Introduction
Citrullus colocynthis belongs to the
cucurbitaceae family and is a well-recognized plant in
traditional medicine. The plant has been previously utilized in
rural areas as a purgative, antidiabetic, insecticide and
antitumoral agent (1). In a
previous study, the beneficial long-term effects of Citrullus
colocynthis seed extracts on glucose homeostasis and body
weight maintenance were documented in streptozotocin-induced
diabetic rats (1).
The aim of the present study was to explore the
direct in vitro effects of several distinct Citrullus
colocynthis seed extracts on glucose-stimulated insulin release
from rat isolated pancreatic islets.
Materials and methods
Citrullus colocynthis extracts
Six extracts from Citrullus colocynthis seeds
were tested; a crude aqueous, defatted aqueous, ethyl acetate,
H2O-methanol and n-butanol extract and an extract
containing the major component of the ethyl acetate, n-butanol and
H2O-methanol extracts, named fraction A.
Extract preparation
The preparation of the crude aqueous and defatted
extracts was reported in a previous study (1). For the hydromethanolic extract, 50 g
of seeds was ground and degreased in hexane. This material was
heated and stirred 3 times for 3 h in a H2O-methanol
mixture (30/70). Following filtration and centrifugation, the
recovered solution was divided into 2 volumes; one was solidified
to form a hygroscopic red-orange residue (H2O-methanol
extract; 4.5% dry matter). The second volume underwent
liquid-liquid extraction 3 times with ethyl acetate and n-butanol,
for the preparation of the ethyl acetate (orange powder; 1.1% dry
matter) and n-butanol (brown powder; 1.2% dry matter) extracts,
respectively.
Thin layer chromatography
Following separation by thin layer chromatography,
ethyl acetate, n-butanol and H2O-methanol extracts
revealed the presence of a major single spot. Fractionation of
these extracts on a column silica gel, using the elution system
methanol/water (80/20), enabled material collection from these
fractions (observed under UV light at 254 and 336 nm), which,
following solidification, was referred to as fraction A (217
mg).
Analysis of insulin release
For measurement of insulin release, groups of 8
islets prepared by the collagenase method (2) were incubated for 90 min at 37°C in
1.0 ml salt-balanced medium (3)
containing 2.5 mg/ml bovine serum albumin and equilibrated against
a mixture of O2-CO2 (95-5, v-v). The insulin
content of the incubation media was measured by radioimmunoassay
(4). The present study was
approved by the Commission d’Ethique et du Bien-Etre Animal
(Faculty of Medicine, Brussels Free University, Brussels,
Belgium).
Analysis of lactate dehydrogenase
(LDH)
LDH output in the incubation medium and the final
LDH content of the islets were measured by the CytoTox-ONE™
homogeneous membrane integrity assay (Promega Corp., Madison, WI,
USA) and expressed as nmol of lactate converted to pyruvate per min
and per 100 islets.
Statistical analysis
All results are presented as mean ± SEM with the
number of individual observations (n) or degree of freedom (df).
Statistical significance of differences between mean values was
assessed using a Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Control insulin input
When measured within triplicate experiments, the
control insulin output recorded in the absence of any extract was
27.3±1.4, 63.2±2.1 and 233.9±11.7 μU/islet/90 min (n=27–30) at 2.8,
8.3 and 16.7 mM D-glucose, respectively. The overall mean values,
derived from separate experiments conducted at specific hexose
concentrations was 56.5±2.5 (n=140) and 254.1±10.7 (n=54)
μU/islet/90 min at 8.3 and 16.7 mM D-glucose, respectively. At 8.3
(P>0.21) and 16.7 mM D-glucose (P>0.23), no significant
difference was identified between insulin output in the former and
latter experiments.
