Introduction
There are two main forms of strabismus, concomitant
strabismus and incomitant strabismus. Concomitant strabismus is
characterized by an angle of deviation that remains the same in all
directions of gaze, whichever eye is fixing. Strabismus is one of
the most common problems in pediatric ophthalmology, affecting 3–5%
of the worldwide population (1,2),
2–4% of the white population and 0.6% of Asians and Africans
(3–5).
The pathogenesis of strabismus, particularly
concomitant strabismus, remains poorly understood. It is believed
that neural abnormalities and genetic and environmental factors are
responsible for the occurrence of strabismus (6–8).
Clinical observations have shown that the hypergenesis or
hypoplasia of muscles may cause ocular muscles to become
unbalanced; this may reflect a change in the neural input to an
anatomically normal muscle and lead to strabismus, for example, the
correlation between the overacted inferior oblique muscles and
incomitant strabismus (9–12). Anatomic anomalies exist in 90% of
the strabismus cases that occur before the patient is 6 years old.
Furthermore, our previous study confirmed the abnormal expression
of structural proteins in some of the 324 extraocular muscles of
278 patients with strabismus, including concomitant strabismus
(unpublished data). Therefore, we aimed to acquire more information
concerning the correlation between muscle development-related genes
and concomitant strabismus.
Myogenic differentiation 1 (MYOD1), myogenin (MYOG),
retinoblastoma 1 (RB1), cyclin-dependent kinase (CDK) inhibitor 1A
(P21), CDK inhibitor 1C (P57) and insulin-like growth factor 1
(IGF1) are the key regulatory genes involved in the initiation and
regulation of myogenesis (http://www.ans.iastate.edu/research/reecy/reecyfocus.html).
Muscle creatine kinase (MCK) is a marker of the late stage of
muscle differentiation (13).
MYOD1 and MYOG are two key myogenic factors. MYOD1,
which is only expressed in skeletal muscle and its precursors
(14), regulates muscle cell
differentiation by regulating the cell cycle and is a prerequisite
for myogenic initiation. MYOG encodes a specific transcription
factor for inducing myogenesis, which acts at a late stage of
myogenesis and is essential for the formation of functional
skeletal muscle (15,16).
The cell cycle regulatory factors participate in
myogenesis by interacting with myogenic factors. The RB1 protein,
pRb, is able to strengthen the activity of MYOD and MYOG during
skeletal myogenesis as a transcriptional co-factor of MyoD
(13,17–19).
Furthermore, pRb controls entry into the late stages of the muscle
differentiation (13). p21 is
essential for the cell cycle and differentiation in the adult
myogenic progenitor cell (MPC) population (20). p57 is able to stabilize MYOD by
direct binding (21–23). They are all induced by MYOD and
play important roles in the cell cycle of muscle terminal
differentiation (24,25). IGF1 is able to promote skeletal
muscle differentiation and repair muscle damage (26–28).
In order to illuminate the correlation between
myogenesis and concomitant strabismus, we analyzed these 7 genes in
18 extraocular muscles of 18 patients with concomitant strabismus
and 12 normal control muscles from one patient. The results
demonstrated abnormal expression patterns of these 7 genes in the
majority of the diseased extraocular muscles.
Materials and methods
Samples
The resected extraocular muscles were obtained from
patients with strabismus during resection surgery at the Zhongshan
Ophthalmic Center, Guangzhou, China. A total of 18 extraocular
muscles from concomitant strabismus patients were analyzed in this
study, including 10 medial rectus (MR) muscle tissues from 10
patients with concomitant exotropia (XT) and 8 lateral rectus (LR)
muscle tissues from 8 patients with concomitant esotropia (ET).
