Introduction
Cells continuously produce free radicals and
reactive oxygen species (ROS) as part of their metabolic processes.
These free radicals are neutralized by an elaborate antioxidant
defense system consisting of enzymes, including catalase (CAT),
superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), and
numerous non-enzymatic antioxidants, including vitamin E, vitamin
A, vitamin C, glutathione and uric acid (1,2).
Intense exercise increases oxygen consumption and may produce an
imbalance between ROS and antioxidant levels, inducing oxidative
stress as a result of increased ROS production (3). Indeed, exercise-induced oxidative
stress leads to the destruction of tissue and cell macromolecules,
including lipids, proteins and nucleic acids (4–6). It
has also been suggested that exercise-induced oxidative stress may
be associated with muscle fatigue, muscle damage and a decrease in
physical performance (7–9). Antioxidants are substances that help
to reduce the severity of oxidative stress, either by forming a
less active radical or by quenching the reaction. It has been
demonstrated that exogenous antioxidants may prevent oxidative
damage since they are able to detoxify certain peroxides by
scavenging the ROS produced during exhaustive exercise (10).
Rhodiola rosea (“golden root” or “Arctic
root”) is widely distributed at high altitudes in the Arctic and in
mountainous regions throughout Europe and Asia (11). It has been widely used as a
hemostatic, antitussive, tonic and endermic liniment for the
treatment of burns and contusions in traditional Chinese medicine
(12). Salidroside,
2-(4-hydroxyphenyl)ethyl β-D-glucopyranoside, the main active
compound of Rhodiola rosea, is reported to exert antiviral,
antidiabetic, antifatigue, antiaging, neuroprotective and
hepatoprotective effects (13–16).
In addition, several studies have indicated that salidroside is an
effective antioxidant and free radical scavenger (17–19).
However, the effects of salidroside on the oxidative stress induced
by exhaustive exercise have not yet been elucidated. In this study,
exhaustive exercise was selected as the method of inducing
oxidative stress in rats and the effects of salidroside on the
exhaustive exercise-induced oxidative stress in the rat liver
tissue were investigated.
Materials and methods
Chemicals and reagents
Salidroside (purity 99%) was purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China). All kits including malondialdehyde
(MDA), CAT, SOD, GSH-Px and liver glycogen were purchased from the
Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All
other chemicals and reagents were of analytical grade and were
obtained from the usual commercial sources. Deionized water was
used to prepare all solutions and in all experiments.
Animals and grouping
Male Sprague-Dawley (SD) rats (220–250 g) were
purchased from the Animal Experiment Center of Tianjin University
(Tianjin, China). The animals were housed at a temperature of
18–20°C and a humidity of 65–69% and were submitted to a 12-h
light/dark cycle. The rats had unrestricted access to tap water and
standard rat chow. All procedures involving the use of laboratory
animals were carried out in accordance with the National Institutes
of Health guidelines. Following an adaptation period of a week, 40
animals were randomly divided into four groups of ten rats each:
control (C), low-dose salidroside-treated (LT), middle-dose
salidroside-treated (MT) and high-dose salidroside-treated (HT)
groups. The rats in the treated groups received salidroside (25, 50
and 100 mg/kg, respectively) intragastrically (ig) and the rats in
the control group received drinking water ig for 4 weeks.
Exhaustive swimming exercise
After 4 weeks, the rats performed an exhaustive
swimming exercise in an acrylic plastic pool (90×45×45 cm) filled
with water maintained at a temperature of 36±2°C. The water depth,
35 cm, was set so that the rats could not rest by supporting their
tails on the bottom of the pool. Each rat had a weight attached (5%
body weight) to its tail for the duration of the swim-to-exhaustion
exercise. The animals were assessed as being exhausted when they
failed to rise to the surface of the water to breathe within 7 sec
(20,21). The exhaustive swimming time was
recorded in min for each rat.
Biochemical parameters analysis
At the end of exhaustive swimming exercise, all rats
were immediately anesthetized with ethyl ether. Liver tissue was
extracted and frozen in liquid nitrogen for storage at -80°C until
required for MDA, CAT, SOD, GSH-Px and glycogen analysis. All
biochemical parameters were tested following the recommended
procedures provided in the assay kits.
Statistical analysis
The data are expressed as the mean ± SD based on the
indicated number in the experiment. All analyses of data were
carried out using the Statistical Package for Social Sciences
(version 11.0; SPSS, Inc., Chicago, IL, USA). The results were
analyzed using one-way analysis of variance followed by a Student’s
t-test for comparison between various treatment groups. P<0.05
was considered to indicate a statistically significant result.
