-shE cells were seeded at a
density of 3×103 cells/well in a 96-well plate
containing 0.2 ml Leibovitz L15 medium (Gibco) with 10% FBS.
Subsequently, 20 μl 3-(4,5-dimethylthiazol-2-yl)-5-
(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner
salt (MTS; Promega) reagent was added to each well and the cells
were incubated at 37°C for 4 h. The OD values were measured at 490
nm on a microplate reader (Bio-Rad, Hercules, CA, USA) and assessed
every day for up to 7 days.
Two-dimensional (2D) and
three-dimensional (3D) colony formation assay
The growth ability of SW480-shC and SW480-shE cells
was examined using 2D colony formation assay. Approximately 500
SW480-shC or SW480-shE cells were seeded into each well of a 6-well
plate. After incubation at 37°C for 14 days, the cells were washed
with PBS twice, fixed with methanol and then stained with 0.1%
crystal violet. The number of colonies containing >30 cells was
counted under a microscope. In 3D colony formation assay, 300 cells
of each cell line were seeded into each well of an ultra-low
attachment 6-well plate (Corning) and cultured in serum-free
DMEM/F12 (Gibco) containing 1 μg/ml insulin (Haotian
Biotechnology), 20 ng/ml bFGF (Invitrogen), 20 ng/ml EGF
(Invitrogen), 0.1% BSA (Haotian Biotechnology) and 2% B27
(Invitrogen). After 14 days of incubation, the number of
colospheres containing >30 cells was counted. Experiments were
performed in triplicate.
Flow cytometry analysis
SW480-shC and SW480-shE cells were trypsinized and
fixed in 70% ethanol. DNA content of incorporated propidium iodide
(PI) was scanned on a flow cytometer. The cell populations in
G0/G1, S and G2/M phases were determined using the ModFit LT
software.
CD24 and CD44 are widely used for isolating CSCs
from solid tumors, including CRC (14,15).
Combining these 2 markers enhances the purity of CSCs. For
comparing the proportion of CD24+/CD44+ cells
in the SW480-shC and SW480-shE cell populations, cells were
trypsinized, washed with phosphate-buffered saline (PBS) and
resuspended in 2% FBS in PBS at 1×106 cells/100 μl. Each
suspension was incubated with mouse anti-human CD24-FITC (BD
Pharmingen) and CD44-APC antibodies (Miltenyi Biotec) for 20 min at
4°C. The isotype control and blank control were set in the
analysis. Then, the cells were washed twice with PBS and filtered
on a 300 mesh screen to exclude the cell mass. Cells labeled with
CD24+/CD44+ markers were finally analyzed
using a FACSCanto II flow cytometer (BD Biosciences). The FACSDiva
software was used to quantify the fluorescence signals and to set
the logical electronic-gating parameters.
Tumorigenesis assay
SW480-shC and SW480-shE cells (1×105)
suspended in 200 μl Leibovitz L15 medium were implanted into the
backs of 4-week-old female nude mice. The mice were either injected
with SW480-shC or SW480-shE cells to analyze their ability to
initiate tumor xenografts. Nude mice were bred and maintained under
defined conditions at the Laboratory Animal Research Center of
Zhejiang Chinese Medicine University under conditions approved by
the local animal care committee.
Statistical analysis
Unless otherwise indicated, all the results were
analyzed by a two-tailed unpaired Student’s t-test.
Results
Knockdown of CDH1 induces EMT in SW480
cells
We established a cell line model by CDH1
knockdown in SW480 cells. SW480 cells were transfected with
CDH1 shRNA-pSilencer 4.1-CMV (shE vector) or pSilencer
4.1-CMV (shC vector). Western blot analysis showed that E-cadherin
protein expression was downregulated when the shE vector was
transfected into the SW480 cells successfully (Fig. 1B). By contrast, the transfection of
the shC vector had no effect on protein expression.
SW480-shE cells presented with mesenchymal
morphology with disrupted intercellular adhesion and a reorganized
cytoskeleton into an EMT-like formation (Fig. 1C). The results obtained from
western blot analysis showed that the SW480-shE cells expressed
high levels of mesenchymal cell marker proteins, including
N-cadherin and Vimentin (Fig. 1B).
These results collectively proved that SW480-shE cells had
undergone EMT.
SW480-shE cells have reduced cellular
proliferation
The cell cycles of the SW480-shC and SW480-shE cells
were then analyzed by flow cytometry and PI staining. The SW480-shE
cells had a significantly increased number of cells in the G0/G1
phase compared to the SW480-shC cells (72.59 vs. 62.71%) (Fig. 2A).