Some clinical information of the individuals are showed in Table I. Twelve normal control samples,
including binocular MR, LR, superior rectus (SR), inferior rectus
(IR), superior oblique (SO) and inferior oblique (IO) muscle
samples, were obtained from one presumably healthy male 6 h after
sudden mortality. The samples were immediately frozen in liquid
nitrogen and stored at −80°C. Informed consent adhering to the
tenets of the Declaration of Helsinki was obtained from all
participants or their guardians before the study. This study was
approved by the Institutional Review Board of Zhongshan Ophthalmic
Center.
 | Table IClinical information of the
individuals. |
Table I
Clinical information of the
individuals.
| Patient | Gender | Age (year) | Deviation | Eye | Muscle |
|---|
| 1 | Female | 14 | ET | Right | LR |
| 2 | Male | 10 | ET | Right | LR |
| 3 | Male | 11 | ET | Left | LR |
| 4 | Male | 15 | ET | Right | LR |
| 5 | Male | 9 | ET | Right | LR |
| 6 | Male | 20 | ET | Right | LR |
| 7 | Male | 7 | ET | Right | LR |
| 8 | Female | 18 | ET | Left | LR |
| 9 | Female | 15 | ET | Right | MR |
| 10 | Female | 16 | XT | Left | MR |
| 11 | Female | 36 | XT | Right | MR |
| 12 | Male | 16 | XT | Left | MR |
| 13 | Male | 22 | XT | Left | MR |
| 14 | Male | 10 | XT | Left | MR |
| 15 | Male | 7 | XT | Right | MR |
| 16 | Male | 6 | XT | Right | MR |
| 17 | Female | 8 | XT | Left | MR |
| 18 | Female | 18 | XT | Left | MR |
Real-time quantitative RT-PCR
Total RNA was isolated using a commercial reagent
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA) and 1 μg
RNA was reverse transcribed to cDNA using a commercial reagent kit
(PrimeScript® RT reagent kit; Takara Bio, Inc., Shiga,
Japan). The cDNA (1 μl)was diluted (1:10) for real-time PCR.
Real-time PCR was performed using a reagent kit (SYBR®
Premix Ex Taq™, Takara Bio, Inc.) following the manufacturer’s
instructions for the ABI Prism 7000 sequence detection system
(Applied Biosystems, Foster City, CA, USA). The primers used for
real-time PCR are shown in Table
II. GAPDH were used as an internal control to normalize the
gene expression levels. Relative transcript abundance was
quantified by the 2−ΔΔCT method. Data represent the mean
of triplicate measurements and are reported as the mean fold change
(x-fold) + SD.
 | Table IIPrimers for real-time PCR. |
Table II
Primers for real-time PCR.
| Gene name | Primer sequences
(5′→3′) | Tm (°C) |
|---|
| MYOD1 |
F-CGCTCCAACTGCTCCGACGG | 66 |
|
R-GACACCGCCGCACTCTTCCC | |
| MYOG |
F-GAGCTCACCCTGAGCCCCGA | 66 |
|
R-GCAGGCACTGGCCTGGACAG | |
| RB1 |
F-AGCTGTGGGACAGGGTTGTGTC | 64 |
|
R-CAACCTCAAGAGCGCACGCC | |
| MCK |
F-GCCACGGGGGCTACAAACCC | 66 |
|
R-GTGGGGGCAACGTGTAGCCC | |
| P21 |
F-GCTGTCCCCTGCAGCAGAGC | 65 |
|
R-TGCATCCAGGAGGCCCGTGA | |
| P57 |
F-CTCTTGCGCGGGGTCTGCTC | 63 |
|
R-AACGGCGCGGCGATCAAGAA | |
| IGF1 |
F-GGGCGCCTCAGACAGGCATC | 65 |
|
R-CAGGCTTGAGGGGTGCGCAA | |
| GAPDH |
F-CCCGCTTCGCTCTCTGCTCC | 65 |
|
R-ACCAGGCGCCAATACGACC | |
| β-actin |
F-CGAGCACAGAGCCTCGCCTTTGC | 66 |
|
R-ACATGCCGGAGCCGTTGTCGAC | |
Results
Expression patterns of 6 myogenesis
regulatory factors are similar in the normal extraocular
muscles
In this study, we first examined the expression of 6
myogenesis regulatory factors in normal samples, i.e., all 12
extraocular muscles from the normal eye, including bilateral MR,
LR, SR, IR, SO and IO muscles (Fig
1). We found a similar expression pattern in all the muscles,
including the synergistic, antagonistic and yoke muscles. In the
synergistic muscles, including MR-SR-IR (Fig. 1A, C and D) and LR-SO-IO (Fig. 1B, E and F), MYOD1 levels were
invariably higher and MYOG levels were lower than those of other
genes. For the other factors, the levels of IGF1 were close to
those of β-actin in most samples. The expression levels of the cell
cycle regulatory factors RB1, P21 and P57 were low in the MR, LR
and IO muscles but comparable to those of β-actin in the other 3
types of muscles. The expression pattern was similar in the
antagonistic muscles and the yoke muscles (Fig. 1). Although there was a divergence
in expression levels among the 6 myogenesis-related regulatory
factors, there was a similar expression pattern in all the 12
normal binocular extraocular muscles.
