Introduction
The frequency and severity of joint loading are
important determinants for the development of joint destruction
characterized by degeneration of articular cartilage. The excessive
motion and exposure of load on the joints are known to clinically
and experimentally cause articular cartilage injury (1–8).
Thus, sports associated with repetitive impact and torsional
loading on the joints increase the risk of articular cartilage
degeneration, which results in the clinical symptoms of
osteoarthritis (OA) (6,7). The disease process of OA involves
degradation and functional loss of joint cartilage. Previous
studies with experimental OA models have revealed that the early
changes in the biochemical properties and metabolism of cartilage
matrix may be detected prior to the appearance of radiological
changes (3). Therefore, a number
of biomarkers with greater reliability and sensitivity have been
established for the early diagnosis or prognosis of OA (9–16).
Furthermore, such biomarkers are used for evaluating the actions of
disease-modifying drugs since they specifically reflect alterations
in the metabolism of cartilage and associated tissues, including
bone and synovium (15). In this
context, C-terminal cross-linked telopeptides of type II collagen
(CTX-II) and C-terminal propeptides of type II procollagen (CPII)
have been used as cartilage-specific type II collagen degradation
and synthesis markers, respectively (12–14).
In addition, N-terminal telopeptides of bone-specific type I
collagen (NTx) and bone alkaline phosphatase (BAP) have been used
as markers of bone resorption and formation, respectively.
Among sports of various intensities and frequencies
of joint loading, bicycle racing is associated with characteristic
biomechanics (i.e., the relatively fixed body position) and low
impact on the skeleton, which results in relatively low strain
magnitudes and low osteogenic stimulus to the bones. In addition,
bicycle racers with low bone mineral density (BMD) have been
identified to be at risk of developing osteoporosis at a younger
age, as these individuals experience bone loss due to the low
mechanical loading nature of the sport. Thus, the effect of bicycle
racing on the joints is largely evaluated using BMD and/or bone
biomarkers rather than cartilage biomarkers (17–21).
Nutritional supplements, including glucosamine,
chondroitin and collagen, are used for joint health to treat or
prevent sports-related cartilage injuries (i.e., OA) in athletes
(22–27). Among these, glucosamine, a
naturally occurring amino monosaccharide, has been widely used to
treat OA in humans (28–30). Glucosamine is present in the
connective and cartilage tissues as a component of
glycosaminoglycans (proteoglycans) and contributes to maintaining
the strength, flexibility and elasticity of these tissues.
Glucosamine inhibits degradation and stimulates synthesis of
glycosaminoglycans, thereby exhibiting chondroprotective actions
(27,31). In addition, glucosamine inhibits
the expression of collagen-degrading enzymes, matrix
metalloproteinases (MMPs), but augments the synthesis of type II
collagen in chondrocytes (32,33).
Therefore, glucosamine has been hypothesized to exert a
chondroprotective action on cartilage injuries by retaining not
only proteoglycans, but also type II collagen in the articular
cartilage. Previously, we revealed that glucosamine administration
reduced enhanced levels of CTX-II but retained CPII levels in
soccer players, indicating that glucosamine exerts a
chondroprotective action in athletes (soccer players) by preventing
type II collagen degeneration but maintaining type II collagen
synthesis (34). Based on these
observations, we hypothesized that glucosamine administration may
affect cartilage metabolism in bicycle racers. In the present
study, the effects of glucosamine administration on cartilage and
bone metabolism was investigated in bicycle racers, using
cartilage- and bone-specific biomarkers, including CTX-II, CPII,
NTx and BAP.
Materials and methods
Subjects
A total of 41 competitive bicycle racers belonging
to the Japan Keirin School [Shizuoka, Japan; all males, aged
between 19 and 22 years old (mean ± SD, 20.3±0.9)] were recruited.