We compared the proliferation rate between SW480-shC
and SW480-shE cells using MTS analysis. As shown in the growth
curve, the proliferative ability of the SW480-shE cells was
significantly inhibited compared to the SW480-shC cells over a
7-day test period (Fig. 2B). The
results demonstrated that the knockdown of CDH1 reduced the
cell proliferative ability in vitro.
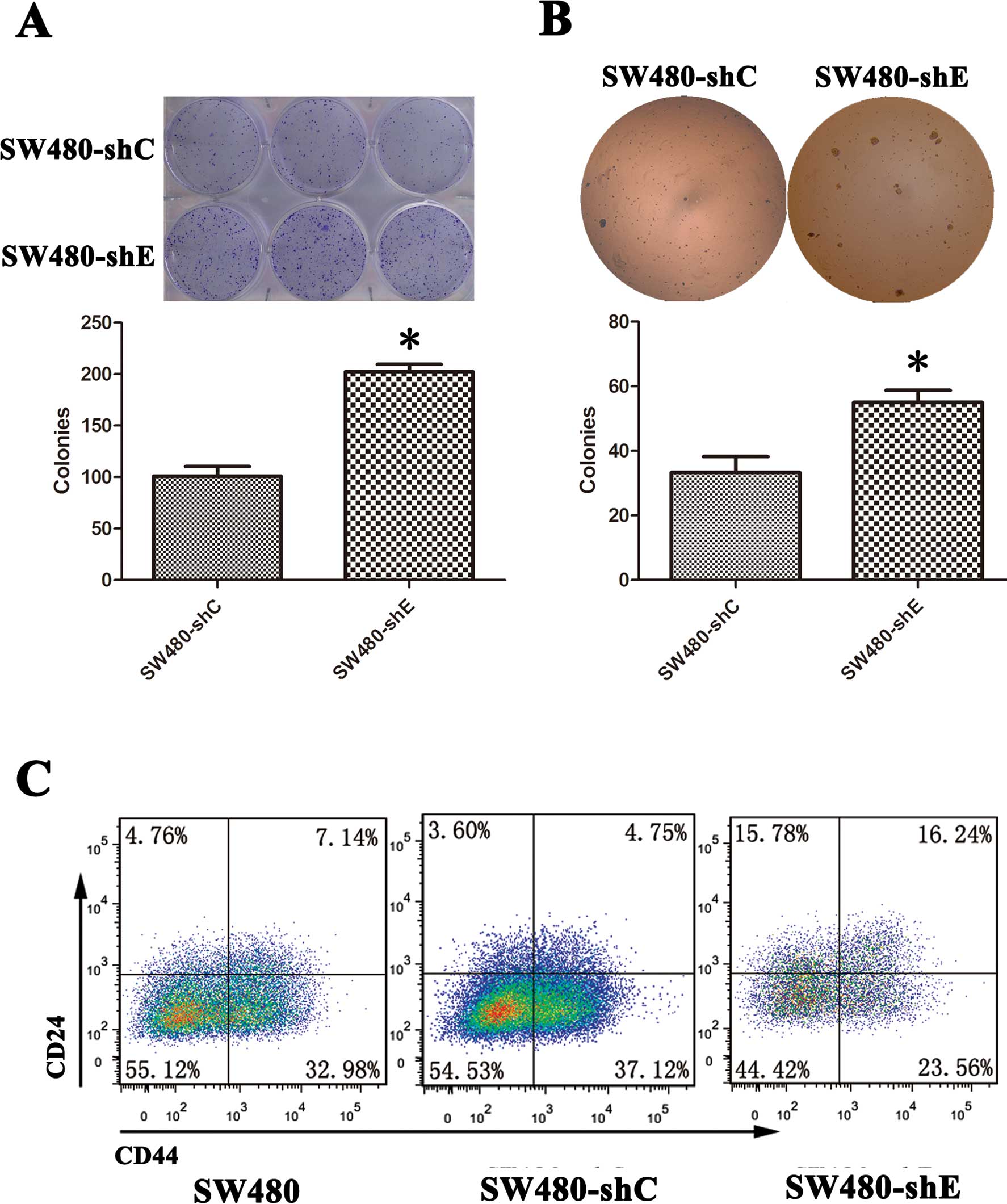
SW480-shE cells have significantly
enhanced colony formation
Certain evidence indicates that EMT generates cells
with properties of stem cells (5,7). One
feature of ‘stemness’ is the ability to form colonies. In the
present study, we examined this hypthesis using colony formation
assay. The colony-forming ability of the SW480-shC and SW480-shE
cells was examined. The results showed that colonies were visible
after 2 weeks and the number and size of colonies were
significantly greater in the SW480-shE cells compared to the
SW480-shC cells (Fig. 3A and
B).
