Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common tumor worldwide but, due to its poor prognosis, it ranks as
the third most common cause of mortality from cancer (1). Surgical therapy, chemotherapy and
radiation have been used for the treatment of HCC. However, HCC
remains one of the more difficult cancers to treat. Although
chemotherapy is a common therapeutic strategy following surgery, it
is toxic to normal tissues and this limits its use (2). Therefore, it is important to develop
safer and more effective drugs for the treatment of HCC.
Danshen has been used to treat various diseases,
including heart disease, hepatitis and cancer, in China for a long
time (3). Tanshinone (Tan) is the
major lipid-soluble pharmacological constituent of danshen
(4). The diterpenoid Tan, which
includes Tan I, Tan IIA, Tan IIB, dihydrotanshinone I and
cryptotanshinone, has also roused extensive attention. Tanshinones
have a variety of biological activities. For example, Tan I is able
to enhance learning and memory, while Tan IIA has antioxidative,
anti-inflammatory, antiproliferative and antitumor properties
(5,6). Previous studies have shown that Tan
IIA possesses cytotoxic activity against multiple human cancer
cells, inducing apoptosis and differentiation in certain human
cancer cells, including HeLa and colo205 cells (7,8).
However, the applications of Tan have been limited due to its poor
solubility, instability and low bioavailability. The poor water
solubility is likely to induce opsonization and cause rapid
clearance from the blood circulation following intravenous
injection. Therefore, there is a need for a better drug delivery
system (DDS) for Tan.
Numerous methods have been used to improve the
absorption and bioavailability of poorly water-soluble drugs. One
of the more attractive types of system is a microemulsion (ME)
which is composed of fine oil-in-water droplets in an aqueous
medium (9). As a DDS approach, MEs
are clear, stable, isotropic mixtures of oil, water and surfactant,
frequently in combination with a cosurfactant (10). MEs offer an interesting and
potentially quite powerful alternative carrier system for drug
delivery due to their high solubilization capacity, transparency,
thermodynamic stability, ease of preparation and high diffusion and
absorption rates when compared with solvents without the surfactant
system (11).
In the present study, Tan was encapsulated into an
ME to provide a Tan ME formulation. The apoptosis- and
differentiation-inducing effects of Tan ME were investigated in H22
cells in vitro and in vivo.
Materials and methods
Chemicals and reagents
Tan was purchased from Chiatai Qingchunbao
Pharmaceutical Co., Ltd. (Hangzhou, China). 5-Fluorouracil (5-Fu)
was purchased from Tianjin Jinyao Amino Acid Co., Ltd. (Tianjin,
China). RPMI-1640, FBS and penicillin-streptomycin were purchased
from Hyclone Corporation (Logan, UT, USA). Rabbit anti-mouse Bax
polyclonal and mouse anti-human Bcl-2 monoclonal antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). FITC-conjugated goat anti-mouse IgG and TRITC-conjugated goat
anti-mouse IgG were purchased from KPL Inc. (Gaithersburg, MD,
USA). TRIzol reagent was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). The Takara RNA PCR kit (AMV)
Ver3.0 was purchased from Takara Biotechnology Co., Ltd. (Dalian,
China).
Animals and cell lines
H22 murine hepatoma cells were purchased from the
Department of Pathology, Dalian Medical University. Male and female
Balb/c mice, weighing 20.0±2.0 g, were obtained from the Animal
Facility of Dalian Medical University. The animals were maintained
in a room with a controlled environment (12-h light/dark cycle,
24±2°C). They were also given free access to standard laboratory
diet and water. All experiments were approved by the Animal
Research Ethics Committee of Dalian Medical University.
