Introduction
Obstructive uropathy refers to the presence of
structural or functional changes in the urinary tract that impede
the normal flow of urine. Hydronephrosis denotes dilation of the
urinary tract. Obstructive nephropathy is a relatively common
entity that is treatable and often reversible. It occurs at all
ages, from infants to elderly subjects. It is the result of
functional or anatomic lesions located in the urinary tract.
Obstructive nephropathy is one of the most serious diseases in
children and has been the most common cause of end-stage renal
failure (1). The kidney is
important in blood filtration, concentration, excretion of waste
products and electrolytes, and reabsorption of nutrients, thus
serving as a main organ for maintaining homeostatic conditions
(2). Active transport of these
metabolites through the renal tubules increases the opportunities
for tubule cells to produce or come in contact with harmful agents,
such as reactive oxygen species (ROS), indicating that the kidney
is at high risk of oxidative stress due to its metabolic action.
Oxidative stress refers to a disrupted redox equilibrium between
the production of free radicals and the ability of cells to protect
against damage caused by these molecules. Oxidative stress has been
implicated as a major cause of tissue injuries in a variety of
human diseases, including obstructive nephropathy. Free radicals
specifically play important roles in the oxidative stress pathway
(3).
The medicinal benefits of drinking green tea have
been known in Asian countries since ancient times. The consumption
of green tea has attracted much recent attention due to its
emerging beneficial health effects. Green tea is one of the most
popular and widely consumed beverages and is rich in a variety of
antioxidant catechin polyphenols (4–6). The
major polyphenols present in green tea are (−)-epicatechin,
(−)-epicatechin-3-gallate, (−)-epigallocatechin and
(−)-epigallocatechin-3-gallate (EGCG), which comprise 30–42% of
solid green tea extract (6). Among
these components, EGCG is the most abundant and most active
catechin derivative, with potent antioxidant and chemopreventive
activities (7). Since acute
obstructive nephropathy is associated with increased oxidative
stress in the tissue, it is likely that the protective effects of
catechins are mainly due to their anti-oxidative properties.
Catechins are known as effective scavengers of ROS, which are also
involved in modulation of gene expression (8). Recently, much attention has been
focused on EGCG, which has been reported to possess anti-oxidative,
anti-inflammatory and anti-carcinogenic effects (4,9,10).
Therefore, in order to ascertain whether oxidative
stress was involved in the mechanism of obstructive nephropathy in
rats and to evaluate the effect of EGCG as a representative
polyphenol, we investigated the effect of EGCG on oxidative stress
in UUO rats with obstructive nephropathy and probed the underlying
mechanisms. The chemical structure of EGCG is illustrated in
Fig. 1.
Materials and methods
Animals
Male, Sprague Dawley (SD) rats (120–150 g, 6–8 weeks
old; China Medical University Experimental Animal Research Center,
Shenyang, China) were used in these experiments. The rats were
housed in standard cages (four animals per cage) and fed standard
laboratory chow and tap water ad libitum. A constant
photoperiod (14 h light and 10 h dark) and constant temperature of
20°C were maintained. Procedures for care and handling of animals
used in this study were approved by the ethics committee of China
Medical University and carried out in accordance with guidelines
established by the China Medical University Experimental Animal
Research Centre.
Experimental design
In total, 150 adult male rats were randomly divided
into 5 groups (n=30 each): control group (group N), a normal group
of rats that underwent sham surgery; unilateral ureteral
obstruction (UUO) group (group C), where the unilateral ureter was
ligated resulting in an obstructive nephropathy model; EGCG group
(group T), where rats were intraperitoneally injected with the EGCG
at dosage of 2.5 mg/kg (T1), 5 mg/kg (T2) and
10 mg/kg (T3)/day (each dose, n=30), following
unilateral ureteral ligation. When the 72-h experiment was
performed, the normal and control groups received physiological
saline.
Animal surgery and sampling
Briefly, rats were anesthetized, laparotomy was
performed, and the left ureter identified and ligated at two points
along the ureter 1 cm apart with 4/0 silk, 3–5 mm below the renal
hilum. The sham-surgery was performed in the same way; the rats of
the normal group (ten rats) were also laparatomized, the ureter
exposed, but no ligature was made. Following closure of the
abdomen, the animals were returned to the cage with free access to
standard lab chow and water ad libitum. Rats were allowed to
recover for 72 h prior to tissue collection. Unilateral obstructive
nephropathy was confirmed by sacrificing all the rats 72 h after
surgery. The obstructed kidneys were then fixed for 4 h in 4%
formalin and embedded in paraffin for immunohistochemistry
analysis. The remaining portions of each sample were diced finely
in the presence of liquid nitrogen, and stored until analysis.