Results and Discussion
Effect of salidroside on the exhaustive
swimming time of rats
Swimming was selected as a model for exercise
performance, since swimming appears to be natural behavior for
rodents and humans (22,23). As shown in Fig. 1, the exhaustive swimming times of
the rats in the LT, MT and HT groups were significantly prolonged
compared with those in the control group (P<0.05), and were
1.52, 1.80 and 1.96 times longer than in the control group,
respectively. These results suggest that salidroside is able to
elevate the exercise tolerance of rats.
Effect of salidroside on the liver
glycogen levels of rats
The contribution of glycogen to energy production
during exhaustive exercise is necessary since glycogen may be
degraded rapidly to produce ATP aerobically and anaerobically
(24,25). The energy for exercise is derived
initially from the breakdown of glycogen and later from circulating
glycogen released by the liver and from non-esterified fatty acids.
Therefore, increased liver glycogen storage levels are conducive to
enhancements of endurance and locomotory capacity (26). As shown in Fig. 2, the liver glycogen levels of the
rats in the LT, MT and HT groups were significantly increased
compared with those in the control group (P<0.05). These results
suggest that salidroside is able to significantly increase the
liver glycogen levels of the rats following exhaustive exercise by
improving the glycogen reserve, reducing the glycogen consumption
during exercise or both.
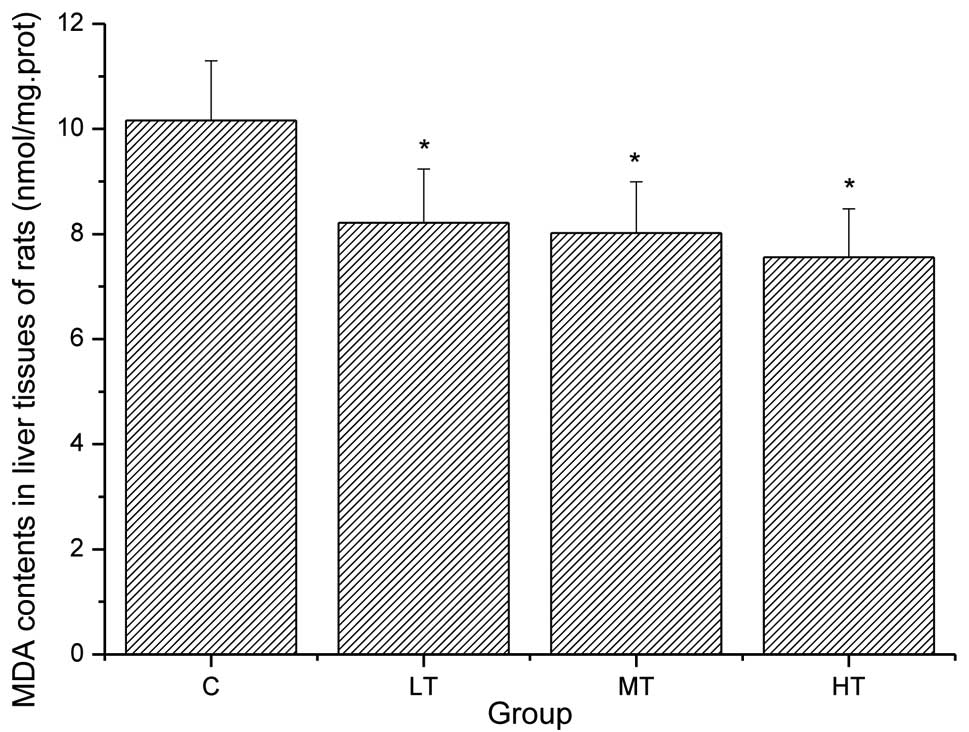
Effect of salidroside on the MDA levels
in the liver tissues of rats
Oxidative stress induced by exhaustion exercise may
produce ROS. The production of ROS is harmful to the mitochondria
in the cell as the ROS affect the functioning of the mitochondria,
causing them to lose their efficacy as components of the electron
transport chain, which may also lead to aging or fatigue. This type
of oxidization injury to the mitochondria results in lipid
peroxidation and causes cell damage (27). MDA, a metabolite of phospholipid
peroxidation, is a popular marker of living body oxidative damage
(28). As shown in Fig. 3, the MDA levels in the liver
tissues of the rats in the LT, MT and HT groups were significantly
decreased compared with those in the C group (P<0.05). These
results suggest that salidroside reduces lipid peroxidation.
