Introduction
Postoperative cognitive dysfunction (POCD) refers to
a decline in cognitive function following surgery, characterized by
an impairment of memory, concentration and comprehension, and a
decreased ability to process information (1,2). The
underlying molecular mechanism of POCD has not been fully
identified; however, accumulating hypotheses have been proposed
based on experimental evidence indicating that anesthetics and
surgery may act as potential causes for POCD (3). As neuroinflammation has been
demonstrated to be associated with cognitive defects in many
central nervous system (CNS) diseases, it may also play a crucial
role in POCD (4). It has been
hypothesized that anesthetics lead to neuroinflammation through
upregulation of the release of pro-inflammatory factors, such as
tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β)
(5,6). Furthermore, as surgery has been
demonstrated to cause a profound systemic inflammatory response, in
an approximate association with the magnitude of tissue damage, it
may also act as a key factor in POCD (7).
Peripheric pro-inflammatory cytokines may enter the
CNS through deficient or damaged areas of the blood-brain barrier
(BBB) and subsequently trigger a series of neuroinflammation over
the entire surface of the brain (8). As resident immunocytes, microglia
rapidly alter the expression of various molecules in response to
changes in the brain, to maintain the balance of the internal
environment. Once activated, microglia highly express masses of
receptors, subsequently initiating various signaling pathways and
eventually releasing numerous pro-inflammtory factors (9). Toll-like receptor 4 (TLR4), a type of
pattern recognition receptor (PRR) mainly expressed on microglia,
may be activated by numerous endogenous and exogenous factors, and
is involved in innate and adaptive immunity by producing numerous
cytokines and pro-inflammatory factors via myeloid differentiation
factor 88 (MyD88)-dependent and MyD88-independent pathways
(10,11). As accumulating evidence has
demonstrated that the activation of TLR4 leads to
neuroinflammation, we hypothesized that TLR4 may contribute to POCD
through the induction of neuroinflammation.
In the present study, to evaluate whether TLR4 was
associated with POCD, we investigated the cognitive functions of
aged rats following surgery, as well as the activation of microglia
and their expression of TLR4. We also examined certain key
downstream factors and compared their changes before and after
surgery.
Materials and methods
Animals and groups
Sixty Sprague-Dawley female rats (22–23 months old,
weighing 450–550 g) were purchased from the Dongchuang Laboratory
Animal Centre (Hunan, China) and fed separately in a light-,
temperature- and humidity-controlled environment with free access
to food and water.
Experiments were conducted in accordance with the
guidelines for care and use of laboratory animals of the Ethics
Committee of Central South University (Hunan, China). The rats were
randomly assigned to four groups. In the control group (group C;
n=15), the rats did not undergo surgery. The remaining 45 rats were
divided into 3 parallel operation groups (groups S1, S3 and S7;
n=15, respectively). All the rats were trained in the Morris water
maze (MWM) for 6 days. Subsequently, rats in the operation groups
underwent splenectomy under isoflurane anesthesia, and were tested
by the MWM again on postoperative days 1, 3 and 7,
respectively.
Tissue treatment
The experimental animals were sacrificed under
anesthesia, following the spatial working tests. Hippocampal
tissues of 8 rats in each group were quickly separated and stored
in liquid nitrogen prior to use. The remaining animals in each
group were perfused transcardially with cold physiological saline
followed by cold 4% paraformaldehyde solution in 0.1 M PBS. Then,
the brain tissue was dissected and stored in 1% sodium azide in
double distilled water (DDW), and subsequently embedded in optimum
cutting temperature (OCT) tissue freezing medium and sectioned for
immunofluorescence.
MWM
The rats were trained with the platform in a fixed
location for three trials per day, for six consecutive days. The
subjects were placed on the platform 30 sec prior to the start of
each trial, and released into the water from one of three randomly
assigned release points (NW, SW and NE). The platform was located
in the SE. In each trial, one rat was allowed to swim until it
landed on the platform. If a rat failed to find the platform within
60 sec, it was picked up and placed on the platform for 15 sec. The
animal was maintained on the platform for 30 sec between trials.