Measurement of ROS, GSH, GSSG, total GSH
and GSH/GSSG
Briefly, tissue was homogenized in TES/SB buffer
consisting of 20 mM Tris, 1 mM EDTA, 250 mM sucrose, 20 mM sodium
borate and 2 mM serine. The homogenates were centrifuged at 4°C for
10 min at 10,000 × g, and the supernatants were obtained. The
content of ROS, GSH, GSSG and total glutathione was measured by
commercial kits (Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer’s instructions.
Immunohistochemistry
Sections (5 μm thickness) were prepared and
subjected to the immunoperoxidase method. Endogenous peroxidase was
eliminated by treatment with 3% H2O2/10%
methanol phosphate buffered saline (PBS) for 20 min at room
temperature. After washing with water and 0.05 M PBS (pH 7.4),
slides were blocked with 5% bovine serum albumin (BSA) in PBS for
20 min at room temperature to prevent non-specific protein binding.
The slides were then incubated with 1:100 diluted specific primary
antibody for Nrf2 and γ-GCS (Santa Cruz Biotechnology, Santa Cruz,
CA, USA) in PBS containing 5% BSA overnight at 4°C. The sections
were rinsed in 0.05 M PBS containing 0.1% BSA. After washing with
PBS, the slides were incubated with a biotinylated goat peroxidase
conjugated secondary antibody and 0.1% DAB substrate, using the
standard streptavidin-biotin-based method. For the negative
control, slides were processed without primary antibody. The
optical densities of Nrf2 and γ-GCS bands from the membranes were
determined by densitometric scanning using a Nikon Eclipse Scanjet
E800 photo scanner and MoticMed System 6.0 software. The value of
the optical density of each protein band was expressed as the mean
± standard deviation.
Nrf2 and γ-GCS gene expression
When rats were sacrificed, renal tissue was
immediately frozen in liquid nitrogen for RNA extraction. Total RNA
was isolated from rat renal tissues with TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). Total RNA was reverse-transcribed
using the PrimeScript™ RT Reagent kit (Takara Biotechnology Dalian,
China) according to the manufacturer’s instructions. Quantitative
real-time PCR was performed for Nrf2, γ-GCS and GAPDH using the
following primers pairs: Nrf2, sense 5′-TTGATTGACATCC TTTGG-3′ and
antisense 5′-GTTCCTTCTGGAGTTGCT-3′; γ-GCS, sense
5′-ATCTACCACGCAGTCAAG-3′ and antisense 5′-CCGCCATTCAGTAACAAC-3′;
GAPDH, sense 5′-TGTGTCCGTCGTGGATCTGA-3′ and antisense
5′-ATGGTGGTGAAGACGCCAGTA-3′. Real time-PCR analysis was performed
using an ABI 7500 with the following thermal cycling conditions: 1
cycle at 95°C for 10 sec, followed by 40 cycles at 95°C for 5 sec
and 60°C for 34 sec. All samples were run in triplicate. The
amplification results were detected and analyzed using the SDS
real-time PCR detection system. The gene signals were standardized
against the corresponding GAPDH signal, and results were expressed
as the ratio of each molecule to GAPDH.
Western blot analysis
The frozen renal tissue was immediately homogenized,
the protein was solubilized in radioimmunoprecipitation buffer, and
the total protein was separated on a 12% acrylamide SDS-PAGE gel
and electroblotted to a nitrocellulose membrane. The membrane was
blocked with 5% (w/v) fat-free milk and then incubated with a
monoclonal antibody to Nrf2 and γ-GCS (rat origin, 1:1000) (Santa
Cruz Biotechnology) overnight at 4°C, followed by incubation with
horseradish peroxidase-conjugated secondary antibodies (1:1000) for
1 h at room temperature. Membranes were exposed to chemiluminescent
reagents and then to X-ray film. The average intensity of the bands
was determined using Tanon Image software. Data were collected in
terms of average intensity of bands of Nrf2 and γ-GCS per average
intensity of bands of β-actin, and imported to a spreadsheet
(Excel; Microsoft, USA).