CD24/CD44 expression is upregulated in
SW480-shE cells
CD24 and CD44 are widely accepted surface markers
used for isolating CSCs from solid tumors, including CRC (16). We detected the proportion of
CD24+/CD44+ cells in both the SW480-shC and
SW480-shE cells by flow cytometry. The result revealed that the
proportion of CD24+/CD44+-expressing cells
increased from 4.75 to 16.24% in the SW480-shE cells, while no
significant difference was observed in the SW480-shC cells
(Fig. 3C). This result suggested
that the knockdown of CDH1 made the SW480 cells more
stem-like, giving them the property of ‘stemness’.
CDH1 knockdown promotes
tumorigenesis
SW480-shC and SW480-shE cells (1×105)
were injected subcutaneously into the backs of nude mice. The mice
were divided into 2 groups: those injected with SW480-shC cells and
those injected with SW480-shE cells. In the SW480-shE group,
xenograft tumors could be detected in 4 out of 5 mice at 3 weeks
after inoculation, while no visible tumor could be observed at 5
weeks in the SW480-shC group. A significant difference in tumor
growth was observed between the SW480-shC and SW480-shE xenograft
mice (Fig. 4). From these results,
it is evident that the knockdown of CDH1 gene promoted the
tumorigenesis of CRC.
Discussion
To date, the existence of CSCs has been confirmed in
tumors from many organs, including breast, brain, prostate,
pancreas, head and neck, colon, lung, skin, liver and ovary
(17,18). CSCs are a small subpopulation of
cancer cells, which expand the CSC pool and differentiate into
cancer progenitor cells by symmetric or asymmetric division
(19). CSCs are quiescent,
resistant to therapy and responsible for sustaining tumor growth
and recurrence (4,5,20,21).
Although there have been many advances in CSC research, the origin
of CSCs and the differences between CSCs and differentiated cancer
cells remain unclear.
There is growing evidence suggesting that EMT plays
an important role in tumor development, not only in tumor
recurrence, but also in tumor metastasis (22). In CRC, EMT occurs at the invasive
front and produces single migratory cells with decreased E-cadherin
expression (8,10). Recent evidence shows that cells
which undergo EMT acquire stem cell-like properties (5,7). It
has been reported that the proportion of CSCs increases in breast
cancer cells with the induction of EMT by the knockdown of the
human CDH1 gene (5).
However, a stable model of CSCs via CDH1 knockdown in CRC
has not yet been established.
In this study, we provide evidence to support the
hypthesis that EMT participates in CRC progression. As shown in a
previous study, non-motile, polarized epithelial cells dissolved
their cell-cell junctions and converted into individual, motile,
non-polarized cells after EMT (23).
In the present study, we show that SW480-shE cells
may be regarded as EMT-induced mesenchymal cells, and that these
cells possess CSC properties. With CDH1 knockdown,
E-cadherin expression in SW480-shE cells decreased, accompanied by
an increase in N-cadherin and Vimentin expression. SW480-shE cells
presented with disrupted intercellular adhesion and a reorganized
cytoskeleton into an EMT-like formation. These cells were
comparatively quiescent, grew slower and had a greater
colony-formating ability, as shown by both 2D and 3D cultures,
compared to the SW480-shC cells. In an in vivo experiment,
SW480-shE cells also displayed stronger tumorigenic ability, which
is a main characteristic of CSCs. Based on the fact that CD24 and
CD44 are widely used for isolating CSCs from solid tumors,
including CRC (16), we used these
markers in our study (24,25); our results demonstrated that the
combination of these 2 markers enhanced the purity of CSCs and
enriched the CD24+CD44+ subpopulation
significantly in the SW480-shE cells. In conclusion, SW480-shE
cells possess most of the properties of CSCs.
Since CSC resistance to chemotherapy is the barrier
to successful tumor treatment, a previous study used the EMT model
for enriching breast CSCs, and high-throughput screening for
anticancer drugs with CSC-specific toxicity (5). Generally, CSCs are obtained through
FACS, spheroid culture and TGFβ-induced EMT in CRC (26). However, these CSCs cannot be used
for long-term culture due to differentiation. Therefore, a more
effective and stable model of CSCs is required for the study of
CSCs and screening for CSC-sensitive chemotherapeutic drugs.