Effect of aqueous extract on insulin
output
At a concentration of 45 μg/ml, the aqueous extract
was identified to significantly increase insulin output at all
D-glucose concentrations (2.8, 8.3 and 16.7 mM). At 16.7 mM,
insulin output was 279.3±18.2 and 325.4±13.2 μU/islet/90 min in the
absence (n=24) and presence (n=27; P<0.05) of the aqueous
extract, respectively. Relative to control insulin outputs observed
at each D-glucose concentration (100.0±3.2%; n=82), results
recorded at the same concentration of aqueous extract (45 μg/ml)
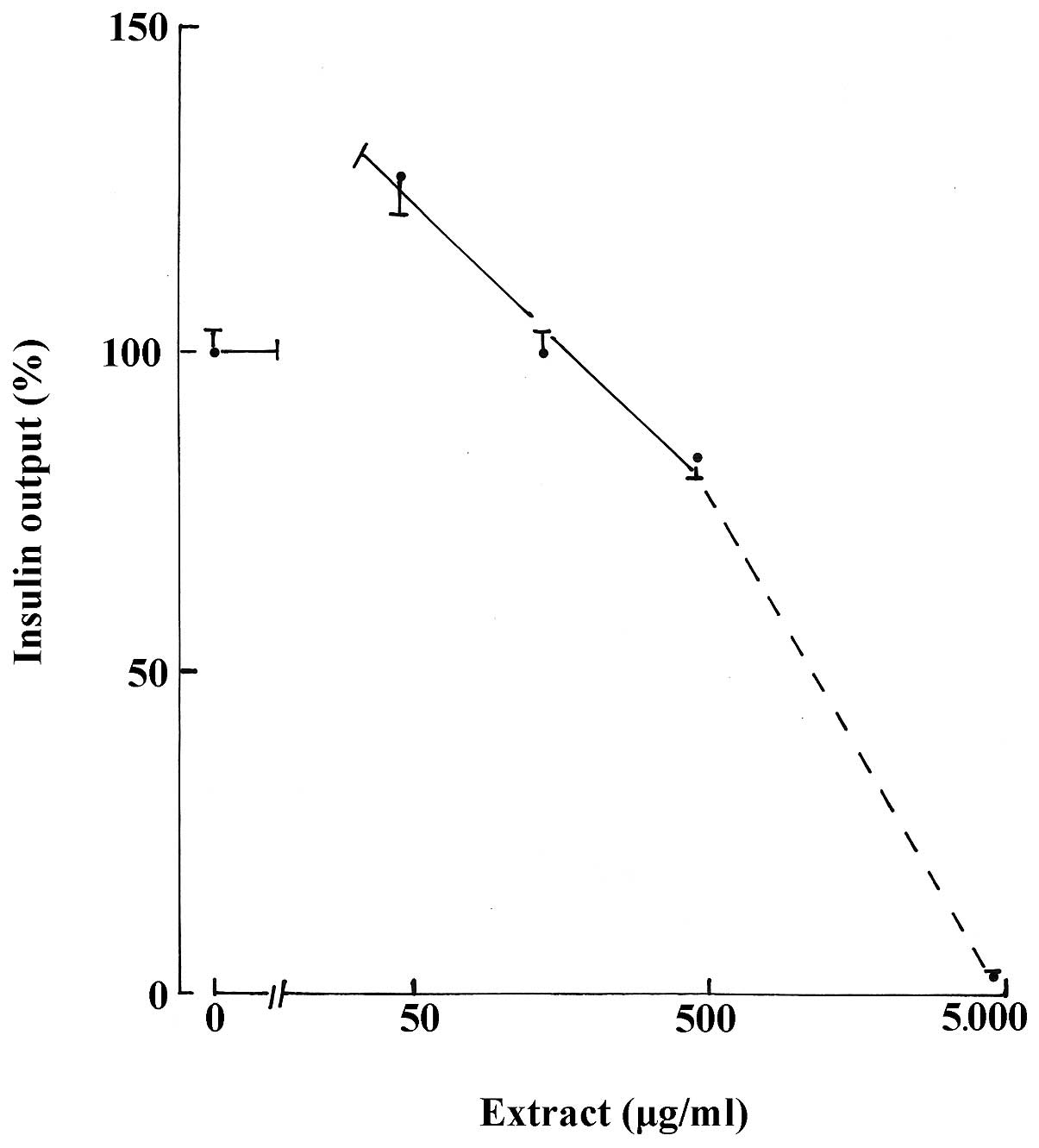
were 126.7±5.2% (n=82; P<0.001). As demonstrated in Table I, a progressive decrease in insulin
output was observed with higher concentrations of the aqueous
extract. In the 45–450 μg/ml range, the decrease yielded, in
semi-logarithmic coordinates, a slope of less pronounced incline
than that identified at higher concentrations of the aqueous
extract (Fig. 1).
 | Table IRelative values for insulin output ±
SEM (%) in the presence of aqueous extract. |
Table I
Relative values for insulin output ±
SEM (%) in the presence of aqueous extract.