Individual heights and weights were measured to determine body mass
index (BMI; Table I). The study
protocol was approved by the local ethics committee (Juntendo
University Shizuoka Hospital, Shizuoka, Japan) and was conducted in
accordance with the Declaration of Helsinki and Ethical Guidelines
for Epidemiological Research. Subjects provided written informed
consent prior to participation in the study. All racers were living
in dormitories with similar sleeping patterns and meal times.
Subjects were actively training for competitive cycling during the
study period over six sessions a week (Monday to Saturday) for 5
h/day. Participants did not exhibit symptoms of joint injuries,
including pain, stiffness and disability and were not consuming
medications likely to affect cartilage and bone metabolism during
the study period.
 | Table IBaseline data of subjects in the
placebo and glucosamine groups. |
Table I
Baseline data of subjects in the
placebo and glucosamine groups.
| | Glucosamine |
|---|
| |
|
|---|
| Variable | Placebo | (1.5 g/day) | (3 g/day) |
|---|
| Age, years | 22.0±3.4 | 21.9±3.7 | 21.1±3.7 |
| Height, cm | 172.1±5.4 | 172.1±3.3 | 171.6±5.1 |
| Body weight, kg | 73.6±7.7 | 73.3±6.8 | 73.3±6.7 |
| CTX-II, ng/mmol
Cr | 545.2±331.0 | 450.6±93.2 | 594.4±369.7 |
| CPII, ng/ml | 1,401.2±542.9 | 1,007.1±329.3 | 1,113.1±370.6 |
| NTX, nmol BCE/mmol
Cr | 56.2±23.9 | 65.2±16.9 | 63.9±24.7 |
| BAP, ng/ml | 17.7±7.4 | 20.3±6.2 | 25.0±20.9 |
Glucosamine administration
To evaluate the effect of glucosamine administration
on the biomarkers for cartilage and bone metabolism, individuals
were orally administered glucosamine hydrochloride (500 mg/capsule;
supplied by Koyo Chemical Co., Ltd., Tokyo, Japan) at doses of 1.5
g/day (1.5 g following supper) or 3 g/day (1.5 g following
breakfast and 1.5 g following supper) or cornstarch (0.9 g/day) as
a placebo for 3 months. Groups were classified and matched for age,
height, weight and BMI. Serum and urine samples were collected
prior to (at month 0) and following the glucosamine administration
(at month 3).
Measurement of biomarkers
To measure biomarkers of cartilage and bone
metabolism, second morning void urine and fasting blood samples
were obtained from all subjects. Urine and serum samples were
collected following an overnight fast and stored in aliquots at
−80°C until use. To evaluate cartilage metabolism, urinary CTX-II
and serum CPII were measured. For evaluation of bone metabolism,
urinary NTx and serum BAP were analyzed. An assay for CTX-II was
performed using a Urine CartiLaps® ELISA kit
(Immunodiagnostic System, Inc., Tyne and Wear, UK). CTX-II is
cleaved by collagenases during degradation of type II collagen and
is used as a type II collagen degradation marker (12). An assay for CPII was performed with
a Procollagen type II C-propeptide ELISA kit (Ibex Pharmaceuticals,
Inc., Mont-Royal, QC, Canada). CPII is cleaved from type II
procollagen during processing of synthesized procollagen and is
used as a type II collagen synthesis marker (14). NTx and BAP are used as bone
resorption and formation markers, respectively. Urinary NTx,
creatinine (Cr) and serum BAP were measured by BML, Inc. (Tokyo,
Japan) based on the ELISA (NTx and BAP) and enzyme (Cr) assays.
Concentrations of urinary CTX-II and NTx were corrected by Cr and
expressed as ng/mmol Cr and nmol bone collagen equivalent
(BCE)/mmol Cr, respectively. Concentrations of CPII and BAP were
expressed as ng/ml and mg/l, respectively.
Tolerability and safety were assessed throughout the
study on the basis of the incidence and severity of
intervention-related adverse events (side-effects) as well as
abnormal changes in blood pressure, pulse rate and laboratory
tests, including hematology and biochemical profiles.