The rats in groups S1, S3 and S7 underwent surgery on the seventh
day. On postoperative days 1, 3 and 7, rats were subjected to a
reversal test, in which the platform was relocated to the opposite
quadrant of the pool. The reversal learning revealed whether the
animals were able to extinguish their initial learning of the
platform’s position and acquire a direct path to the new goal
position. Swimming distance, speed and latency to the platform were
recorded by video tracking mounted on the ceiling, and digital
images were analyzed by water maze software (Smart Junior Software,
Panlab, Barcelona, Spain).
Immunofluorescence
Sections were blocked in 3% normal goat serum at
room temperature for 1 h, and then incubated with rabbit anti-rat
TLR4 antibody (1:100; Abbiotec, LLC, San Diego, CA, USA) antibody
at 4°C overnight. Following washing wih PBS, sections were
incubated with biotinylated goat anti-rabbit (TLR4) or horse
anti-rabbit (ox-42) IgG secondary antibody at 37°C for 2 h,
followed by reaction with red fluorescein for 2 h after washing.
Prior to blocking in 3% normal horse serum at room temperature for
1 h again, washing was carried out. Incubation with mouse anti-rat
ox-42 antibody (1:50; Millipore, Billerica, MA, USA) was carried
out at 4°C overnight. Subsequently, biotinylated horse anti-rabbit
IgG secondary antibody and green fluorescein were applied
successively for 2 h for each antibody, and washing was conducted
prior to each step. Sections were observed through a fluorescence
microscope equipped with a digital camera.
Protein extraction and western blot
analysis
Total RNA was extracted from homogenization of 200
mg hippocampus tissue samples using cold RIPA lysis buffer. The
protein concentrations were measured by a bicichoninic acid (BCA)
protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
The samples (20 μg) were loaded and separated by SDS-PAGE and then
electrophoretically transferred to PVDF membranes (Pall
Corporation, Port Washington, NY, USA). The membranes were probed
with various antibodies, including TLR4, MyD88,
TIR-domain-containing adapter-inducing interferon-β (TRIF), TNF-α
and IL-1β, and visualized with peroxidase and an enhanced
chemiluminescence system (Pierce Biotechnology, Inc.). The
concentrations of primary antibodies are as follows: Rabbit
anti-TLR4 (1:500; Abbiotec LLC), rabbit anti-MyD88 (1:1,000),
rabbit anti-TRIF (1:1,000), rabbit anti- IL-1β (1:1000) and rabbit
anti-TNF-α (1:800; Cell Signaling Technology, Inc., Beverly, MA,
USA).
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from homogenization of 200
mg hippocampus tissue samples using TRIzol reagent (Invitrogen,
Life Technologies, Carlsbad, CA, USA) and RT-PCR was then
performed. Briefly, synthesis of the first strand of complementary
DNA was conducted using the RNA PCR kit (AMV) Version 3.0. RT-PCR
was performed at 30°C for 10 min, 42°C for 30 min and terminated by
heating to 99°C for 5 min. TLR4, MyD88, TRIF, TNF-α and IL-1β were
then amplified by PCR. Primer sequences and amplification sizes are
provided in Table I. β-actin was
used as an internal reference to determine the relative expression
levels of mRNA.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Primer (length) | Sequence |
|---|
| TLR4 (523bp) | 5′-3′:
GAAGCCTCGTGCTCCCTGGC
3′-5′: GAAGCCTCGTGCTCCCTGGC |
| MyD88 (163bp) | 5′-3′:
GCCGGAGCTTTTCGACGCCT
3′-5′: GAGCTCGCTGGCGATGGACC |
| TRIF (273bp) | 5′-3′:
CTGATGCTCACCTGCGGCCA
3′-5′: CAGCCGGGCATCCTTGCACT |
| TNF-α (161bp) | 5′-3′:
TGACCCCCATTACTCTGACC