| D-glucose (mM) | |
|---|
|
| |
|---|
| Aqueous extract
(μg/ml) | 2.8 | 8.3 | 16.7 | Pooled data |
|---|
| Nil | 100.0±5.6 (28) | 100.0±5.7 (30) | 100.0±5.5(24) | 100.0±3.2(82) |
| 45 | 125.6±6.4
(29)i | 136.5±12.5
(30)g | 117.1±5.6
(27)f | 126.7±5.2
(86)i |
| 135 | 112.3±9.1
(28)c | 85.6±3.4 (30)f | 100.9±7.4
(30)b | 99.3±4.1 (88)a |
| 450 | 81.3±5.1 (30)g | 80.3±2.9 (30)h | 87.2±5.7 (28)e | 82.8±2.7 (88)i |
| 4,500 | 5.7±1.7 (30)i | 2.2±1.2 (30)i | 0.0±0.0 (30)i | 2.6±0.7 (90)i |
Effect of aqueous extract on LDH
content
Relative to the final LDH content of islets
incubated for 90 min in the presence of 8.3 mM D-glucose
(100.0±4.3%; n=4), the release of LDH in the incubation, after 30
and 90 min of incubation in the concomitant presence of KCN (2.0
mM) was >21.8±7.2% (n=3) and >33.1% (n=1), respectively.
Incubations for 30 and 90 min in the absence of KCN revealed values
corresponding to LDH release of 0.39±0.16% (n=4) and 0.82±0.23%
(n=4). Release of LDH, following incubation in the presence of
increasing concentrations of aqueous extract for 30 and 90 min, did
not exceed 0.42±0.06 and 0.72±0.08, 0.86±0.11 and 0.81±0.06, and
0.66±0.18 and 0.81±0.11% at 50, 150 and 450 μg/ml, respectively
(n=4 in all cases). Therefore, variations in insulin output evoked
by the aqueous extract (45–450 μg/ml) in islets exposed to 8.3 mM
D-glucose did not appear to correlate with a specific cytotoxic
action.
Effect of defatted aqueous extract on
insulin output
The defatted aqueous extract, when tested at 8.3 mM
D-glucose, yielded results comparable with those recorded with the
untreated aqueous extract. At 50 μg/ml, the defatted aqueous
extract increased insulin release to 126.0±12.2% (n=20) compared
with the corresponding control value (100.0±6.5%; n=20), the former
percentage was not identified as significantly different to the
untreated aqueous extract (136.5±12.5%; n=30; P>0.55). However,
at higher concentrations (150–450 μg/ml) of the defatted aqueous
extract, islet insulin output expressed relative to control data
was identified as significantly higher (102.5±4.7%; n=40;
P<0.001) than that found with the untreated aqueous extract
(83.0±2.3%; n=60).
Effect of ethyl acetate extract on
insulin output
At 25 μg/ml, the ethyl acetate extract was
identified to significantly increase insulin output (P<0.02) in
the presence of 8.3 or 16.7 mM D-glucose (Table II). At these concentrations of
D-glucose, relative magnitudes of increments were calculated as
14.1±4.7 (df=57) and 24.2±7.0% (df=58), respectively. These
increments were not identified to differ significantly (P≥0.13)
from those recorded at identical concentrations of D-glucose in the
presence of aqueous extract tested at a 45-μg/ml concentration,
i.e., 36.5±13.7 (df=58) and 17.1±7.9% (df=49) at 8.3 and 16.7 mM
D-glucose, respectively.
 | Table IIAbsolute values for insulin output
(μU/islet/90 min) in the presence of ethyl acetate extract. |
Table II
Absolute values for insulin output
(μU/islet/90 min) in the presence of ethyl acetate extract.
| D-glucose (mM), mean
± SEM (%) |
|---|
|
|
|---|
| Ethyl acetate extract
(μg/ml) | 2.8 | 8.3 | 16.7 |
|---|
| Nil | 27.3±1.4 (27) | 63.2±2.1 (30) | 233.9±11.7 (30) |
| 25 | 25.4±1.5 (30)a | 72.1±2.2 (29)b | 286.8±13.3
(30)c |
Effect of H2O-methanol extract
on insulin output
In the presence of 8.3 mM D-glucose, the
H2O-methanol extract, tested at concentrations of 56,
167 and 500 μg/ml, caused a progressive increase in insulin output
(Fig. 2). Observed increases were
only identified to be significant at H2O-methanol
extract concentrations of 167 and 500 μg/ml (P≤0.04).