 | Figure 1Expression of 6 myogenesis regulatory
genes in all 12 normal extraocular muscles. The relative level of
all the six genes: MYOD1, myogenic differentiation 1; MYOG,
myogenin; RB1, retinoblastoma 1; MCK, muscle creatine kinase; IGF1,
insulin-like growth factor 1 in all 6 extraocular muscles of same
eye ball were analyzed: (A) MR, medial rectus; (B) LR, lateral
rectus; (C) SR, superior rectus; (D) IR, inferior rectus; (E) SO,
superior oblique; and (F) IO, inferior oblique. The total RNA was
from all 12 extraocular muscles from the right eye (R) and the left
eye (L) of the same control. Using the specific primers, the
expression levels of 6 factors and β-actin were quantified by
real-time quantitative RT-PCR. β-ACT, β-actin. |
Abnormal expression of 7
myogenesis-related genes in 18 extraocular muscles of patients with
concomitant strabismus
Generally, we obtained the resectional MR muscle
from the XT patients and LR muscle from the ET patients by surgery.
Therefore, we detected the expression of the 7 myogenesis-related
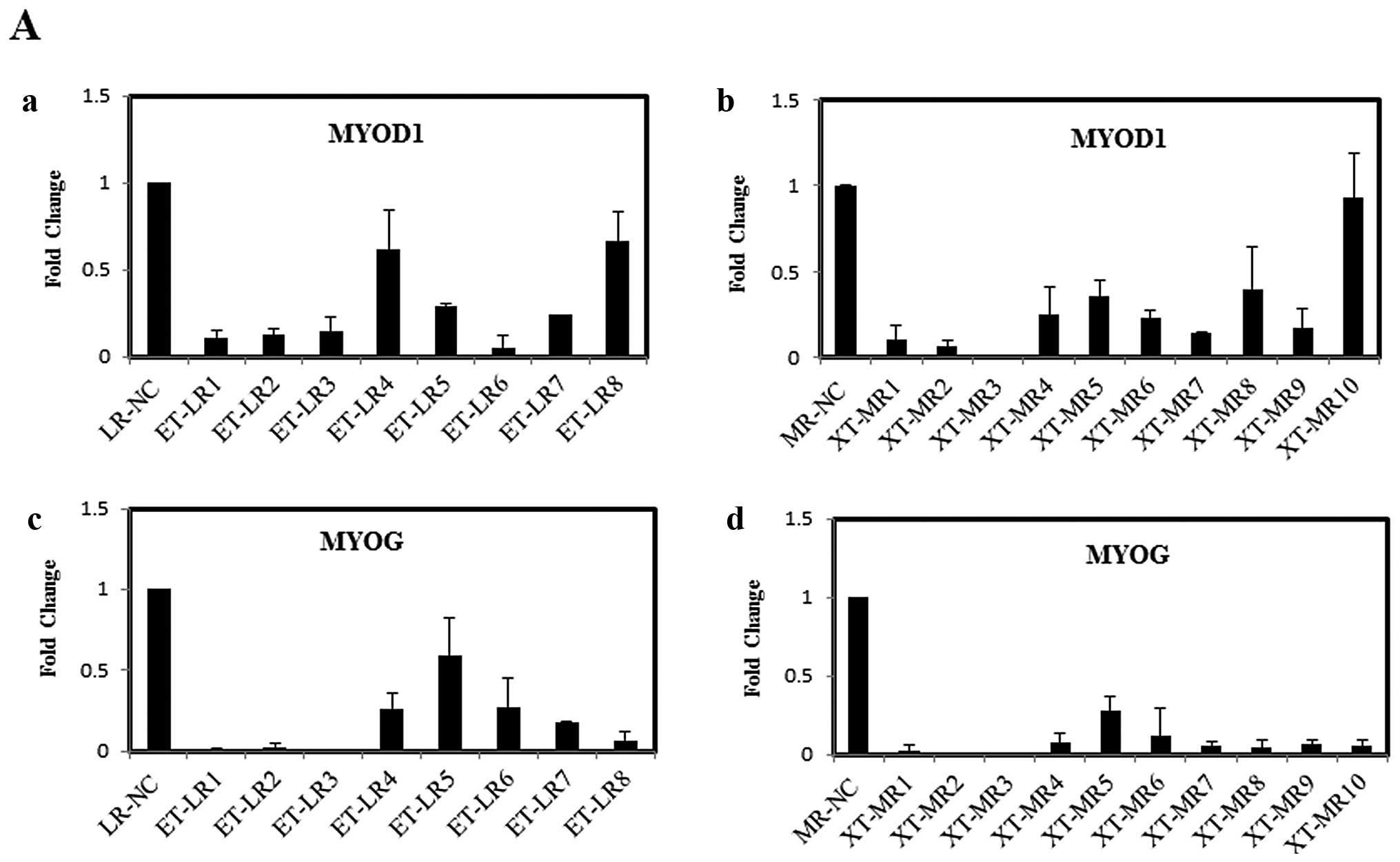
genes in XT-MR and ET-LR muscles in this study. Fig. 2 shows the abnormal expression of
all 7 factors in the diseased muscles.
The expression levels of MYOD1 and MYOG were
markedly reduced in almost all of the samples (Fig. 2A). The expression levels of MYOD1
were reduced significantly in 7 of the 8 LR and 8 of the 10 MR
samples, and those of MYOG decreased in 7 of the 8 LR and all 10 MR
samples. Although the levels of MYOD1 and MYOG were downregulated,
the relative expression pattern between them was similar to that in