3′-5′: GGCCACTACTTCAGCGTCTC |
| IL-1β (245bp) | 5′-3′:
CTCCATGAGCTTTGTACAAGG
3′-5′: TGCTGATGTACCAGTTGGGG |
Statistical analysis
Statistical analysis was performed using SPSS 17.0
statistical software (SPSS Inc., Chicago, IL, USA). Data from the
RT-PCR and western blot analysis were analyzed by a one-way ANOVA,
in which age and operation were the dependent variables. A one-way
repeated measures ANOVA was used to analyze the training behavioral
parameters. A separate one-way ANOVA was performed to analyze the
effects of surgery on working memory performance during the
reversal testing. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Aged rats showed a transient cognitive
deficit in the early days following surgery
Rats in all groups were trained to search the
platform in the MWM for 6 days prior to surgery. Latency and
distance were applied as two key parameters to evaluate their
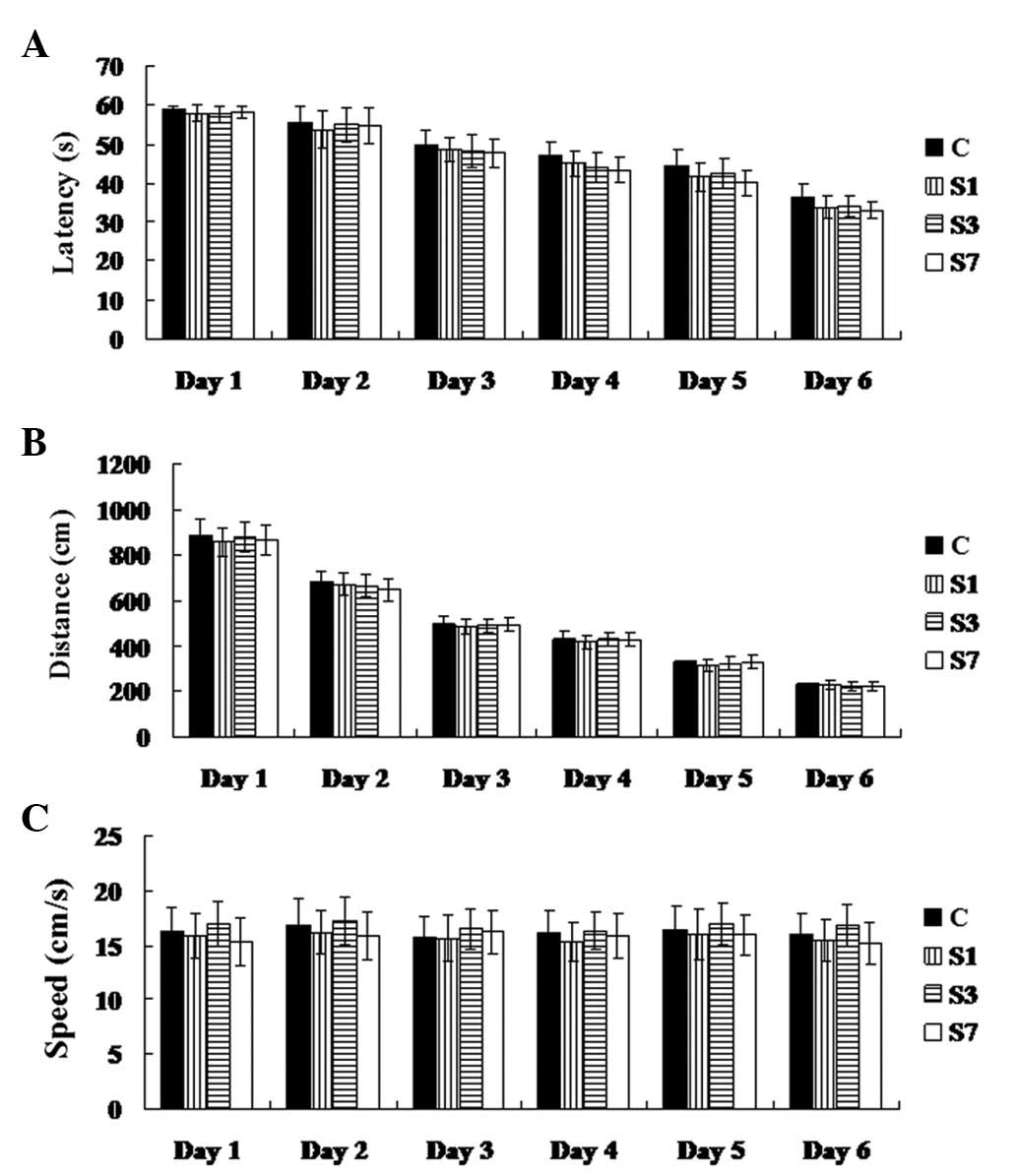
spatial memory ability. As demonstrated in Fig. 1, latency and distance gradually
decreased during the training, suggesting that their spatial memory
was built. On the 6th day following training, almost all rats were
able to rapidly find the platform in the MWM each time. On
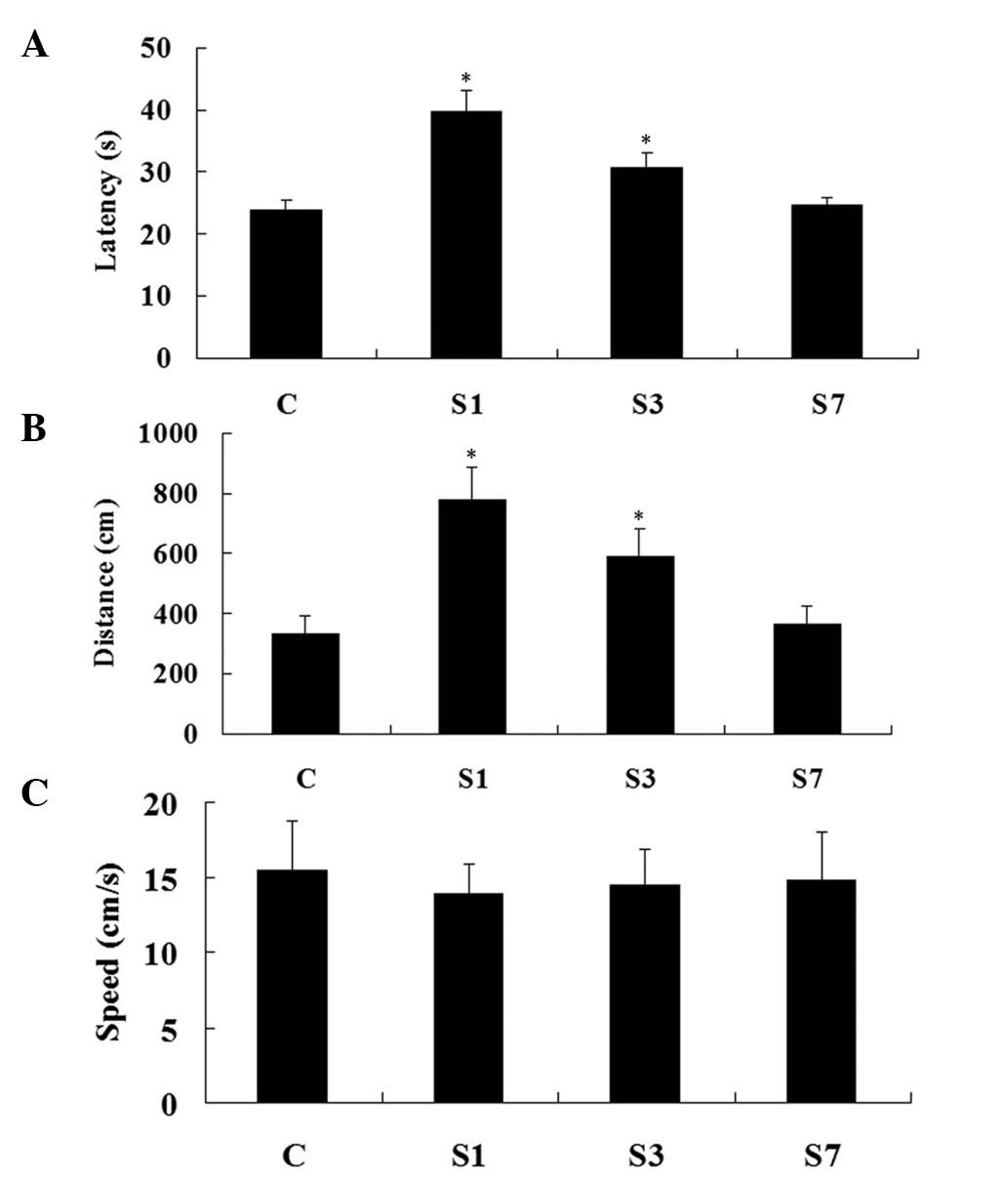
postoperative day 1, the distance and latency in group S1 were
increased compared with group C (P<0.05). On postoperative day
3, although decreased, the distance and latency in group S3
remained higher than those of group C (P<0.05). On postoperative
day 7, these two key parameters returned to normal (compared with
group C, P>0.05). Swimming speeds in these four groups
demonstrated no significant difference (P>0.05) (Fig. 2). Accordingly, these data suggest
that the rats suffered a transient cognitive deficit following
surgery.