Effect of n-butanol extract on insulin
output
The n-butanol extract was tested in the presence of
8.3 mM D-glucose at increasing concentrations over a 9-fold range
(40, 116 and 350 μg/ml). At the lowest concentration of n-butanol
extract (40 μg/ml), insulin release was decreased (P<0.05) to
79.6±7.2% (n=20) of the corresponding mean values in the absence of
the extract (100.0±6.5%; n=20). When the concentration of n-butanol
was increased to 116 and 350 μg/ml, a modest progressive increase
in insulin output was observed. However, the insulin output
measured at 350 μg/ml n-butanol extract (97.0±11.2%; n=20) was not
identified as significantly different (P>0.8) from the mean
corresponding control value (Fig.
2).
Effect of fraction A extract on insulin
output
Fraction A extract (0.5 mg/ml) treatment resulted in
an increased insulin output from islets incubated in the presence
of 8.3 mM D-glucose to 171.2±12.3% (n=20; P<0.001) compared with
control (100.0±8.2%; n=20). The relative magnitude of the latter
increase (71.2±14.8%; df=38) was not identified as significantly
different (P>0.41) from that recorded at the same concentration
(0.5 mg/ml) of the H2O-methanol extract at 8.3 mM
D-glucose (55.4±12.4%; df=38). However, insulin output in the
presence of fraction A did exceed (P<0.04) the relative
magnitude of the increase in insulin output caused by the aqueous
extracts (32.3±9.8%; df=98) tested at a lower concentration (0.05
mg/ml) in the presence of 8.3 mM D-glucose. A 4-fold increase in
the concentration of fraction A extract up to 2.0 mg/ml decreased
insulin output to 70.7±9.4% (n=20; P<0.025) of control
(100.0±8.2%; n=20). The output of insulin in the presence of 2.0
mg/ml fraction A extract was 40.5±4.4% (n=20; P<0.001) of the
mean corresponding value identified within the same experiment(s)
in the presence of 0.5 mg/ml fraction A extract (100.0±5.1%; n=20).
This decrease was more pronounced (P<0.01) than that recorded at
the same concentration of D-glucose (8.3 mM), when the
concentration of aqueous extract underwent a 9- to 10-fold increase
from 45–50 to 450 μg/ml. In this case the output of insulin
averaged at the highest of these concentrations 69.5±2.9% (n=50;
P<0.001) of that recorded within the same experiment(s) at the
lower concentration of the aqueous extracts (100.0±5.2%; n=50).
Therefore, despite a 2.25- to 2.50-fold higher increase in extract
concentration, the relative magnitude of the decrease in insulin
output was 2-fold lower (P<0.01) with aqueous (30.5±6.0%; df=98)
than with the fraction A extract (59.5±6.7%; df=38).
Discussion
The present study demonstrates that under selected
experimental conditions, the majority of extracts examined in this
study (aqueous, ethyl acetate, H2O-methanol and fraction
A) exhibit positive insulinotropic actions. In the case of the
aqueous extract, a positive insulinotropic action was observed with
2.8, 8.3 and 16.7 mM D-glucose. However, in the presence of ethyl
acetate extract a significant increase in insulin output was only
observed at 8.3 and 16.7 mM D-glucose. H2O-methanol,
n-butanol and fraction A extracts were only tested at 8.3 mM
hexose.
When the concentration of the extract was
progressively increased, positive insulinotropic action was often
reduced and glucose-stimulated insulin secretion was inhibited.
Specifically, this was observed in the presence of the aqueous
extract at 2.8, 8.3 and 16.7 mM and fraction A at 8.3 mM
D-glucose.
An explanation for this dual effect may involve
participation of distinct chemical compounds in the modulation of
the insulinotropic action of D-glucose and may differ between
distinct extracts. For example, inhibition of glucose-stimulated
insulin output at a low concentration (40 μg/ml) was only
identified in the n-butanol extract and inhibition was reduced at
higher concentrations of the extract (116 and 350 μg/ml).
The following observations are consistent with this
hypothesis. Firstly, the relative magnitude of the highest
increment in insulin output differed with distinct extracts. At 8.3
mM D-glucose, the relative magnitude was 71.2±14.8, 55.4±12.4,
31.2±9.8 and 24.1±7.6% in the presence of fraction A,
H2O-methanol, aqueous and ethyl acetate, respectively.