Statistical analysis
Data were analyzed using the SPSS statistical
programs (IBM Japan, Ltd., Tokyo, Japan); Friedman test was used
for the analysis of the differences between the biomarker levels
prior to and following the administration of glucosamine and a
two-way analysis of variance (ANOVA) model was used for the
analysis of the differences among the three groups (placebo, 1.5
and 3 g glucosamine/day). Data are presented as the mean ± SD and
P<0.05 was considered to indicate a statistically significant
difference.
Results
In the present study, the effect of glucosamine, a
chondroprotective agent for OA, on the levels of biomarkers for
type II collagen degradation and synthesis and bone resorption and
formation, was examined in bicycle racers. Prior to glucosamine
administration, CTX-II levels (ng/mmol Cr) were 545.2±331.0,
450.6±93.2 and 594.4±369.7 in placebo (n=13) and 1.5 g
glucosamine/day (n=14) and 3 g glucosamine/day groups (n=14),
respectively (P>0.42). CPII levels (ng/ml) were 1,401.2±542.9,
1,007.1±329.3 and 1,113.1±370.6 (placebo vs. 1.5 g glucosamine/day,
P<0.05; placebo vs. 3 g glucosamine/day, P>0.12; 1.5 g
glucosamine/day vs. 3 g glucosamine/day, P>0.79); NTx levels
(nmol BCE/mmol Cr) were 56.2±23.9, 65.2±16.9 and 63.9±24.7