Microglia were activated and TLR4 was
upregulated in the hippocampus following surgery
As demonstrated in Fig.
3, surgery led to changes in the morphology of the microglia.
The expanded body and the long branches are characteristic of
activation, indicating that the microglia changed from a resting to
an activated state. With an increase in time following surgery, the
morphology of the microglia gradually returned to normal,
suggesting that the microglia altered from an activated to a
resting state.
TLR4 is mainly expressed on microglia in
the brain
As demonstrated in Fig.
4, on postoperative day 1, the number of TLR4(+) microglia in
group S1 was significantly increased compared with that in the
control group (P<0.05). Subsequently, the expression of TLR4
decreased over time, following surgery. On postoperative day 3, its
expression in group S3 remained higher than that of group C
(P<0.05). In addition, only a few TLR4(+) microglia were
detected in the hippocampus on postoperative day 7, similar to that
of group C (P>0.05). To further verify these findings, we
determined the expression of TLR4 using RT-PCR and western blot
analysis, the results of which showed a similar pattern to those of
the immunofluorescence experiments (Fig. 5). Based on these data, the
expression of TLR4 changed with the behavior, suggesting that TLR4
may be related to the cognitive deficit.
Expression of MyD88 and TRIF in the
hippocampus
MyD88 and TRIF are key downstream components of the
TLR4 signaling pathway. As demonstrated in Fig. 5, on postoperative day 1, the
expression of MyD88 and TRIF were significantly upregulated in the
hippocampus at both the mRNA and protein levels, compared with
those in group C (P<0.05), suggesting that both TLR4 downstream
pathways were activated. Moreover, the expression of MyD88 and TRIF
demonstrated a similar decrease to TLR4 over time, following
surgery.
Release of pro-inflammatory factors TNF-α
and IL-1β in the hippocampus
To investigate the neuroinflammation, we determined
two key pro-inflammatory factors in brain, TNF-α and IL-1β. Data
from the RT-PCR and western blot analysis revealed that the
expression levels of TNF-α and IL-1β were increased in the early
days following surgery, peaking on postoperative day 1 (P<0.05).
Subsequently, their expression levels gradually reduced with an
increase in time following surgery. On postoperative day 7, the
expression of TNF-α returned to normal; however, the expression of
IL-1β remained higher than that of group C (P<0.05) (Fig. 4). According to the data thus far,
until postoperative day 7, neuroinflammation and cognitive deficit
had disappeared.
Discussion
POCD is common in elderly patients. Its high
morbidity is attributed to multiple reasons, including
neuroinflammation (12).
Accumulating evidence supports the theory that non-infectious
neuroinflammation is associated with POCD (7). As a type of resident immune cell,
microglia stabilize the microenvironment in the brain. Any changes
disturbing homeostasis in the brain lead to the activation of
microglia, such as invading pathogens or brain injury (13). Activated microglia protect neurons
from damage by phagocytosing pathogens or synthesizing and
secreting pro-inflammatory factors (14). However, once the microglia are
over-activated, excessive pro-inflammatory factors are produced and
released, which leads to an excessive neuroinflammatory response
and is harmful to neurons (15).
It has been universally accepted that drugs, as well as stress from
surgery, are able to disrupt homeostasis of the whole body,
including the brain, which leads to systematic inflammation
(1,16). Peripheric pro-inflammatory factors
are capable of entering BBB-deficient areas and activating the
microglia, which leads to the activation of numerous receptors and
thus various downstream signaling pathways (8,11).
TLR4 is a type of PRR mainly expressed on the
membrane of microglia, responsible for exogenous ligands, such as
lipopolysaccharide (LPS), and endogenous ligands, such as HMGB1 and
heat-shock protein 70 (HSP 70) (17,18).