In conclusion, SW480-shE cells possess most of the
properties of EMT and CSCs, and may be a stable model for cancer
study, especially for screening for CSC-sensitive drugs. With the
deepening research of the molecular mechanisms involved in CRC and
drug research, the prognosis of CRC may significantly improve.
Acknowledgements
The authors thank Dr Wei Yuan for providing the
CDH1-shRNA vector (pSilencer 4.1-CMV) and Dr Zhong Shi for language
editing. This study was supported by grants from the National
Natural Science Foundation of China (no. 91019005), the Zhejiang
Provincial Natural Science Foundation of China (no. Y2110034), and
the Zhejiang Education Department of China (no. Y200909117).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010.
|
|
2
|
Vo DM, Julien LA and Thorson AG: Current
controversies in colon and rectal cancer. Minerva Chir. 65:677–693.
2010.
|
|
3
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36.
2001.
|
|
4
|
Pang R, Law WL, Chu AC, et al: A
subpopulation of CD26+ cancer stem cells with metastatic
capacity in human colorectal cancer. Cell Stem Cell. 6:603–615.
2010.
|
|
5
|
Gupta PB, Onder TT, Jiang G, et al:
Identification of selective inhibitors of cancer stem cells by
high-throughput screening. Cell. 138:645–659. 2009.
|
|
6
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008.
|
|
7
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008.
|
|
8
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009.
|
|
9
|
Fang X, Cai Y, Liu J, et al: Twist2
contributes to breast cancer progression by promoting an
epithelial-mesenchymal transition and cancer stem-like cell
self-renewal. Oncogene. 30:4707–4720. 2011.
|
|
10
|
Prall F: Tumour budding in colorectal
carcinoma. Histopathology. 50:151–162. 2007.
|
|
11
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454.
2002.
|
|
12
|
Natalwala A, Spychal R and Tselepis C:
Epithelial-mesenchymal transition mediated tumourigenesis in the
gastrointestinal tract. World J Gastroenterol. 14:3792–3797.
2008.
|
|
13
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.
|
|
14
|
Du L, Wang H, He L, et al: CD44 is of
functional importance for colorectal cancer stem cells. Clin Cancer
Res. 14:6751–6760. 2008.
|
|
15
|
Hwang WL, Yang MH, Tsai ML, et al: SNAIL
regulates interleukin-8 expression, stem cell-like activity, and
tumorigenicity of human colorectal carcinoma cells.
Gastroenterology. 141:279–291. e271–275. 2011.
|
|
16
|
Du L, Wang H and He L: Correction: Article
on CD44 as a marker of colorectal cancer stem cells. Clin Cancer
Res. 14:7964–7967. 2008.
|
|
17
|
Zhang S, Balch C, Chan MW, et al:
Identification and characterization of ovarian cancer-initiating
cells from primary human tumors. Cancer Res. 68:4311–4320.
2008.
|
|
18
|
Marotta LL and Polyak K: Cancer stem
cells: a model in the making. Curr Opin Genet Dev. 19:44–50.
2009.
|
|
19
|
Baumann M, Krause M and Hill R: Exploring
the role of cancer stem cells in radioresistance. Nat Rev Cancer.
8:545–554. 2008.
|
|
20
|
Jiang X, Gwye Y, Russell D, et al: CD133
expression in chemo-resistant Ewing sarcoma cells. BMC Cancer.
10:1162010.
|
|
21
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
|
|
22
|
Kong D, Li Y, Wang Z and Sarkar FH: Cancer
stem cells and epithelial-to-mesenchymal transition
(EMT)-phenotypic cells: are they cousins or twins? Cancers (Basel).
3:716–729. 2011.
|
|
23
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009.
|
|
24
|
Vermeulen L, Todaro M, de Sousa Mello F,
et al: Single-cell cloning of colon cancer stem cells reveals a
multi-lineage differentiation capacity. Proc Natl Acad Sci USA.
105:13427–13432. 2008.
|
|
25
|
Choi D, Lee HW, Hur KY, et al: Cancer stem
cell markers CD133 and CD24 correlate with invasiveness and
differentiation in colorectal adenocarcinoma. World J
Gastroenterol. 15:2258–2264. 2009.
|
|
26
|
Cufi S, Vazquez-Martin A,
Oliveras-Ferraros C, Martin-Castillo B, Joven J and Menendez JA:
Metformin against TGFbeta-induced epithelial-to-mesenchymal
transition (EMT): from cancer stem cells to aging-associated
fibrosis. Cell Cycle. 9:4461–4468. 2010.
|