Preparation of Tan ME
According to our previous study (12), 160 mg Tan was dissolved in 38 g
ethyl oleate using ultrasound, to form the oil phase of the ME. A
mixture of 5.4 g phospholipid, 6 g glycerol, 10.6 g pluronic F68
and 140 ml sterile water formed the aqueous phase of the ME. The
oil and aqueous phases were heated to 65°C. The oil phase was added
to the aqueous phase with magnetic stirring (Diamond, Jin Tian,
China). The obtained emulsion was sheared using a high shearing
emulsifier (FLUKO, Essen, Germany) for 5 min; an amount of water
was then added to compensate for that lost by evaporation. The ME
was emulsified further using a high pressure homogenizer (Mizuho,
Osaka, Japan) under 85 MPa for 10 min and filtered through a
0.45-μm filter membrane. The ME was transferred to a 1-ml ampoule,
sealed with N2 and flow steam sterilized for 20 min.
Cell culture and chemical treatment
H22 cells were routinely cultured in RPMI-1640
medium with 10% (v/v) FBS, penicillin (100 U/ml) and streptomycin
(100 U/ml) at 37°C in a humidified 5% CO2 incubator.
Exponentially growing cells were exposed to 0.2 or 0.8 μg/ml
concentrations of Tan ME for 48 h. The control groups were cells
grown in medium containing an equivalent amount of empty ME or Tan
solution.
Measurement of Bcl-2 expression by flow
cytometry (FCM)
FCM was performed as reported previously (13). At least 5,000 events were collected
for each sample and washed with phosphate-buffered saline (PBS).
After centrifugation, the cell pellet was resuspended in the
fixation/permeabilization solution at 4°C for 15 min. The cells
were then incubated with the mouse anti-human Bcl-2 monoclonal
antibody at 4°C for 20 min in the dark. After washing twice in PBS,
the cells were incubated with the FITC-conjugated goat anti-mouse
IgG at 4°C for 20 min in the dark. After washing twice in PBS, the
cells were analyzed using a FACScan flow cytometer and Cell Quest
software (BD Biosciences, San Jose, CA, USA).
RT-PCR assay
Total RNA was extracted from the tumor tissues or
cells using TRIzol reagent. The quality and concentration of the
RNA were confirmed by spectrophotometry and electrophoresis on
ethidium bromide-stained agarose gels. The RT-PCR amplification was
performed using the Takara RNA PCR kit (AMV) Ver 3.0. The specific
primers and the amplification conditions are shown in Table I. The initial denaturation step for
all genes was set at 94°C for 5 min. Finally, an additional
extension step at 72°C for 5 min was performed. The PCR products
were analyzed by electrophoresis on a 3% agarose gel with EB
staining using a Gel-Pro analyzer (UVP, Upland, CA, USA).
 | Table IPrimer sequences for PCR and
amplification conditions for each target gene. |
Table I
Primer sequences for PCR and
amplification conditions for each target gene.
| Gene name | Primer sequence (5′
to 3′) | Product (base) | Amplification
conditions |
|---|
| Bax | F:
CGGCGAATTGGAGATGAACTG
R: GCAAAGTAGAAGAGGGCAACC | 161 | Bax: Denaturation at
94°C for 1 min, annealing at 60°C for 1 min and synthesizing at
72°C for 30 sec for 35 cycles |
| Bcl-2 | F:
TACCGTCGTGACTTCGCAGAG
R: GGCAGGCTGAGCAGGGTCTT | 350 | Bcl-2 and β-actin:
Denaturation at 94°C for 1 min, annealing at 62°C for 1 min and
synthesizing at 72°C for 1 min for 35 cycles |
| β-actin | F:
TACCACAGGCATTGTGATGG
R: AATAGTGATGACCTGGCCGT | 310 | |
Animals and experimental protocol
The H22 cells were collected from the ascites of the
mice 8 days after inoculation to prepared a cell suspension of
concentration 5.0×106/ml. The Balb/c mice received a
subcutaneous injection of H22 cells (1.0×106 in 0.2 ml)
in the right axillary region (14). From the second day after
inoculation, the Balb/c mice were randomly divided into 6 groups
(10/group; half male and half female). There were 3 Tan ME
treatment groups comprising 3 intravenous (i.v.) dose levels (2, 4
and 8 mg/kg); a positive control group treated with 5-Fu [25 mg/kg,
intraperitonealy (i.p.)]; a negative control group which received
an i.v. injection of empty ME; and a Tan control group which
received a solution of Tan at an i.v. dose of 8 mg/kg. The
treatments were given daily for 7 consecutive days. Prior to each
treatment, the mice were weighed. At the end of the experiment, all
mice were sacrificed by dislocation and the body, tumor and spleen
weights were determined. The tissue samples were stored at −80°C
until analysis. The tumor inhibition rate and spleen index were
calculated as follows: inhibition rate (%) = (1 - tumor weight of
test group/tumor weight of Tan solution group) ×100; spleen index =
spleen weight (mg)/body weight (g).