Effect of salidroside on the CAT, SOD and
GSH-Px levels in the liver tissues of rats
CAT, SOD and GSH-Px are regarded as the first line
of defense of the antioxidant enzyme system against ROS generated
during exhaustive exercise (29).
SOD dismutates superoxide radicals to form
H2O2 and O2. GPH-Px is an enzyme
responsible for reducing H2O2 or organic
hydroperoxides to water and alcohol, respectively. CAT catalyzes
the breakdown of H2O2 to form water and
O2(30,31). As shown in Table I, the CAT, SOD and GSH-Px levels in
the liver tissues of the rats in the LT, MT and HT groups were
significantly increased compared with those in the C group
(P<0.05). These results suggest that salidroside promotes
increases in the activities of antioxidant enzymes (CAT, SOD and
GSH-Px), which indicates that salidroside has beneficial effects on
the attenuation of the oxidative stress induced by exhaustive
exercise.
 | Table IEffect of salidroside on the CAT, SOD
and GSH-Px levels in rat liver tissue. |
Table I
Effect of salidroside on the CAT, SOD
and GSH-Px levels in rat liver tissue.
| Group | CAT (U/mg·prot) | SOD (U/mg·prot) | GSH-Px
(U/mg·prot) |
|---|
| C | 33.49±5.15 | 102.38±10.01 | 317.68±42.61 |
| LT | 39.87±4.36a | 126.39±11.47a | 389.52±46.27a |
| MT | 42.28±5.23a | 135.41±14.19a | 396.84±54.63a |
| HT | 47.62±5.94a | 137.95±12.41a | 431.19±44.34a |
In conclusion, this study demonstrates that
salidroside is able to elevate the exercise tolerance and increase
the liver glycogen levels of rats following exhaustive exercise.
Salidroside is also able to reduce MDA levels and enhance the
activities of antioxidant enzymes (CAT, SOD and GSH-Px) in the
liver tissues of rats. These findings indicate that salidroside is
effective in preventing oxidative stress following exhaustive
exercise. Therefore, salidroside may be used as an antioxidant
supplement for competing athletes who participate in exhaustive
endurance events.
Acknowledgements
The authors gratefully acknowledge Dr Wang Fei for
help in editing the manuscript. The authors are also grateful to Dr
Zhang Yang for his timely technical support.
References
|
1
|
Urso ML and Clarkson PM: Oxidative stress,
exercise, and antioxidant supplementation (Review). Toxicology.
189:41–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Konrad M, Nieman DC, Henson DA, et al: The
acute effect of ingesting a quercetin-based supplement on
exercise-induced inflammation and immune changes in runners. Int J
Sport Nutr Exerc Metab. 21:338–346. 2011.PubMed/NCBI
|
|
3
|
Miranda-Vilela AL, Akimoto AK, Alves PC,
et al: Dietary carotenoid-rich oil supplementation improves
exercise-induced anisocytosis in runners: influences of
haptoglobin, MnSOD (Val9Ala), CAT (21A/T) and GPX1 (Pro198Leu) gene
polymorphisms in dilutional pseudoanemia (sports anemia). Genet Mol
Biol. 33:359–367. 2010. View Article : Google Scholar
|
|
4
|
Neubauer O, Reichhold S, Nersesyan A, et
al: Exercise-induced DNA damage: is there a relationship with
inflammatory responses (Review)? Exerc Immunol Rev. 14:51–72.
2008.PubMed/NCBI
|
|
5
|
Powers SK, Nelson WB and Hudson MB:
Exercise-induced oxidative stress in humans: cause and consequences
(Review). Free Radic Biol Med. 51:942–950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanimura Y, Shimizu K, Tanabe K, et al:
Exercise-induced oxidative DNA damage and lymphocytopenia in
sedentary young males. Med Sci Sports Exerc. 40:1455–1462. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Powers SK and Jackson MJ: Exercise-induced
oxidative stress: cellular mechanisms and impact on muscle force
production (Review). Physiol Rev. 88:1243–1276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
König D, Wagner KH, Elmadfa I and Berg A:
Exercise and oxidative stress: significance of antioxidants with
reference to inflammatory, muscular, and systemic stress (Review).
Exerc Immunol Rev. 7:108–133. 2001.PubMed/NCBI
|
|
9
|
Williams MH: Dietary supplements and
sports performance: introduction and vitamins. J Int Soc Sports
Nutr. 1:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sen CK: Antioxidants in exercise nutrition
(Review). Sports Med. 31:891–908. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kelly GS: Rhodiola rosea: a
possible plant adaptogen (Review). Altern Med Rev. 6:293–302.