Electromicroscope
From the unligated right and from the ligated left
kidney, large tissue blocks extending from the renal capsule to at
least the upper third of the inner zone were immersed for at least
24 h in the fixative solution, to which 1% glutardialdehyde was
added. This tissue was then embedded into epoxy resin and used for
electron microscopy. Ultrathin (80 nm) sections were cut with an
ultramicrotome. Ultrathin sections were postfixed with osmium
tetroxyde and contrasted with uranyl acetate and studied with a
CM100 Philips electron microscope.
Statistical analysis
All data were presented as the means ± SEM. One-way
analysis of variance and independent t-test were used for
statistical analysis of the differences between the groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Measurement of ROS, GSH, GSSG, total GSH
and GSSG
Compared with group N, the ROS and GSSG levels of
kidneys in group C were much higher, and the ratio of GSH/GSSG was
much lower. There was no significant difference in GSH compared
with group N. ROS, GSSG and total GSH level were much higher in the
T groups (p<0.01), while much lower than those of group C
(p<0.01; Table I).
 | Table IGSH, GSSG, GSH/GSSG, total glutathione
and ROS in renal tissue (means ± SEM, n=10). |
Table I
GSH, GSSG, GSH/GSSG, total glutathione
and ROS in renal tissue (means ± SEM, n=10).
| | | EGCG |
|---|
| | |
|
|---|
| N | C | T1 | T2 | T3 |
|---|
| GSH (mg/mg pro) | 1.32±0.29 | 1.31±0.11 | 1.34±0.28 | 1.33±0.52 | 1.34±0.47 |
| GSSG (μmol/l) | 0.08±0.04 | 0.73±0.03a | 0.71±0.05a,b | 0.57±0.03a,c | 0.55±0.04a,c |
| Total glutathione
(μmol/l) | 0.59±0.04 | 1.47±0.08a | 1.46±0.06a,b | 1.24±0.06a,c | 1.21±0.05a,c |