When peripheric pro-inflammatory factors enter the brain, some of
these may activate TLR4 signaling pathways (19). It has been demonstrated that TLR4
signaling pathways include two branches; the MyD88-dependent and
TRIF-dependent pathways (20). The
activation of these two pathways induces the phosphorylation of
IκBs and their subsequent degradation, which then promotes the
nuclear translocation of the transcription factor nuclear factor κB
(NF-κB) (21). Once NF-κB enters
into the nucleus, it promotes the transcription of various target
genes, including certain important pro-inflammatory cytokines, such
as IL-1β and TNF-α (21).
At present, whether TLR4 protects or damages the
neurons remains unclear. Although some evidence suggests that TLR
signaling mediates beneficial effects in the CNS, it has also been
demonstrated that TLR-induced activation of microglia and the
release of pro-inflammatory molecules are responsible for
neurotoxic processes in various CNS diseases (8,22,23).
Moreover, numerous studies on CNS diseases, cerebral ischemia and
injury have suggested that expression of TLR4 is associated with
neurodegeneration (13,24,25).
As a result, TLR4-activated microglia may injure the neurons by
releasing excessive cytokines, leading to memory and learning
deficits. In addition, inflammation has been confirmed to
contribute to neurodegeneration at the late stage of Alzheimer’s
disease (AD); however, it may also play a crucial role in the early
stage. Recently, POCD has been regarded as the preliminary state of
AD, due to their similarity in clinical symptoms and pathological
changes (4,26). Therefore, a common or similar
mechanism may exist between them.
As demonstrated in this study, shortly following
surgery, the aged rats showed transient defects in memory and
learning; simultaneously, the expression of TLR4 was significantly
upregulated. The distinct cognitive deficit was evident on
postoperative day 1, as was the peak in expression of TLR4, IL-1β
and TNF-α. Subsequently, this cognitive deficit gradually recovered
over time, accompanied by the expression of TLR4 and
pro-inflammatory cytokines returning to normal. In this study, the
neuroinflammation in the aged brain following surgery was
consistent with that of previous studies, and was likely to be due
to the increased expression of TLR4.
POCD is a global problem, which is becoming worse
along with the increased life-expectancy of society (27). Therefore, it is important, for
humans, to identify the molecular mechanism of POCD. However, few
effective precautions and therapeutic strategies have been well
established. Our findings suggest that the activation of TLR4
contributed to postoperative neuroinflammation in the aged brain by
activating MyD88- and TRIF-dependent signaling pathways, and
ultimately leading to POCD. In summary, the present study
identified the crucial role of TLR4 in POCD, and indicated that
TLR4 may become a novel target for taking precautions and for
therapy.
Acknowledgements
This study is supported by a grant from the National
Natural Science Foundation of China (30871306), the 125 Program of
The third-Xiangya Hospital, Central South University and the
Science-Technology Foundation of Hunan Province, China
(2010sk3111).
References
|
1
|
Coburn M, Fahlenkamp A, Zoremba N and
Schaelte G: Postoperative cognitive dysfunction: Incidence and
prophylaxis. Anaesthesist. 59:177–184; quiz 185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deiner S and Silverstein JH: Postoperative
delirium and cognitive dysfunction. Br J Anaesth. 103(Suppl 1):
i41–i46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seitz DP, Shah PS, Herrmann N, Beyene J
and Siddiqui N: Exposure to general anesthesia and risk of
Alzheimer’s disease: a systematic review and meta-analysis. BMC
Geriatr. 11:832011.
|
|
4
|
Hu Z, Ou Y, Duan K and Jiang X:
Inflammation: a bridge between postoperative cognitive dysfunction
and Alzheimer’s disease. Med Hypotheses. 74:722–724.
2010.PubMed/NCBI
|
|
5
|
Lin D, Cao L, Wang Z, Li J, Washington JM
and Zuo Z: Lidocaine attenuates cognitive impairment after
isoflurane anesthesia in old rats. Behav Brain Res. 228:319–327.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cohendy R, Brougere A and Cuvillon P:
Anaesthesia in the older patient. Curr Opin Clin Nutr Metab Care.
8:17–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosczyk HA, Sparkman NL and Johnson RW:
Neuroinflammation and cognitive function in aged mice following
minor surgery. Exp Gerontol. 43:840–846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teeling JL and Perry VH: Systemic
infection and inflammation in acute CNS injury and chronic
neurodegeneration: underlying mechanisms. Neuroscience.