Immunofluorescence staining
Double immunofluorescence staining was performed as
described previously (6,15). Tumor tissues were removed
immediately en bloc and post-fixed for 24 h in 4% paraformaldehyde
in PBS at 4°C before cryoprotection by bathing in 30% sucrose. They
were then frozen and 6-8-μm sections were prepared using a cryostat
(Leica CM1850; Mannheim, Germany). The sections were rinsed with
PBS 3 times for 5 min each, covered with 1% SDS solution and then
incubated for 5 min at room temperature. After rinsing with PBS 3
times for 5 min each, the sections were treated with 0.3% Triton
X-100, blocked with 10% horse serum and then incubated with mouse
anti-human Bcl-2 monoclonal antibody (1:75) and rabbit anti-mouse
Bax polyclonal antibody (1:75) at 4°C overnight. After a PBS wash,
the slides were incubated with the FITC-conjugated goat anti-mouse
IgG (1:75) and TRITC-conjugated goat anti-mouse IgG (1:75) for 1 h
at room temperature. The nuclei were stained with DAPI. The images
of double-immunostained sections were acquired with a fluorescence
microscope (Nikon, TE2000U, Tokyo, Japan).
Statistical analysis
The results are expressed as the means ± SD.
Comparisons of each group were performed by one-way analysis of
variance (ANOVA) using SPSS 13.0 software. P<0.05 was considered
to indicate a statistically significant result.
Results
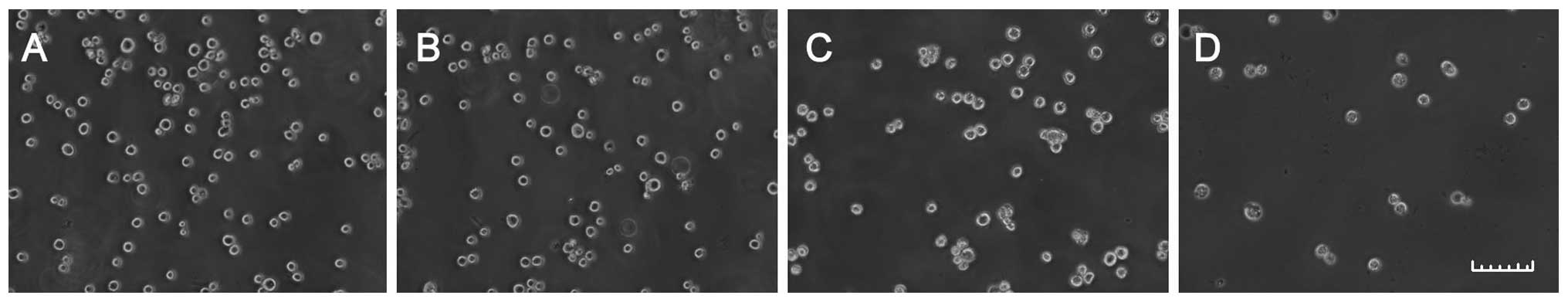
Tan ME induces morphological changes in
H22 cells
Under an inverted light microscope, empty ME-treated
and Tan solution-treated H22 cells grew well and the cell skeletons
were clear. The majority of cells treated with 0.2 or 0.8 μg/ml Tan
ME for 48 h were broken and necrosed (Fig. 1).