2001.
|
|
12
|
Li HB and Chen F: Preparative isolation
and purification of salidroside from the Chinese medicinal plant
Rhodiola sachalinensis by high-speed counter-current
chromatography. J Chromatogr A. 932:91–95. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li F, Tang H, Xiao F, et al: Protective
effect of salidroside from Rhodiolae Radix on
diabetes-induced oxidative stress in mice. Molecules. 16:9912–9924.
2011.
|
|
14
|
Mao GX, Deng HB, Yuan LG, et al:
Protective role of salidroside against aging in a mouse model
induced by D-galactose. Biomed Environ Sci. 23:161–166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu YL, Lian LH, Jiang YZ and Nan JX:
Hepatoprotective effects of salidroside on fulminant hepatic
failure induced by D-galactosamine and lipopolysaccharide in mice.
J Pharm Pharmacol. 61:1375–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Ding Y, Zhou J, et al: The in
vitro and in vivo antiviral effects of salidroside from Rhodiola
rosea L. against coxsackievirus B3. Phytomedicine. 16:146–155.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu P, Hu C, Meehan EJ and Chen L: X-ray
crystal structure and antioxidant activity of salidroside, a
phenylethanoid glycoside. Chem Biodivers. 4:508–513. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y and Liu Y: Study on effects of
salidroside on lipid peroxidation on oxidative stress in rat
hepatic stellate cells. Zhong Yao Cai. 28:794–796. 2005.(In
Chinese).
|
|
19
|
De Sanctis R, De Bellis R, Scesa C, et al:
In vitro protective effect of Rhodiola rosea extract against
hypochlorous acid-induced oxidative damage in human erythrocytes.
Biofactors. 20:147–159. 2004.
|
|
20
|
Oh TW, Oh TW and Ohta F: Dose-dependent
effect of capsaicin on endurance capacity in rats. Br J Nutr.
90:515–520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Misra DS, Maiti R and Ghosh D: Protection
of swimming-induced oxidative stress in some vital organs by the
treatment of composite extract of Withania somnifera,
Ocimum sanctum and Zingiber officinalis in male rat.
Afr J Tradit Complement Altern Med. 6:534–543. 2009.PubMed/NCBI
|
|
22
|
Burneiko RC, Diniz YS, Galhardi CM, et al:
Interaction of hypercaloric diet and physical exercise on lipid
profile, oxidative stress and antioxidant defenses. Food Chem
Toxicol. 44:1167–1172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kramer K, Dijkstra H and Bast A: Control
of physical exercise of rats in a swimming basin. Physiol Behav.
53:271–276. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fallowfield JL and Williams C:
Carbohydrate intake and recovery from prolonged exercise. Int J
Sport Nutr. 3:150–164. 1993.PubMed/NCBI
|
|
25
|
Nicholas CW, Green PA, Hawkins RD and
Williams C: Carbohydrate intake and recovery of intermittent
running capacity. Int J Sport Nutr. 7:251–260. 1997.PubMed/NCBI
|
|
26
|
Tang W, Zhang Y, Gao J, et al: The
anti-fatigue effect of 20(R)-ginsenoside Rg3 in mice by
intranasally administration. Biol Pharm Bull. 31:2024–2027. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang JJ, Shieh MJ, Kuo SL, et al: Effect
of red mold rice on antifatigue and exercise-related changes in
lipid peroxidation in endurance exercise. Appl Microbiol
Biotechnol. 70:247–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu HK, Hsieh CC, Hsu JJ, et al: Preventive
effects of Spirulina platensis on skeletal muscle damage
under exercise-induced oxidative stress. Eur J Appl Physiol.
98:220–226. 2006.
|
|
29
|
Huang CC, Lin TJ, Lu YF, et al: Protective
effects of L-arginine supplementation against exhaustive
exercise-induced oxidative stress in young rat tissues. Chin J
Physiol. 52:306–315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
You LJ, Zhao MM, Regenstein JM and Ren JY:
In vitro antioxidant activity and in vivo
anti-fatigue effect of loach (Misgurnus anguillicaudatus)
peptides prepared by papain digestion. Food Chem. 124:188–194.
2011. View Article : Google Scholar
|
|
31
|
Jin HM and Wei P: Anti-fatigue properties
of tartary buckwheat extracts in mice. Int J Mol Sci. 12:4770–4780.
2011. View Article : Google Scholar : PubMed/NCBI
|