| GSH/GSSG | 5.25±0.07 | 0.59±0.09a | 0.60±0.07a,b | 0.75±0.08a,c | 0.78±0.09a,c |
| ROS (U/mg pro) | 1617±124 | 3782±207a | 3779±183a,b | 3273±176a,c | 3114±191a,c |
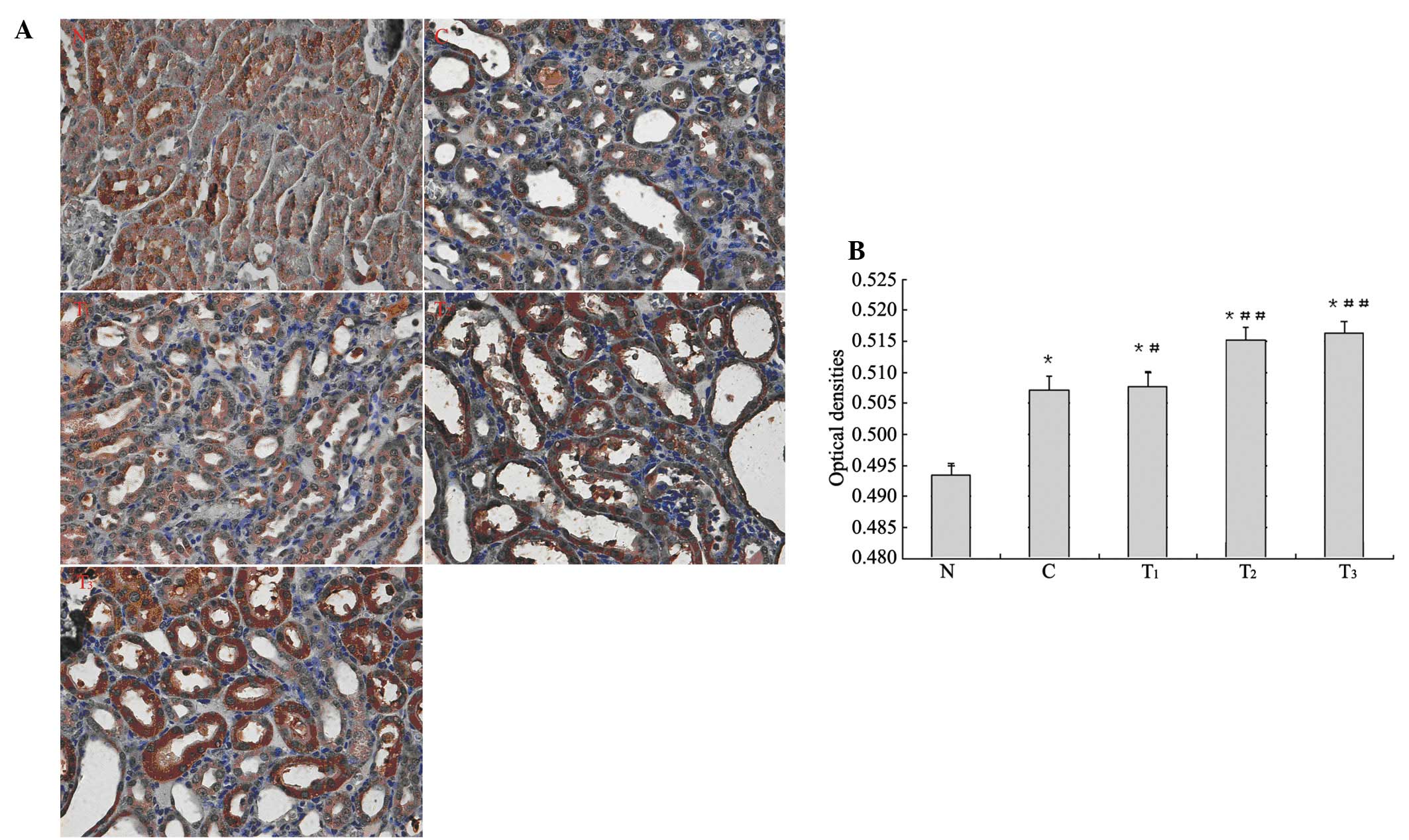
Immunohistochemistry
The expression of Nrf2 protein was mainly focused on
the renal tubular epithelial cells of rat kidney cortex and
medulla. Microscopic observation showed stained nuclei of the renal
tubular epithelial cells and dark brown particles. In the
immunohistochemical analysis of Nrf2 expression, higher average
optical density was found in group C and the treatment groups
compared with group N (p<0.01). Compared with group C, higher
average optical density in the T1 group was observed
(p<0.05), and even higher average optical density in the
T2 and T3 groups was found (p<0.01;
Fig. 2).
The expression of γ-GCS protein was mainly focused
on the renal tubular epithelial cells of the cortex and medulla in
rat kidneys. Microscopic observation showed staining in the
cytoplasm of the renal tubular epithelial cells and dark brown
particles. In the immunohistochemical analysis of γ-GCS expression,
higher average optical density was found in group C and the
treatment groups compared with group N (p<0.01). Compared with
group C, higher average optical density in the T1 group
was observed (p<0.05), and even higher average optical density
in the T2 and T3 groups was found (p<0.01;
Fig. 3).
Nrf2 and γ-GCS mRNA expression
Compared with group N, Nrf2 and γ-GCS mRNA relative
copy number in group C and each treatment group were significantly
enhanced (p<0.01). Compared with C group, Nrf2 and γ-GCS mRNA
relative copy number in the T1 group was enhanced
(p<0.05) and significantly enhanced in T2 and
T3 (p<0.01) groups (Fig.
4).