Tan ME downregulates Bcl-2 protein levels
in vitro
The effects of the different treatments on Bcl-2
protein levels were evaluated by FCM analysis (Fig. 2). High levels of the Bcl-2 protein
were detected by FCM 48 h after empty ME treatment and the Tan
solution-treated group had markedly decreased Bcl-2 protein levels
compared with the empty ME-treated group. Tan ME decreased Bcl-2
protein levels markedly compared with those of the Tan
solution-treated group, in a dose-dependent manner (P<0.01).
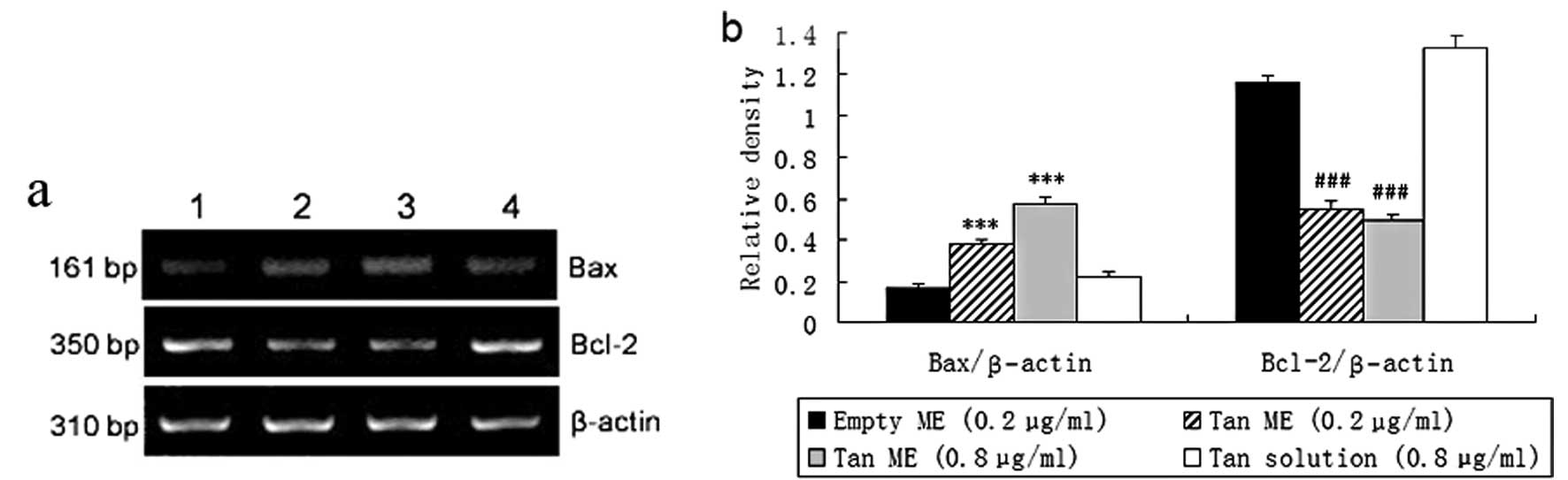
Tan ME downregulates Bcl-2 mRNA levels
and upregulates Bax mRNA levels in vitro
Using semi-quantitative RT-PCR, we first examined
the effects of Tan ME on the mRNA levels of Bcl-2 and Bax in
cultured H22 cells. As shown in Fig.
3, Bax mRNA expression levels were low in the Tan solution
group, but 0.2 and 0.8 μg/ml Tan ME increased the mRNA levels of
Bax. In the Tan solution group, Bcl-2 mRNA was highly detectable
and 0.2 and 0.8 μg/ml Tan ME markedly decreased the levels of Bcl-2
mRNA (P<0.001).