(P>0.28); BAP levels (mg/l) were 17.7±7.4, 20.3±6.2 and
25.0±20.9 (P>0.25) in placebo and 1.5 and 3 g glucosamine/day
groups, respectively (Table I).
Thus, there were no significant differences in the CTX-II, NTx and
BAP levels among the placebo, 1.5 and 3 g glucosamine/day groups
prior to glucosamine administration, although the CPII level in the
placebo group was significantly higher than that of the 1.5 g
glucosamine/day group (P<0.05).
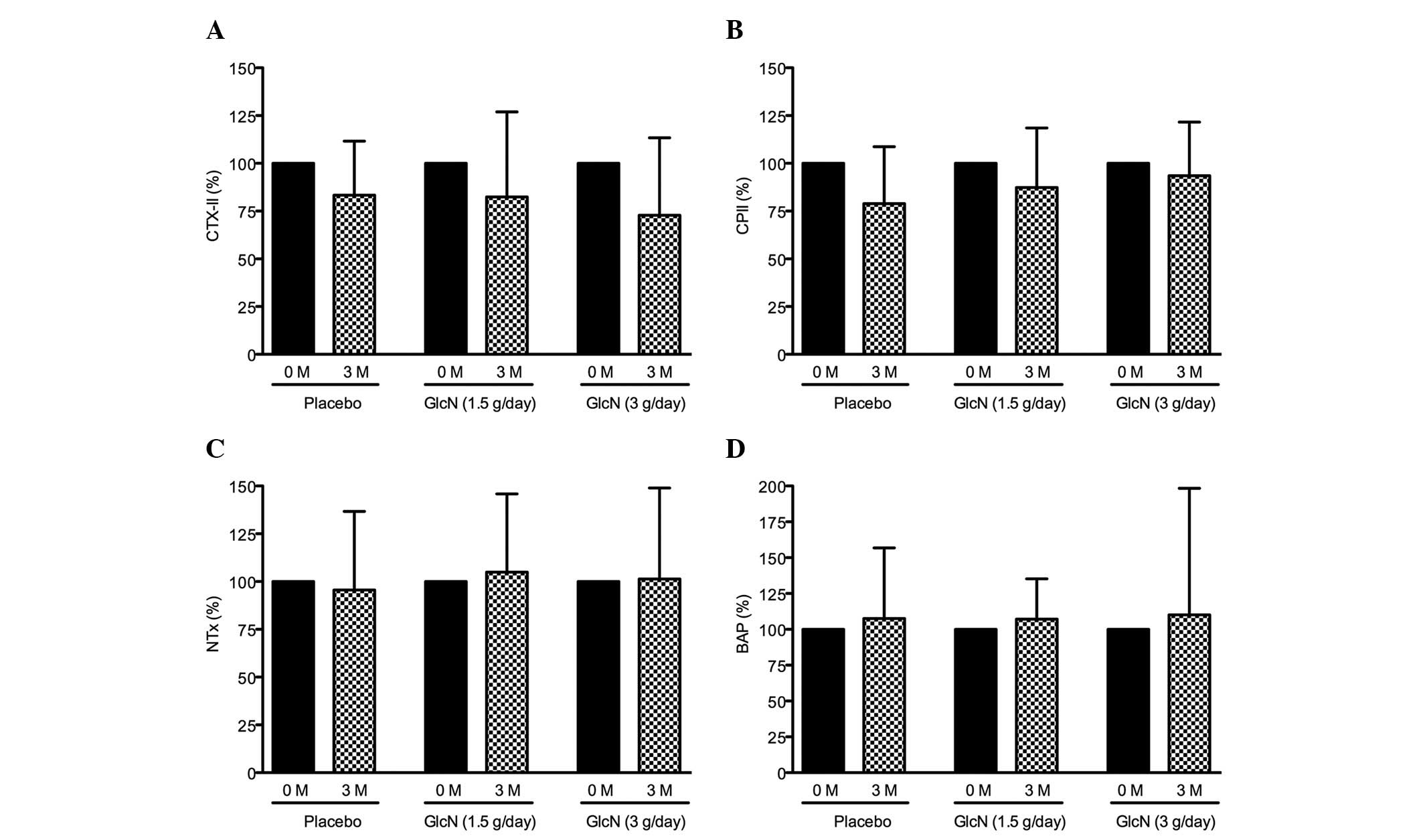
Following the administration of glucosamine, CTX-II
levels were decreased by 18 and 23% from the baseline (prior to
administration) in the 1.5 and 3 g glucosamine/day groups,
respectively. CTX-II was also decreased (17%) in the placebo group
after 3 months (Fig. 1A). CPII
levels were decreased by 13 and 7% in the 1.5 and 3 g
glucosamine/day groups, respectively, and was also decreased by 22%
in the placebo group after 3 months (Fig. 1B). By contrast, NTx levels were
slightly increased, by 5 and 1% in the 1.5 and 3 g glucosamine/day
groups, respectively, and decreased by 4% in the placebo group
(Fig. 1C). BAP levels were
slightly increased by 8, 7 and 10% in the placebo, 1.5 and 3 g
glucosamine/day-groups, respectively (Fig. 1D).