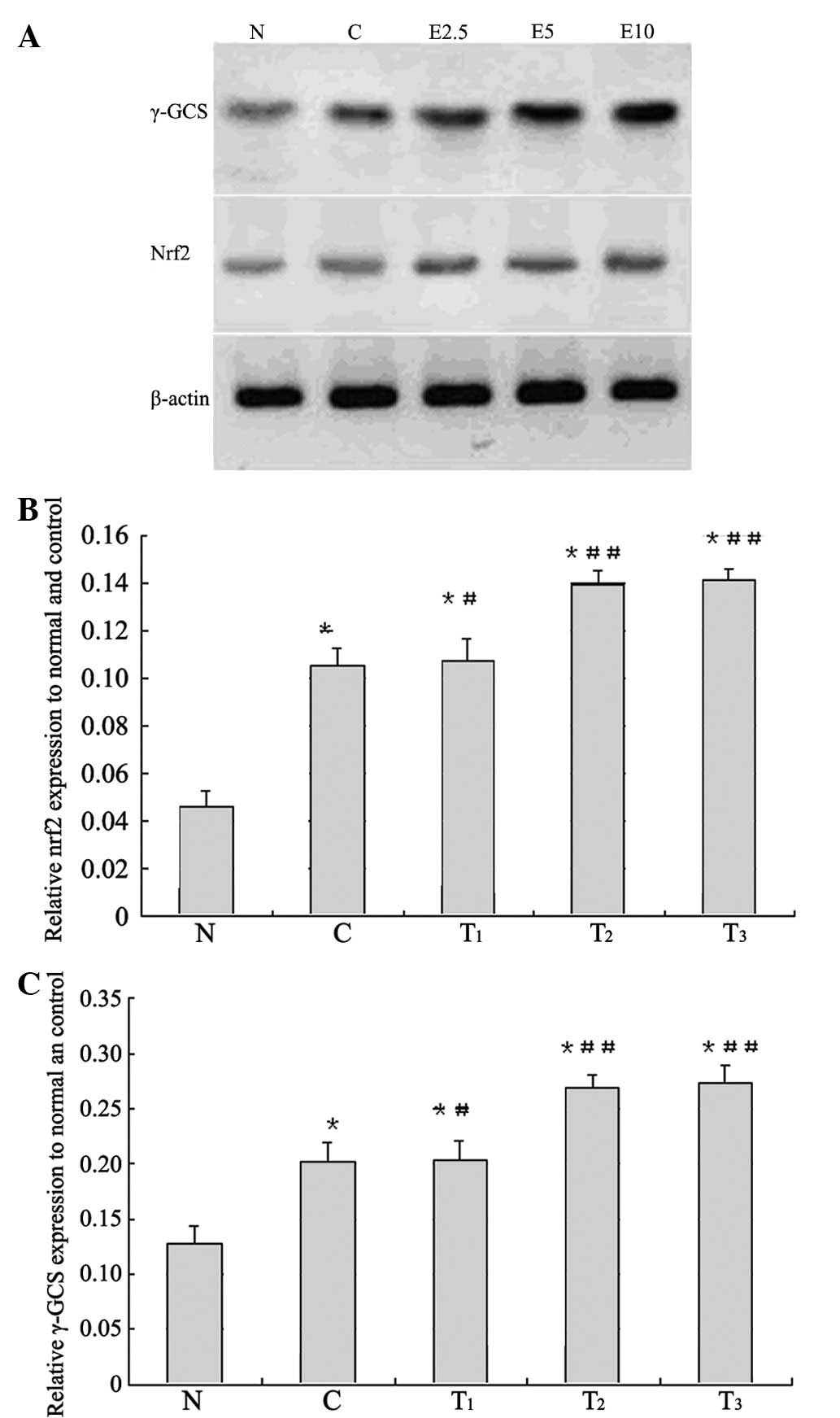
γ-GCS and Nrf2 protein expression
To elucidate the expression levels of the
antioxidant enzyme γ-GCS, as well as the upstream regulator Nrf2,
we performed western blot analyses of the renal cortex. The results
are presented in Fig. 5.
Obstructive nephropathy rats showed increases in the levels of Nrf2
and γ-GCS protein expression compared to the normal value
(p<0.01), and the rats administered 2.5, 5 and 10 mg of EGCG
showed higher values compared to the control value, respectively.
The levels of Nrf2 and γ-GCS in the kidney cortex of obstructive
nephropathy control rats were similarly elevated above those in the
kidney cortex of normal animals (p<0.01, respectively), and
treatment with EGCG at 2.5 mg (p<0.05), 5 and 10 mg (p<0.01)
doses significantly enhanced the obstructive nephropathy-induced
increases, respectively.
Electron microscopy
The ultrastructure of kidneys in rats from each
group showed that the renal tissue was injured markedly in the
model group (C), and the apoptosis of renal tubular epithelial
cells induced by obstruction was alleviated in EGCG-treated groups
(T2 and T3) (Fig. 6).
Discussion
As an antioxidant, EGCG has drawn widespread
attention in studies of kidney disease. EGCG has been known as the
most potent Nrf2 activator among the green tea polyphenols, as
evidenced by its pronounced ability to induce ARE-luciferase
reporter gene transactivation (11). Nrf2, a bZIP transcription factor,
is sequestered in the cytoplasm by kelch-like ECH-associated
protein 1 (Keap1). Exposure of cells to the antioxidant response
element (ARE) inducers results in the dissociation of Nrf2 from
Keap1 and facilitates translocation of Nrf2 to the nucleus, where
it heterodimerizes with small Maf protein, and binds to ARE,
eventually resulting in the transcriptional regulation of target
genes including both phase I (oxidation and reduction) and phase II
biotransformation (conjugation) (12–15).