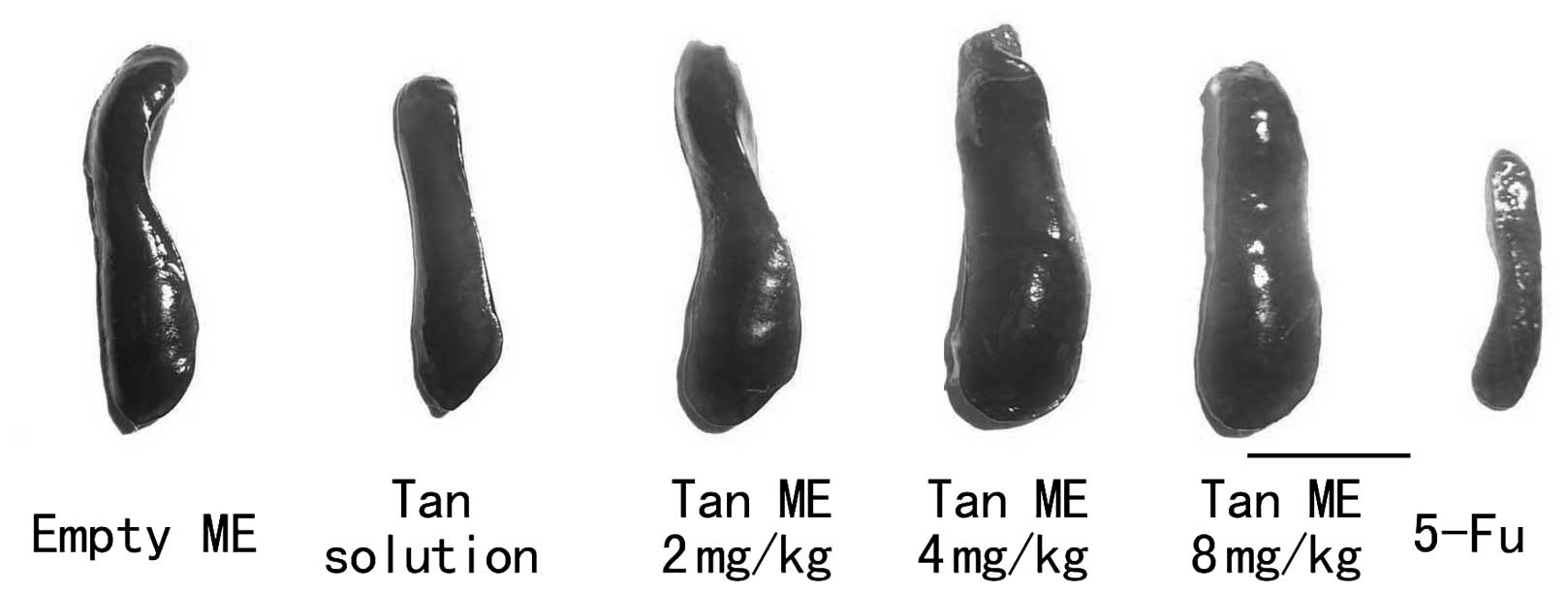
Tan ME inhibits tumor growth in vivo
The treatment with 8 mg/kg Tan ME demonstrated
significant inhibitory effects on the growth of the inoculated H22
cells in the mice; the inhibition rate was 47.66%. No significant
differences in the tumor growth between the 2 and 4 mg/kg Tan
ME-treated groups and the Tan solution-treated group were observed
(Table II). As a positive
control, 5-Fu (25 mg/kg) significantly inhibited tumor growth but
it also decreased the mean body weight and spleen index of the mice
(Figs. 4 and 5).
 | Table IIEffects of body weight, spleen index
and inhibition rate for H22 hepatic carcinoma in mice treated with
Tan ME. |
Table II
Effects of body weight, spleen index
and inhibition rate for H22 hepatic carcinoma in mice treated with
Tan ME.