normal tissues: the levels of MYOD1 were clearly higher than those
of MYOG.
The expression of the three cell cycle regulators
was different in diseased tissues (Fig. 2B). The expression levels of RB1
were reduced in 5 of the 8 LR and 7 of the 10 MR samples but
increased in the other samples (Fig.
2B-a and b). The expression levels of P21 were decreased in all
18 samples, particularly in the MR tissue, but the expression
levels of P57 were increased in 7 of the 8 LR and 7 of the 10 MR
samples (Fig. 2B-c-f).
The expression levels of the other two factors, IGF1
and MCK, were reduced in most of the samples compared with their
levels in the normal controls (Fig.
2C). The IGF1 levels were reduced in 6 of the 8 LR and most of
the 10 MR tissues. MCK levels were decreased in 6 of the 8 LR and 8
of the 10 MR tissues, but increased in the others.
These results reveal the abnormal expression of
these myogenesis genes in the extraocular muscles of patients with
concomitant strabismus.
Discussion
An obstacle to the understanding of eye muscle
pathology is the lack of a practical way to obtain tissue samples
of the entire muscle from normal individuals and patients with
strabismus (29). In this study,
we examined the mRNA levels of 7 myogenesis-related genes in 18
extraocular muscles from 18 patients with concomitant strabismus
and 12 normal control samples from one presumably healthy
individual. We found that a similar expression pattern of these
genes was presented in all the normal tissues. However, their
expression was abnormal in the extraocular muscles of patients with
concomitant strabismus.