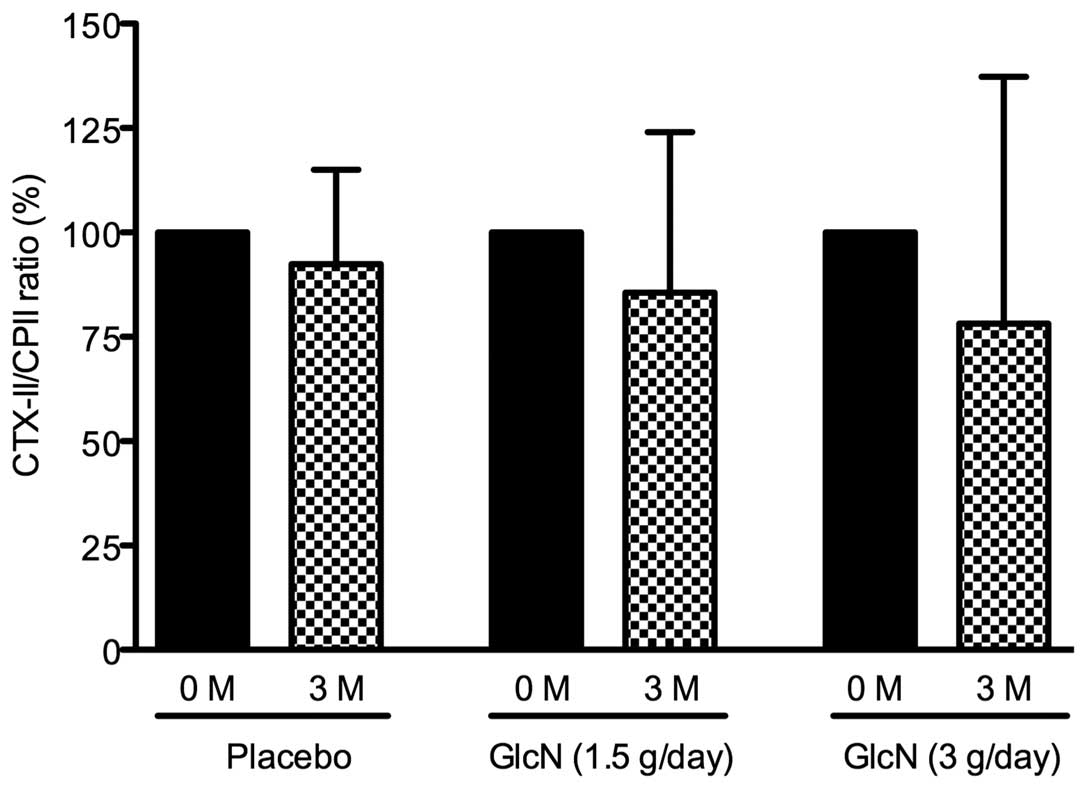
At present, it is hypothesized that the ratio of
type II collagen degradation to synthesis is suitable for the
prediction of the progression of joint damage in patients with knee
OA (35,36). Based on this concept, the effect of
glucosamine administration on the CTX-II/CPII ratio in bicycle
racers was evaluated. The CTX-II/CPII ratio was decreased by 14 and
23% in the 1.5 and 3 g glucosamine/day groups, respectively but
only by 4% in the placebo group. However, these changes were not
identified to be statistically significant (Fig. 2).
In addition, no significant changes were observed in
body weight, BMI, blood pressure, pulse rate and results of
laboratory tests, including hematological and biochemical profiles
(aspartate aminotransferase, alanine aminotransferase, creatinine,
blood urea nitrogen, creatine kinase, total cholesterol and
glucose) in sera prior to and following glucosamine administration
(data not shown).
Discussion
The majority of athletes (>70%) are known to
routinely utilize nutritional supplements, including glucosamine,
chondroitin, collagen, vitamins, calcium and magnesium (22–27).
In the present study, the effect of glucosamine, a
chondroprotective agent for OA, on cartilage and bone metabolism
was investigated in bicycle racers, using cartilage- and
bone-specific biomarkers, including CTX-II, CPII, NTx and BAP.
The mean CTX-II levels were 529.7±295.8 ng/mmol Cr
and NTx levels were 66.9±22.4 nmol BCE/mmol Cr in bicycle racers
prior to glucosamine administration (n=41). Previously, we revealed
that CTX-II levels (1,348.0±212.9 ng/mmol Cr, mean ± SEM) and NTx
(92.9±8.5 nmol BCE/mmol Cr) in soccer players (n=18) were
significantly higher than the levels of CTX-II (509.5±87.6 ng/mmol
Cr, mean ± SEM) and NTx (56.0±8.6 nmol BCE/mmol Cr) in non-athlete
controls (n=10) (34). Based on
these results, CTX-II and NTx levels in bicycle racers are lower
compared with soccer players and almost equal to the levels of
non-athlete controls, indicating that bicycle racing yields a lower
impact on the joints compared with playing soccer.