158:1062–1073. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glezer I, Simard AR and Rivest S:
Neuroprotective role of the innate immune system by microglia.
Neuroscience. 147:867–883. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crack PJ and Bray PJ: Toll-like receptors
in the brain and their potential roles in neuropathology. Immunol
Cell Biol. 85:476–480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okun E, Griffioen KJ, Lathia JD, Tang SC,
Mattson MP and Arumugam TV: Toll-like receptors in
neurodegeneration. Brain Res Rev. 59:278–292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haseneder R, Kochs E and Jungwirth B:
Postoperative cognitive dysfunction. Possible neuronal mechanisms
and practical consequences for clinical routine. Anaesthesist.
61:437–443. 2012.(In German).
|
|
13
|
Shao J, Liu T, Xie QR, et al: Adjudin
attenuates lipopolysaccharide (LPS)-and ischemia-induced microglial
activation. J Neuroimmunol. 254:83–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kamer AR, Galoyan SM, Haile M, et al:
Meloxicam improves object recognition memory and modulates glial
activation after splenectomy in mice. Eur J Anaesthesiol.
29:332–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai ZY, Yan Y and Chen R: Minocycline
reduces astrocytic reactivation and neuroinflammation in the
hippocampus of a vascular cognitive impairment rat model. Neurosci
Bull. 26:28–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moller JT, Cluitmans P, Rasmussen LS, et
al: Long-term postoperative cognitive dysfunction in the elderly
ISPOCD1 study. ISPOCD investigators International Study of
Post-Operative Cognitive Dysfunction. Lancet. 351:857–861. 1998.
View Article : Google Scholar
|
|
17
|
Zhang Z, Zhang ZY, Wu Y and Schluesener
HJ: Immunolocalization of Toll-like receptors 2 and 4 as well as
their endogenous ligand, heat shock protein 70, in rat traumatic
brain injury. Neuroimmunomodulation. 19:10–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krynetskaia NF, Phadke MS, Jadhav SH and
Krynetskiy EY: Chromatin-associated proteins HMGB1/2 and PDIA3
trigger cellular response to chemotherapy-induced DNA damage. Mol
Cancer Ther. 8:864–872. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng Z, Yuan R, Song M, et al: The
toll-like receptor 4-mediated signaling pathway is activated
following optic nerve injury in mice. Brain Res. Oct 24–2012.(Epub
ahead of print). View Article : Google Scholar
|
|
20
|
Brown J, Wang H, Hajishengallis GN and
Martin M: TLR-signaling networks: an integration of adaptor
molecules, kinases, and cross-talk. J Dent Res. 90:417–427. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Xu H and Sun B: Lipopolysaccharide
regulates MMP-9 expression through TLR4/NF-κB signaling in human
arterial smooth muscle cells. Mol Med Report. 6:774–778.
2012.PubMed/NCBI
|
|
22
|
Perry VH, Nicoll JA and Holmes C:
Microglia in neurodegenerative disease. Nat Rev Neurol. 6:193–201.
2010. View Article : Google Scholar
|
|
23
|
Song M, Jin J, Lim JE, et al: TLR4
mutation reduces microglial activation, increases Abeta deposits
and exacerbates cognitive deficits in a mouse model of Alzheimer’s
disease. J Neuroinflammation. 8:922011.PubMed/NCBI
|
|
24
|
Hua F, Ma J, Ha T, et al: Activation of
Toll-like receptor 4 signaling contributes to hippocampal neuronal
death following global cerebral ischemia/reperfusion. J
Neuroimmunol. 190:101–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pascual M, Balino P, Alfonso-Loeches S,
Aragon CM and Guerri C: Impact of TLR4 on behavioral and cognitive
dysfunctions associated with alcohol-induced neuroinflammatory
damage. Brain Behav Immun. 25(Suppl 1): S80–S91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie Z and Tanzi RE: Alzheimer’s disease
and post-operative cognitive dysfunction. Exp Gerontol. 41:346–359.
2006.
|
|
27
|
Hartholt KA, van der Cammen TJ and Klimek
M: Postoperative cognitive dysfunction in geriatric patients. Z
Gerontol Geriatr. 45:411–416. 2012. View Article : Google Scholar : PubMed/NCBI
|