Together with several other genes related to
myogenesis, myogenic factors during muscle regeneration, including
MYOD, myogenic factor (Myf)5, myogenin and Myf4, are essential for
skeletal muscle determination and the differentiation of skeletal
muscle tissue. MYOD knockout mice are deficient in skeletal muscle
regeneration (30–32). In activated skeletal satellite
cells, differentiating signals upregulate MYOD and activate the
transcription of MYOD target genes, result in myogenic
differentiation (33). In the
current study, we found that the level of MYOD1 was always higher
than that of MYOG in normal tissues and also in the diseased
tissues. Therefore, we suspected that this expression pattern is
required for normal muscle maturation. Moreover, this pattern was
also present in all the synergistic and antagonistic muscles, as
well as in the yoke muscles. This may maintain a balance among all
the extraocular muscles and may be necessary for maintaining normal
eye movement.
In the extraocular muscles of the concomitant
strabismus patients, the expression levels of MYOD1 and MYOG were
reduced by 70–80%. Repressed MYOG expression is responsible for
impaired myoblast differentiation (34). The repressed levels of MYOD1 and
MYOG imply that the myogenesis process, including differentiation,
fusion and growth, was impaired in the concomitant strabismus
patients. This is likely to disrupt the formation of functional
muscles and the development of extraocular muscles and, therefore,
lead to the gradual development of concomitant strabismus. However,
downregulation of myogenin may reactivate the cell cycle, probably
through the downregulation of the endogenous expression of MYOD
(35). Therefore, the repression
of MYOD1 and MYOG levels may be a separate self-healing mechanism
of the impaired muscles.
Other regulatory factors functioning in muscle
differentiation, specifically RB1, P21, P57 and IGF1, constitute a
regulatory network with myogenic factors that interact with each
other directly or indirectly. pRb interacts with MYOD and MYOG, and
controls entry into the later stages of the muscle differentiation
process (13,36–38).
The CDK inhibitors, P21 and P57, block the cell cycle by repressing
the activity of CDKs. The regulatory factors, particularly the cell
cycle inhibitors, are theoretically highly expressed in normal
skeletal muscle. However, our data revealed that the levels of RB1,
P21 and IGF1, particularly those of P21, were reduced in the
majority of the diseased tissues with respect to their levels in
the normal controls. The levels of another cell cycle inhibitor,
P57, were increased in most of the diseased samples. Therefore,
decreased expression of RB1 and P21 may be the reason for muscle
overdevelopment and increased expression of P57 may be a
compensation for decreased RB1, P21 and IGF1 levels. As MYOD is
able to induce the expression of p57 in cells lacking p21 (24,39),
repressed P21 and increased P57 levels may be caused by the
reduction of MYOD1 levels. Regardless of what caused the changes in
the 3 genes, the balance of cell cycle regulation was disrupted in
the differentiation and growth of the extraocular muscles in the
patients with concomitant strabismus.
The regulatory network of myogenic factors and other
muscle growth regulators maintains the normal development and
maturation of skeletal muscle, including extraocular muscle. Our
results confirmed the abnormality in the levels of these
myogenesis-related genes, which may contribute to concomitant
strabismus and augment the pathology of the extraocular muscle of
the concomitant strabismus patients. Additional samples and
candidate genes should be studied in the future. Further studies
are required to identify the mechanism involving these genes and
determine the overall correlation between the myogenic process and
the occurrence of strabismus.
Acknowledgements
The authors thank all patients for their
participation. This study was supported by grants from the National
Natural Science Foundation of China (81170891).
Abbreviations:
|
MR
|
medial rectus
|
|
LR
|
lateral rectus
|
|
SR
|
superior rectus
|
|
IR
|
inferior rectus
|
|
SO
|
superior oblique
|
|
IO
|
inferior oblique
|
|
XT
|
concomitant exotropia
|
|
ET
|
concomitant esotropia
|
|
NC
|
normal control
|
References
|
1
|
Ziakas NG, Woodruff G, Smith LK and
Thompson JR: A study of heredity as a risk factor in strabismus.