In addition, we reported that glucosamine may exert
a chondroprotective action in soccer players by preventing type II
collagen degradation but maintaining type II collagen synthesis
(34). The present study also
revealed that synthesis of type II collagen (CPII) was not changed
by glucosamine administration (13 and 7% reduction in 1.5 and 3 g
glucosamine and 21% reduction in placebo groups), however, the
degradation of type II collagen (CTX-II) was reduced by glucosamine
(18 and 27% reduction in 1.5 and 3 g glucosamine/day and 17%
reduction in placebo groups). Consistent with these changes, the
ratio of CTX-II (type II collagen degradation)/CPII (type II
collagen synthesis) was reduced by glucosamine administration (14
and 22% reduction in 1.5 and 3 g glucosamine/day and 8% reduction
in placebo groups). Furthermore, the effect of glucosamine was
dose-dependent. These observations indicate that glucosamine may
similarly exhibit a chondroprotective action in bicycle racers by
preventing type II collagen degradation but maintaining
synthesis.
In addition to the chondroprotective action,
glucosamine is reported to have the potential to modulate bone
metabolism. In this context, we previously identified that
glucosamine induces osteoblastic but suppresses osteoclastic cell
differentiation in mouse osteoblastic cells and therefore may
increase bone matrix deposition and decrease bone resorption,
eventually promoting bone formation (37). In addition, glucosamine
administration was previously demonstrated to suppress reduction of
bone mineral density in a rat experimental OA model (38). The present study indicated that
glucosamine administration did not markedly affect NTx (type I
collagen degradation) levels but significantly increased BAP (a
bone formation marker) levels at 3 g/day (P<0.05), when analyzed
by Friedman test. These results indicate that glucosamine may have
a potential to modulate bone metabolism, particularly bone
formation, in humans.
The actions of glucosamine on OA and knee injury in
athletes have been largely investigated using a dose of 1.5 g/day
in short-and long-term treatments without apparent adverse effects
(27,30). Thus, glucosamine may be a safe and
nontoxic agent. Consistent with this, the present study revealed
that none of the physical measurement parameters (body weight and
body mass index), physiological examinations (systolic/diastolic
blood pressure and pulse rate) and laboratory tests (hematology and
blood chemistry) were significantly changed, even at a dose of 3
g/day, following glucosamine administration.
In conclusion, the present study, by the analysis of
biomarkers, including CTX-II, CPII, NTx and BAP, demonstrated that
cartilage (type II collagen degradation and synthesis) and bone
(bone resorption and formation) metabolism in bicycle racers is
almost the same as that of non-athletes but lower than that of
soccer players. However, the administration of glucosamine reduced
type II collagen degradation but maintained synthesis in bicycle
racers as well as soccer players. Therefore, glucosamine may exert
a chondroprotective action by preventing type II collagen
degradation in athletes of various sports, including soccer players
and bicycle racers.
References
|
1
|
O’Kane JW, Hutchinson E, Atley LM and Eyre
DR: Sport-related differences in biomarkers of bone resorption and
cartilage degradation in endurance athletes. Osteoarthritis
Cartilage. 14:71–76. 2006.PubMed/NCBI
|
|
2
|
Roos H, Dahlberg L, Hoerrner LA, Lark MW,
Thonar EJ, Shinmei M, Lindqvist U and Lohmander LS: Markers of
cartilage matrix metabolism in human joint fluid and serum: the
effect of exercise. Osteoarthritis Cartilage. 3:7–14. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qi C and Changlin H: Effects of moving
training on histology and biomarkers levels of articular cartilage.
J Surg Res. 135:352–363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi C, Changlin H and Zefeng H: Matrix
metalloproteinases and inhibitor in knee synovial fluid as
cartilage biomarkers in rabbits: the effect of high-intensity
jumping exercise. J Surg Res. 140:149–157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim HJ, Lee YH and Kim CK: Biomarkers of
muscle and cartilage damage and inflammation during a 200 km run.