| Group | Dose (mg/kg) | Body weight (g) | Spleen index
(mg/g) | Tumor weight (g) | Inhibition rate
(%) |
|---|
|
|---|
| Pre-medication | Post-medication |
|---|
| Empty ME | 8 | 20.10±1.93 | 22.75±2.05 | 8.01±0.70 | 1.12±0.18 | - |
| 5-Fu | 25 | 20.48±1.56a | 17.98±1.88 | 3.65±0.40b | 0.37±0.07b | 65.79 |
| Tan solution | 8 | 17.87±0.51 | 21.20±2.65 | 8.10±1.24 | 1.08±0.30 | - |
| 2 | 19.40±1.15 | 22.20±4.39 | 9.93±1.35 | 1.09±0.17 | −0.89 |
| Tan ME | 4 | 19.93±1.79 | 21.93±3.56 | 8.93±0.48 | 1.01±0.12 | 6.33 |
| 8 | 18.45±1.08 | 19.60±2.26 | 9.60±1.12 | 0.56±0.08a | 47.66 |
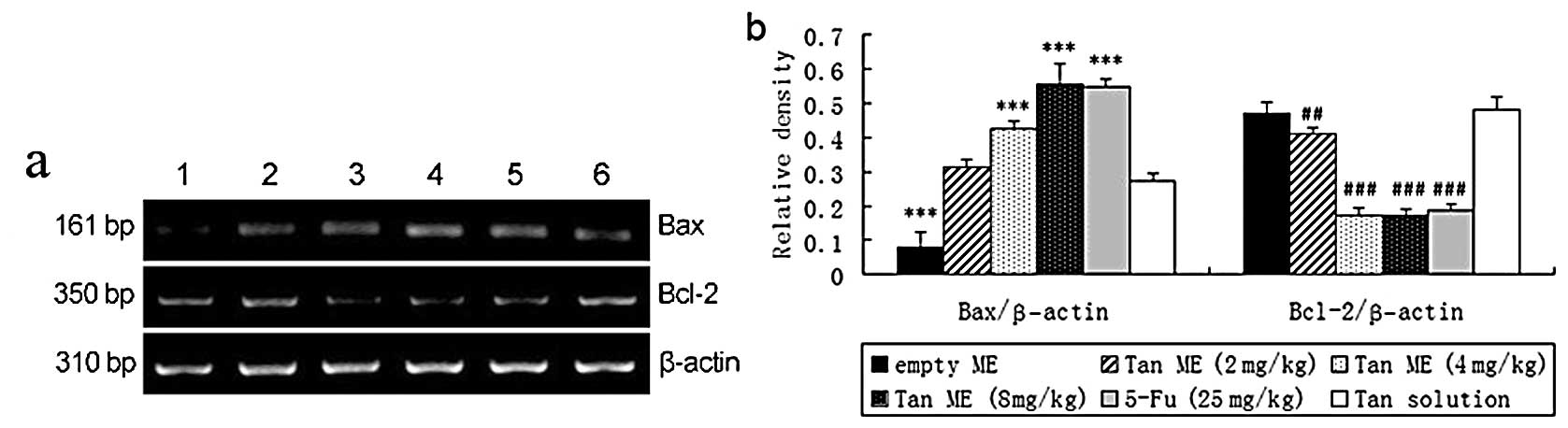
Tan ME treatment downregulates Bcl-2 and
upregulates Bax mRNA levels in H22 tumors in vivo
As shown in Fig. 6,
Tan ME increased Bax mRNA levels in a dose-dependent manner
(P<0.001). Compared with the Tan solution-treated group, the
Bcl-2 mRNA levels were significantly decreased in the tumors from
the mice treated with 2 mg/kg Tan ME (P<0.01) or 4 or 8 mg/kg
Tan ME (P<0.001).