Eye (London). 16:519–521. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arora A, Williams B, Arora AK, McNamara R,
Yates J and Fielder A: Decreasing strabismus surgery. Br J
Ophthalmol. 89:409–412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu DN: Prevalence and mode of inheritance
of major genetic eye diseases in China. J Med Genet. 24:584–588.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robaei D, Rose KA, Kifley A, Cosstick M,
Ip JM and Mitchell P: Factors associated with childhood strabismus:
findings from a population-based study. Ophthalmology.
113:1146–1153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abrahamsson M, Magnusson G and Sjöstrand
J: Inheritance of strabismus and the gain of using heredity to
determine populations at risk of developing strabismus. Acta
Ophthalmol Scand. 77:653–657. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mustari MJ and Ono S: Neural mechanisms
for smooth pursuit in strabismus. Ann N Y Acad Sci. 1233:187–193.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilmer JB and Backus BT: Genetic and
environmental contributions to strabismus and phoria: evidence from
twins. Vision Res. 49:2485–2493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michaelides M and Moore AT: The genetics
of strabismus. J Med Genet. 41:641–646. 2004. View Article : Google Scholar
|
|
9
|
Kushner BJ: Incomitant strabismus: does
extraocular muscle form denote function? Arch Ophthalmol.
128:1604–1609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang YH, Ma KT, Lee JB and Han SH:
Anterior transposition of inferior oblique muscle for treatment of
unilateral superior oblique muscle palsy with inferior oblique
muscle overaction. Yonsei Med J. 45:609–614. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu X, Mai G, Yu H, Deng D, Lin X, Chen J
and Wu H: Study on ocular torsion of V patterns strabismus. Yan Ke
Xue Bao. 19:160–164. 2003.PubMed/NCBI
|
|
12
|
Mellott ML, Scott WE, Ganser GL and Keech
RV: Marginal myotomy of the minimally overacting inferior oblique
muscle in asymmetric bilateral superior oblique palsies. J AAPOS.
6:216–220. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Novitch BG, Spicer DB, Kim PS, Cheung WL
and Lassar AB: pRb is required for MEF2-dependent gene expression
as well as cell-cycle arrest during skeletal muscle
differentiation. Curr Biol. 9:449–459. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weintraub H, Davis R, Tapscott S, et al:
The myoD gene family: nodal point during specification of the
muscle cell lineage. Science. 251:761–766. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hasty KA, Wu H, Byrne M, et al:
Susceptibility of type I collagen containing mutated alpha 1(1)
chains to cleavage by human neutrophil collagenase. Matrix.
13:181–186. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Apponi LH, Corbett AH and Pavlath GK:
RNA-binding proteins and gene regulation in myogenesis. Trends
Pharmacol Sci. 32:652–658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu W, Schneider JW, Condorelli G, Kaushal
S, Mahdavi V and Nadal-Ginard B: Interaction of myogenic factors
and the retinoblastoma protein mediates muscle cell commitment and
differentiation. Cell. 72:309–324. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Skapek SX, Pan YR and Lee EY: Regulation
of cell lineage specification by the retinoblastoma tumor
suppressor. Oncogene. 25:5268–5276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas DM, Carty SA, Piscopo DM, Lee JS,
Wang WF, Forrester WC and Hinds PW: The retinoblastoma protein acts
as a transcriptional coactivator required for osteogenic
differentiation. Mol Cell. 8:303–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hawke TJ, Meeson AP, Jiang N, Graham S,
Hutcheson K, DiMaio JM and Garry DJ: p21 is essential for normal
myogenic progenitor cell function in regenerating skeletal muscle.