Eur J Appl Physiol. 99:443–447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buckwalter JA and Lane NE: Does
participation in sports cause osteoarthritis? Iowa Orthop J.
17:80–89. 1997.PubMed/NCBI
|
|
7
|
Buckwalter JA and Lane NE: Athletics and
osteoarthritis. Am J Sports Med. 25:873–881. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Creighton DL, Morgan AL, Boardley D and
Brolinson PG: Weight-bearing exercise and markers of bone turnover
in female athletes. J Appl Physiol. 90:565–570. 2001.PubMed/NCBI
|
|
9
|
Garnero P, Rousseau JC and Delmas PD:
Molecular basis and clinical use of biochemical markers of bone,
cartilage and synovium in joint diseases. Arthritis Rheum.
43:953–968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garnero P, Piperno M, Gineyts E, Christgau
S, Delmas PD and Vignon E: Cross sectional evaluation of
biochemical markers of bone, cartilage and synovial tissue
metabolism in patients with knee osteoarthritis: relations with
disease activity and joint damage. Ann Rheum Dis. 60:619–626. 2001.
View Article : Google Scholar
|
|
11
|
Poole AR: Biochemical/immunochemical
biomarkers of osteoarthritis: utility for prediction of incident or
progressive osteoarthritis. Rheum Dis Clin North Am. 29:803–818.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Christgau S, Garnero P, Fledelius C, Moniz
C, Ensig M, Gineyts E, Rosenquist C and Qvist P: Collagen type II
C-telopeptide fragments as an index of cartilage degradation. Bone.
29:209–215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poole AR, Ionescu M, Fitzcharles MA and
Billinghurst RC: The assessment of cartilage degradation in vivo:
development of an immunoassay for the measurement in body fluids of
type II collagen cleaved by collagenases. J Immunol Methods.
294:145–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shinmei M, Ito K, Matsuyama S, Yoshihara Y
and Matsuzawa K: Joint fluid carboxy-terminal type II procollagen
peptide as a marker of cartilage collagen biosynthesis.
Osteoarthritis Cartilage. 1:121–128. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garnero P and Delmas PD: Biomarkers in
osteoarthritis. Curr Opin Rheumatol. 15:641–646. 2003. View Article : Google Scholar
|
|
16
|
Rousseau JC and Delmas PD: Biological
markers in osteoarthritis. Nat Clin Pract Rheumatol. 3:346–356.
2007. View Article : Google Scholar
|
|
17
|
Warner SE, Shaw JM and Dalsky GP: Bone
mineral density of competitive male mountain and road cyclists.
Bone. 30:281–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smathers AM, Bemben MG and Bemben DA: Bone
density comparisons in male competitive road cyclists and untrained
controls. Med Sci Sports Exerc. 41:290–296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stewart AD and Hannan J: Total and
regional bone density in male runners, cyclists and controls. Med
Sci Sports Exerc. 32:1373–1377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nichols JF, Palmer JE and Levy SS: Low
bone mineral density in highly trained male master cyclists.
Osteoporos Int. 14:644–649. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rector RS, Rogers R, Ruebel M and Hinton
PS: Participation in road cycling vs running is associated with
lower bone mineral density in men. Metabolism. 57:226–232. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang SH, Johnson K and Pipe AL: The use
of dietary supplements and medications by Canadian athletes at the
Atlanta and Sydney Olympic Games. Clin J Sport Med. 16:27–33. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Froiland K, Koszewski W, Hingst J and
Kopecky L: Nutritional supplement use among college athletes and
their sources of information. Int J Sport Nutr Exerc Metab.
14:104–120. 2004.PubMed/NCBI
|
|
24
|
Schwenk TL and Costley CD: When food
becomes a drug: nonanabolic nutritional supplement use in athletes.