Tan ME treatment modulates the
distributions of Bcl-2 and Bax in H22-transplanted tumors
We found little staining of Bax and strong staining
of Bcl-2 in the Tan solution-treated tumor tissue by
immunohistochemistry. Compared with the Tan solution group, 4 and 8
mg/kg Tan ME markedly increased Bax and decreased Bcl-2 in a
dose-dependent manner (Fig.
7).
Discussion
In our study, Tan was encapsulated into an ME.
Phospholipid, ethyl oleate, glycerol and pluronic F68 are
biocompatible components of the Tan ME that can be safely used for
i.v. injection (16,17). The surfactant enhances the
permeability of the ME. Our previous study found that empty ME
itself had effects on tumor cells (12). Therefore, in the present study, we
used two control groups, an empty ME group and a Tan solution
group. The results showed that there was no significant difference
between the empty ME and Tan solution groups, but there were
significant dose-dependent differences in the three Tan ME-treated
groups, demonstrating that the ME enhances the antitumor effect of
Tan.
As a positive control, 5-Fu had a significant
inhibitory effect on the growth of the inoculated H22 cells in mice
(the inhibition rate was 65.79%) but it significantly decreased the
body weight and spleen index of the mice. Compared with the empty
ME and Tan solution groups, Tan ME had no significant effects on
spleen index and body weight, indicating that Tan ME caused no
major side effects in the mice.
It is now well established that apoptosis is a
complex biological process involving many pathways. The activation
of apoptosis pathways is a key mechanism by which cytotoxic drugs
kill tumor cells (18). The
induction of apoptosis is now considered to be an important method
for the assessment of the clinical effectiveness of antitumor drugs
(19). Certain evidence suggests
that one of the most important regulators of the apoptotic pathway
is the Bcl-2 family of genes, including Bcl-2 and Bax genes
(20). One mechanism in the cell
death program is the upregulation of the proapoptotic Bax protein.
An increase in Bax expression levels effects a change in the
permeability of mitochondrial membranes. This leads to the release
of cytochrome c and the activation of caspases, which finally
proteolyze cellular components. Bcl-2 is one of the key genes known
to downregulate Bax and p53 and therefore has the potency to
inhibit this apoptotic pathway (21). Su and Lin found that Tan IIA
inhibited the proliferation of MDA-MB-231 cells in a dose- and
time-dependent manner. One of the mechanisms may be through the
upregulation of the expression of Bax and the downregulation of
Bcl-2 expression and the induction of apoptosis (22).
In conclusion, we demonstrate that, as a drug
delivery system, the ME enhances the antitumor effect of Tan. Tan
ME had significant anticancer effects in vitro and in
vivo, providing a basis for the further development of this
novel formulation for a new class of anticancer drugs.
Acknowledgements
This study was supported by a grant from the Key
Science and Technology Program of Liaoning Province, China (Grant
No. 2005225013-9).
References
|
1
|
Voiculescu M, Winkler RE, Moscovici M and
Neuman MG: Chemotherapies and targeted therapies in advanced
hepatocellular carcinoma: from laboratory to clinic. J
Gastrointestin Liver Dis. 17:315–322. 2008.PubMed/NCBI
|
|
2
|
Zhang HT, Luo H, Wu J, et al: Galangin
induces apoptosis of hepatocellular carcinoma cells via the
mitochondrial pathway. World J Gastroenterol. 16:3377–3384. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tian HL, Yu T, Xu NN, et al: A novel
compound modified from tanshinone inhibits tumor growth in vivo via
activation of the intrinsic apoptotic pathway. Cancer Lett.