Am J Physiol Cell Physiol. 285:C1019–C1027. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park CW and Chung JH: Age-dependent
changes of p57(Kip2) and p21(Cip1/Waf1) expression in skeletal
muscle and lung of mice. Biochim Biophys Acta. 1520:163–168. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reynaud EG, Leibovitch MP, Tintignac LA,
Pelpel K, Guillier M and Leibovitch SA: Stabilization of MyoD by
direct binding to p57(Kip2). J Biol Chem. 275:18767–18776. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang P, Wong C, Liu D, Finegold M, Harper
JW and Elledge SJ: p21(CIP1) and p57(KIP2) control muscle
differentiation at the myogenin step. Genes Dev. 13:213–224. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo K, Wang J, Andrés V, Smith RC and
Walsh K: MyoD-induced expression of p21 inhibits cyclin-dependent
kinase activity upon myocyte terminal differentiation. Mol Cell
Biol. 15:3823–3829. 1995.PubMed/NCBI
|
|
25
|
Vaccarello G, Figliola R, Cramerotti S,
Novelli F and Maione R: p57Kip2 is induced by MyoD through a
p73-dependent pathway. J Mol Biol. 356:578–588. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kandalla PK, Goldspink G, Butler-Browne G
and Mouly V: Mechano growth factor E peptide (MGF-E), derived from
an isoform of IGF-1, activates human muscle progenitor cells and
induces an increase in their fusion potential at different ages.
Mech Ageing Dev. 132:154–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matheny RW Jr, Nindl BC and Adamo ML:
Minireview: Mechano-growth factor: a putative product of IGF-I gene
expression involved in tissue repair and regeneration.
Endocrinology. 151:865–875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hill M and Goldspink G: Expression and
splicing of the insulin-like growth factor gene in rodent muscle is
associated with muscle satellite (stem) cell activation following
local tissue damage. J Physiol. 549:409–418. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Spencer RF and Porter JD: Biological
organization of the extraocular muscles. Prog Brain Res. 151:43–80.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cornelison DD, Olwin BB, Rudnicki MA and
Wold BJ: MyoD(−/−) satellite cells in single-fiber culture are
differentiation defective and MRF4 deficient. Dev Biol.
224:122–137. 2000.
|
|
31
|
White JD, Scaffidi A, Davies M, McGeachie
J, Rudnicki MA and Grounds MD: Myotube formation is delayed but not
prevented in MyoD-deficient skeletal muscle: studies in
regenerating whole muscle grafts of adult mice. J Histochem
Cytochem. 48:1531–1544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schuierer MM, Mann CJ, Bildsoe H, Huxley C
and Hughes SM: Analyses of the differentiation potential of
satellite cells from myoD−/−, mdx, and PMP22 C22 mice. BMC
Musculoskelet Disord. 6:152005.
|
|
33
|
Mokalled MH, Johnson AN, Creemers EE and
Olson EN: MASTR directs MyoD-dependent satellite cell
differentiation during skeletal muscle regeneration. Genes Dev.
26:190–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin X, Yang X, Li Q, et al: Protein
tyrosine phosphatase-like A regulates myoblast proliferation and
differentiation through MyoG and the cell cycling signaling
pathway. Mol Cell Biol. 32:297–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mastroyiannopoulos NP, Nicolaou P, Anayasa
M, Uney JB and Phylactou LA: Down-regulation of myogenin can
reverse terminal muscle cell differentiation. Plos One.
7:e298962012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burkhart DL and Sage J: Cellular
mechanisms of tumour suppression by the retinoblastoma gene. Nat
Rev Cancer. 8:671–682. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen TT and Wang JY: Establishment of
irreversible growth arrest in myogenic differentiation requires the
RB LXCXE-binding function. Mol Cell Biol. 20:5571–5580. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sellers WR, Novitch BG, Miyake S, et al:
Stable binding to E2F is not required for the retinoblastoma
protein to activate transcription, promote differentiation, and
suppress tumor cell growth. Genes Dev. 12:95–106. 1998. View Article : Google Scholar
|
|
39
|
Figliola R and Maione R: MyoD induces the
expression of p57Kip2 in cells lacking p21Cip1/Waf1: overlapping
and distinct functions of the two cdk inhibitors. J Cell Physiol.
200:468–475. 2004. View Article : Google Scholar : PubMed/NCBI
|