The molecular mechanism underlying antioxidant enzyme induction by
EGCG has been the subject of extensive investigations. One of the
most plausible mechanisms responsible for activation of Nrf2
involves phosphorylation of serine/threonine residues of Nrf2 by
protein kinases, which facilitates enhanced nuclear translocation
of Nrf2 and subsequent ARE binding (16). The phenomenon of nuclear
translocation was also observed by Nrf2 immunohistochemical
staining in this study, that is, Nrf2-positive cells transferred
from the cytoplasm to the nuclei of renal tubular epithelial cells.
These findings of EGCG upregulation and nuclear translocation on
Nrf2 correspond with other studies (16–18).
EGCG has been shown to induce expression of glutathione
S-transferase, glutathione peroxidase, γ-GCS and heme oxygenase-1,
which are involved in the elimination or inactivation of ROS and
electrophiles implicated in multi-stage oxidative stress. γ-GCS may
be considered to be one of the major antioxidant enzymes, as it is
the rate-limiting enzyme in GSH synthesis and GSH has been
postulated to be one of the most important antioxidants (19,20).
Nrf2 gene knockout of rats may lead to the reduction of γ-GCS
subunit expression and GSH synthesis in fibroblast cells and liver
cells (21). These results thus
provide new insights into the anti-oxidative mechanisms of EGCG.
These findings suggest that EGCG-induced expression of some
representative antioxidant enzymes may provide the rats with
acquired antioxidant defense capacity, allowing them to survive
oxidative stress.
The role of this study was to ascertain the possible
roles of Nrf2 in renal cellular defense against oxidative stress,
and whether EGCG could ameliorate the development of obstructive
nephropathy. The data indicated that oxidant stress could improve
the protein expression of Nrf2 and γ-GCS, and the expression could
be enhanced by EGCG. The protein expression of both Nrf2 and γ-GCS
is mainly located in renal tubular epithelial cells. It is thus
clear that oxidative stress of rats with UUO mainly injured the
renal tubule, which suggests that renal oxidative stress may
increase the downstream target gene γ-GCS through upregulation of
Nrf2.
Nrf2 plays a pivotal role in cell defenses against
oxidative stress in the kidney by controlling the intracellular
antioxidant states, and the possibility of Nrf2 participation in
prevention of systemic oxidative stress is proposed in this study.
In addition, EGCG-treated groups showed suppressed oxidative stress
and acute renal damage caused by UUO. Our study demonstrates that
UUO rats were much more susceptible to the expression of Nrf2 and
γ-GCS, and EGCG induces Nrf2-mediated expression of γ-GCS in renal
tissue. Therefore, we inferred that EGCG activated Keap1,
facilitated the release of Nrf2 for nuclear translocation, and
finally induced expression of some representative antioxidant
enzymes in renal tissue.
The expression of Nrf2 and γ-GCS were positively
correlated with the doses of EGCG administered by intraperitoneal
injection, and high-dose EGCG had the best oxidative stress
protective effect. Results from this study also provide important
and novel insights into the molecular mechanisms underlying the
oxidative stress chemoprevention effects of EGCG, as well as the
role of Nrf2 in its biological functions. EGCG showed marked
anti-oxidative activity in the management of obstructive
nephropathy in a dose-dependent manner. Our results indicate that
the development of obstructive nephropathy may be effectively
inhibited by EGCG. As one of the most powerful antioxidants with
the advantages of high safety and fewer adverse reactions, EGCG is
expected to be an effective application for the prevention or
treatment of obstructive nephropathy. Of course, these conclusions
are only based on animal experiments, and further studies are
required to establish this type of treatment in humans. A larger
number of clinical experiments must be performed to verify the
chemopreventive effects of EGCG, in order for EGCG to have future
clinical application.
Acknowledgements
This study was supported by grants of Liaoning
Province Natural Science Foundation of China and the Science and
Technology Research Foundation, Department of Education of
Heilongjiang Provincial (11551158). The authors thank the Key
Laboratory of Congenital Malformations of China Ministry of Health
for providing the experiment site and instruments. The authors also
wish to express their gratitude to Li Ma for her technical
assistance and Dr Jia-ning Miao for his assistance with
histopathological-related research.