297:18–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du JR, Li X, Zhang R and Qian ZM:
Tanshinone inhibits intimal hyperplasia in the ligated carotid
artery in mice. J Ethnopharmacol. 98:319–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kai G, Xu H, Zhou C, et al: Metabolic
engineering tanshinone biosynthetic pathway in Salvia
miltiorrhiza hairy root cultures. Metab Eng. 13:319–327. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown D, Lydon J, McLaughlin M,
Stuart-Tilley A, Tyszkowski R and Alper S: Antigen retrieval in
cryostat tissue sections and cultured cells by treatment with
sodium dodecyl sulfate (SDS). Histochem Cell Biol. 105:261–267.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su CC and Lin YH: Tanshinone IIA
down-regulates the protein expression of ErbB-2 and up-regulates
TNF-α in colon cancer cells in vitro and in vivo. Int
J Mol Med. 22:847–851. 2008.PubMed/NCBI
|
|
8
|
Zhou L, Chan WK, Xu N, et al: Tanshinone
IIA, an isolated compound from Salvia miltiorrhiza Bunge,
induces apoptosis in HeLa cells through mitotic arrest. Life Sci.
83:394–403. 2008.PubMed/NCBI
|
|
9
|
Han DH, Jin ZH, Jin YZ, Yin XZ, Shen YY
and Gao ZG: Thermal reversible microemulsion system for poorly
water-soluble YH439 for oral delivery. Chem Pharm Bull (Tokyo).
58:11–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lawrence MJ and Rees GD:
Microemulsion-based media as novel drug delivery systems. Adv Drug
Deliv Rev. 45:89–121. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jadhav KR, Shaikh IM, Ambade KW and Kadam
VJ: Applications of microemulsion based drug delivery system. Curr
Drug Deliv. 3:267–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan Q, Fan GJ, Yang PM and Zhao JY: Effect
of tanshinone microemulsion on reversing MDR in human tumor cells.
Zhongguo Zhong Yao Za Zhi. 29:1079–1081. 2004.(In Chinese).
|
|
13
|
Cibelli M, Fidalgo AR, Terrando N, et al:
Role of interleukin-1beta in postoperative cognitive dysfunction.
Ann Neurol. 68:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan D, Bao HY, Bau T, Li Y and Kim YH:
Antitumor components from Naematoloma fasciculare. J
Microbiol Biotechnol. 19:1135–1138. 2009.
|
|
15
|
Sawada K, Kalam-Azad A, Sakata-Haga H, Lee
NS, Jeong YG and Fukui Y: Striking pattern of Purkinje cell loss in
cerebellum of an ataxic mutant mouse, tottering. Acta Neurobiol Exp
(Warsaw). 69:138–145. 2009.PubMed/NCBI
|
|
16
|
Nornoo AO, Osborne DW and Chow DS:
Cremophor-free intravenous microemulsions for paclitaxel I:
formulation, cytotoxicity and hemolysis. Int J Pharm. 349:108–116.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai YH, Hsieh YH, Huang YB, Chang JS,
Huang CT and Wu PC: Microemulsions for intravesical delivery of
gemcitabine. Chem Pharm Bull (Tokyo). 58:1461–1465. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Debatin KM: Apoptosis pathways in cancer
and cancer therapy. Cancer Immunol Immunother. 53:153–159. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu XD, Fan RF, Zhang Y, et al:
Down-regulation of telomerase activity and activation of caspase-3
are responsible for tanshinone I-induced apoptosis in monocyte
leukemia cells in vitro. Int J Mol Sci. 11:2267–2280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Guo QL, You QD, et al: The
anticancer activities of wogonin in murine sarcoma S180 both in
vitro and in vivo. Biol Pharm Bull. 29:1132–1137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kernt M, Neubauer AS, Eibl KH, et al:
Minocycline is cytoprotective in human trabecular meshwork cells
and optic nerve head astrocytes by increasing expression of XIAP,
survivin, and Bcl-2. Clin Ophthalmol. 4:591–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su CC and Lin YH: Tanshinone IIA inhibits
human breast cancer cells through increased Bax to Bcl-xL ratios.
Int J Mol Med. 22:357–361. 2008.PubMed/NCBI
|