At the highest concentration of the n-butanol extract, the output
of insulin recorded at 8.3 mM D-glucose was even 3.0±12.9% lower
than the corresponding control value. This may be due to data
referring to distinct concentrations of each extract. Secondly and
more convincingly, at almost identical concentrations (0.45–0.50
mg/ml) of various extracts and D-glucose (8.3 mM), the relative
magnitude of the changes in insulin output ranged from positive
values of 71.2±14.8 and 55.4±12.4% and a negative value of
12.1±6.1% with fraction A, H2O-methanol and aqueous
extracts, respectively. These variations in secretory response to
distinct extracts tested over a comparable concentration range is
further demonstrated in Fig. 2. In
addition, the relative magnitude of the decrease in insulin output
in response to increased extract concentration was variable, as
illustrated by comparison between the aqueous or fraction A
extracts.
The present results must be compared with those
previously reported by Nmila et al(5). The group analyzed a basic fraction
obtained by ion exchange chromatography of an
H2O-isopropanol extract, which was prepared following
hexane delipidation of a crude Citrullus colocynthis seed
powdered extract. Increased pancreatic flow and insulin release was
observed at 0.1 mg/ml basic fraction in rat isolated pancreas
perfused in the presence of 8.3 mM D-glucose. The authors
hypothesized that this effect is associated with insulinotropic
amino acids in the extract, including leucine, isoleucine and
phenylalanine. However, β-(pyrazol-1-yl-)-L-alanine, a major amino
acid present in Citrullus colocynthis seeds and specific
members of the cucurbitaceae family, is not involved in the
stimulation of insulin secretion (5). Phytochemical testing of extracts of
the present study revealed that the hydromethanolic and ethyl
acetate extracts exhibit higher levels of polyphenols and
flavonoids than aqueous and n-butanol extracts, particularly
quercetin and myrcetin (unpublished data). In previous studies,
exposure of rat isolated islets to specific flavonoids, including
(-)epicathechin or quercetin, resulted in a 44–70% increase in
insulin release (6). It was argued
that such flavonoids may act on islet function, at least in part,
via alteration of Ca2+ fluxes and cyclic nucleotide
metabolism (6).
In conclusion, the present study provides a
preliminary insight into the chemical compounds responsible for the
positive and negative insulinotropic actions of Citrullus
colocynthis seed extracts and may prove useful for the
identification of molecules suitable for the prevention and/or
correction of diabetes mellitus in human subjects.
Acknowledgements
The authors thank C. Demesmaeker for secretarial
help. The present study was supported by a grant from the Belgian
Foundation for Scientific Medical Research (no. 3.45020.07 to
A.S.).
References
|
1
|
Benariba N, Djaziri R, Zerriouh BH,
Boucherit K, Louchami K, Sener A and Malaisse WJ: Antihyperglycemic
effect of Citrullus colocynthis seed aqueous extracts in
streptocotocin-induced diabetic rats. Metab Funct Res Diab.
2:71–76. 2009.
|
|
2
|
Malaisse-Lagae F and Malaisse WJ: Insulin
release by pancreatic islets. Methods in Diabetes Research. Larner
J and Pohl S: I(part B)John Wiley & Sons; New York, NY: pp.
147–152. 1984
|
|
3
|
Malaisse WJ, Maggetto C, Leclercq-Meyer V
and Sener A: Interference of glycogenolysis with glycolysis in
pancreatic islets from glucose-infused rats. J Clin Invest.
9:432–436. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leclercq-Meyer V, Marchand J,
Woussen-Colle MC, Giroix MH and Malaisse WJ: Multiple effects of
leucine on glucagon, insulin and somatostatin secretion from the
perfused rat pancreas. Endocrinology. 116:1168–1174. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nmila R, Rachid H, Gross R, Manteghetti M,
Ribes G, Petit P, Tijane M and Sauvaire Y: Mise en evidence d’un
effet insulino-stimulant de fractions de graines de coloquinte
(Citrullus colocynthis L. Schrader). Biologie & Santé.
2:88–99. 2002.(In French).
|
|
6
|
Hii CS and Howell SL: Effects of
flavonoids on insulin secretion and 45Ca+2
handling in rat islets of Langerhans. J Endocrinol. 107:1–8. 1985.
View Article : Google Scholar : PubMed/NCBI
|