Am J Sports Med. 30:907–916. 2002.PubMed/NCBI
|
|
25
|
Dascombea BJ, Karunaratna M, Cartoon J,
Fergie B and Goodmana C: Nutritional supplementation habits and
perceptions of elite athletes within a state-based sporting
institute. J Sci Med Sport. 13:274–280. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gorsline RT and Kaeding CC: The use of
NSAIDs and nutritional supplements in athletes with osteoarthritis:
prevalence, benefits and consequences. Clin Sports Med. 24:71–82.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ostojic SM, Arsic M, Prodanovic S, Vukovic
J and Zlatanovic M: Glucosamine administration in athletes: effects
on recovery of acute knee injury. Res Sports Med. 15:113–124. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McAlindon TE, Lavalley MP, Gulin JP and
Felson DT: Glucosamine and chondroitin for treatment of
osteoarthritis: a systematic quality assessment and meta-analysis.
JAMA. 283:1469–1475. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reginster JY, Deroisy R, Rovati LC, Lee
RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE and
Gossett C: Long-term effects of glucosamine sulphate on
osteoarthritis progression: a randomized, placebo-controlled
clinical trial. Lancet. 357:251–256. 2001. View Article : Google Scholar
|
|
30
|
Pavelká K, Gatterová J, Olejarová M,
Machacek S, Giacovelli G and Rovati LC: Glucosamine sulfate use and
delay of progression of knee osteoarthritis: a 3-year, randomized,
placebo-controlled, double-blind study. Arch Intern Med.
162:2113–2123. 2002.PubMed/NCBI
|
|
31
|
Igarashi M, Kaga I, Takamori Y, Sakamoto
K, Miyazawa K and Nagaoka I: Effects of glucosamine derivatives and
uronic acids on the production of glycosaminoglycans by human
synovial cells and chondrocytes. Int J Mol Med. 27:821–827.
2011.PubMed/NCBI
|
|
32
|
Nakamura H, Shibakawa A, Tanaka M, Kato T
and Nishioka K: Effects of glucosamine hydrochloride on the
production of prostaglandin E2, nitric oxide and metalloproteases
by chondrocytes and synoviocytes in osteoarthritis. Clin Exp
Rheumatol. 22:293–299. 2004.PubMed/NCBI
|
|
33
|
Derfoul A, Miyoshi AD, Freeman DE and Tuan
RS: Glucosamine promotes chondrogenic phenotype in both
chondrocytes and mesenchymal stem cells and inhibits MMP-13
expression and matrix degradation. Osteoarthr Cartil. 15:646–655.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshimura M, Sakamoto K, Tsuruta A,
Yamamoto T, Ishida K, Yamaguchi H and Nagaoka I: Evaluation of the
effect of glucosamine administration on biomarkers for cartilage
and bone metabolism in soccer players. Int J Mol Med. 24:487–494.
2009.PubMed/NCBI
|
|
35
|
Cahue S, Sharma L, Dunlop D, Ionescu M,
Song J, Lobanok T, King L and Poole AR: The ratio of type II
collagen breakdown to synthesis and its relationship with the
progression of knee osteoarthritis. Osteoarthritis Cartilage.
15:819–823. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sharif M, Kirwan J, Charni N, Sandell LJ,
Whittles C and Garnero P: A 5-yr longitudinal study of type IIA
collagen synthesis and total type II collagen degradation in
patients with knee osteoarthritis - association with disease
progression. Rheumatol. 46:938–943. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Igarashi M, Sakamoto K and Nagaoka I:
Effect of glucosamine, a therapeutic agent for osteoarthritis, on
osteoblastic cell differentiation. Int J Mol Med. 28:373–379.
2011.PubMed/NCBI
|
|
38
|
Wang SX, Laverty S, Dumitriu M, Plaas A
and Grynpas MD: The effects of glucosamine hydrochloride on
subchondral bone changes in any animal model of osteoarthritis.
Arthritis Rheum. 56:1537–1548. 2007. View Article : Google Scholar : PubMed/NCBI
|