References
|
1
|
Klahr S: Obstructive Nephropathy. Internal
Med. 39:355–361. 2000. View Article : Google Scholar
|
|
2
|
Robertson JL: Chemically induced
glomerular injury. A review of basic mechanisms and specific
xenobiotics. Toxicol Pathol. 26:64–72. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zalba G, Fortuño A and Díez J: Oxidative
stress and atherosclerosis in early chronic kidney disease. Nephrol
Dial Transplant. 21:2686–2690. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang CS: Inhibition of carcinogenesis by
tea. Nature. 389:134–135. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujiki H, Suganuma M, Okabe S, et al:
Cancer inhibition by green tea. Mutat Res. 402:307–310. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mukhtar H and Ahmad N: Mechanism of cancer
chemopreventive activity of green tea. Proc Soc Exp Biol Med.
220:234–238. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suganuma M, Okabe S, Sueoka N, et al:
Green tea and cancer chemoprevention. Mutat Res. 428:339–344. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robak J and Gryglewski RJ: Flavonoids are
scavengers of superoxide anions. Biochem Pharmacol. 37:837–841.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang CS, Lambert JD, Hou Z, Ju J, Lu G and
Hao X: Molecular targets for the cancer preventive activity of tea
polyphenols. Mol Carcinog. 45:431–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang CS, Prabhu S and Landau J: Prevention
of carcinogenesis by tea polyphenols. Drug Metabolism Reviews.
33:237–253. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen C, Yu R, Owuor ED and Kong AN:
Activation of antioxidant-response element (ARE), mitogen-activated
protein kinases (MAPKs) and caspases by major green tea polyphenol
components during cell survival and death. Arch Pharm Res.
23:605–612. 2000. View Article : Google Scholar
|
|
12
|
Cho HY, Jedlicka AE, Reddy SP, et al: Role
of NRF2 in protection against hyperoxic lung injury in mice. Am J
Respir Cell Mol Biol. 26:175–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thimmulappa RK, Mai KH, Srisuma S, Kensler
TW, Yamamoto M and Biswal S: Identification of Nrf2-regulated genes
induced by the chemopreventive agent sulforaphane by
oligonucleotide microarray. Cancer Res. 62:5196–5203.
2002.PubMed/NCBI
|
|
14
|
Hu R, Xu C, Shen G, et al: Identification
of Nrf2-regulated genes induced by chemopreventive isothiocyanate
PEITC by oligonucleotide microarray. Life Sci. 79:1944–1955. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shelby MK and Klaassen CD: Induction of
rat UDP-glucurono-syltransferases in liver and duodenum by
microsomal enzyme inducers that activate various transcriptional
pathways. Drug Metab Dispos. 34:1772–1778. 2006. View Article : Google Scholar
|
|
16
|
Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH
and Wung BS: Upregulation of heme oxygenase-1 by
Epigallocatechin-3-gallate via the phosphatidylinositol
3-kinase/Akt and ERK pathways. Life Sci. 78:2889–2897. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang XY, Zhao WP, Li YQ, et al: The role
of NF-E2-related factor 2 in the induction of uridine
5-diphosphate-glucuronosyltransferase 1A and its isoforms by
epigallocatechin gallate in colon cancer cells. Zhonghua Yi Xue Za
Zhi. 86:82–87. 2006.PubMed/NCBI
|
|
18
|
Andreadi CK, Howells LM, Atherfold PA and
Manson MM: Involvement of Nrf2, p38, BRaf, and nuclear
factor-kappaB, but not phosphatidylinositol 3-kinase, in induction
of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol.
69:1033–1040. 2006.PubMed/NCBI
|
|
19
|
Dalton TP, Dieter MZ, Yang Y, Shertzer HG
and Nebert DW: The most glutamate cysteine ligase catalytic subunit
(Gclc) gene: embryonic lethal when homozygous, and proposed model
for moderate glutathione deficiency when heterzygous. Biochem
Biophys Res Commun. 20:2792000.
|
|
20
|
Itoh K, Chiba T, Takahashi S, et al: An
Nrf2/small Maf heterodimer mediates the induction of phase II
detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan JY and Kwong M: Impaired expression
of glutathione synthetic enzyme genes in mice with targeteddeletion
of the Nrf2 basic-leucine zipper protein. Biochim Biophys Acta.
1517:19–26. 2000. View Article : Google Scholar : PubMed/NCBI
|