Introduction
Opioids are known to be potent drugs used for acute
and chronic pain, although some effects limit their use, including
respiratory depression and possibly dependence. Non-steroidal
anti-inflammatory drugs (NSAIDs) are also not viable options due to
their weak response and more adverse effects such as
gastrointestinal disturbances and renal damage. Therefore, the
identification of analgesic agents that cause no side-effects and
retain opioid-like potency is required. There are several ways to
identify new targets for this purpose. Previous studies have shown
the involvement of the GABAergic system in the modulation of pain
at the supraspinal and spinal level (1,2).
Oxysophocarpine (OSC) is an alkaloid extracted from
Siphocampylus verticillatus (Campanulaceae) (3). Findings of previous studies showed
that the contents extracted from Siphocampylus verticillatus
have certain pharmacological effects. Rodrigues et
al(4) demonstrated that OSC
has a significant antidepressant-like effect following assessment
using the tail suspension and forced swimming tests in mice.
Trentin et al(5)
demonstrated that the hydroalcoholic extract of Siphocampylus
verticillatus causes long-lasting anti-nociception when
assessed against neurogenic and inflammatory models of nociception
in mice. Findings of a pharmacological and clinical study
demonstrated that sophocarpine has pain-relieving effects (6). Oxysophocarpine and sophocarpine have
a similar molecular structure (7).
We hypothesized that OSC is an anti-nociceptive drug with greater
efficacy, lower toxicity and fewer side-effects. To test this
hypothesis, thermal- and chemical-based behavioral models of
nociception were used and different routes of administration were
assessed to investigate the analgesic effects of OSC and to
determine the primary analgesic sites of OSC in mice. Additionally,
immunohistochemistry was used to investigate whether the
anti-nonciceptive effects of OSC are associated with the expression
of GABAA receptors in the cerebral cortex and the
hippocampus in ICR mice.
Materials and methods
Animals
Institute of Cancer Research (ICR) mice (18–22 g)
were provided by the Experimental Animal Center of Ningxia Medical
University (Yinchuan, China) (certificate no. SYXK Ningxia
2005-0001). Mice were housed at 22±2°C and 50±5% relative humidity
under a 12-h light/dark cycle, and they had access to food and
water ad libitum. The experiments were performed during the
light phase. Mice were acclimatized to the laboratory for ≥1 h
prior to testing. For the test each mouse was used only once. The
mice were randomly divided into the negative control (saline
group), positive control (morphine, aspirin) and different OSC dose
groups (n=10/group).
The drugs were injected intraperitoneally (i.p.) or
intracerebroventricularly (i.c.v.), with the exception of aspirin,
which was administered intragastrically (i.g.). The experiments
were performed in accordance with the Guidelines for the Care of
Laboratory Animals of Ningxia Medical University.
Drugs
OSC was supplied by the Zi Jin Hua Pharmaceutical
Co., (Ningxia University, Ningxia, China) (lot no. 071218, purity
>98%), which was dissolved in 0.9% (w/v) NaCl solution. Morphine
was obtained from the Shenyang Phamaceutical Co. (Shenyang, China),
and aspirin from the Yongning Phamaceutical Factory (Yinchuan,
China); both were dissolved in normal saline. The drugs were
administered i.p. in a volume of 0.1 ml/10 g, with the exception of
aspirin, which was administered i.g. in a volume of 0.2 ml/10 g.
Intracerebroventricular administration was performed in a volume of
10 μl for each mouse. The drug solutions were prepared immediately
prior to initiation of the experiment. The doses of OSC (10, 40 and
80 mg/kg) were selected based on the results of the preliminary
experiments. A single dose of aspirin (400 mg/kg) was chosen
according to a previous study conducted in our laboratory (400
mg/kg) in the formalin model (8),
and various doses of morphine (5, 20, 40 and 50 mg/kg) were chosen
according to previous studies (7,9).
Warm water tail-flick test
The warm water tail-flick test is a common method
used to immobilize the animal in a restrainer with its tail
extending through the hole. The lower 2/3 of the tail was immersed
in hot water maintained at a constant temperature of 50±0.5°C.
Changes in nociception were determined by the changes in the
latency between the tail immersion and withdrawal from the hot
water bath (10). The animals were
immobilized in the tube briefly (25–30 sec) during the tail-flick
measurements. To minimize tissue injury caused by exposure to heat
stimulus, a cut-off time of 15 sec was applied. Pretreatment
latencies were determined twice with an interval of 10 min prior to
drug administration to obtain a stable pre-drug response (baseline
withdrawal latency). Mice with a basal tail-flick latency of 2–5
sec were used. Animals with a significantly different baseline
value from that of the control mice were not included in the study.
The reaction time was recorded for OSC (10, 40 and 80 mg/kg) or
morphine (40 mg/kg). Control animals were administered a similar
volume of 0.9% NaCl solution (10 ml/kg). Following drug
administration, tail immersion latency was measured at 15, 30, 60,
90 and 120 min post-injection. The tail immersion response was
expressed as a percentage of basal latency.
Percentage analgesia was calculated using the
formula: analgesia (%) = [(post-drug tail withdrawal latency −
pre-drug tail withdrawal latency)/(15 sec - pre-drug tail
withdrawal latency)] ×100.
Hot-plate test
The hot-plate test is used in basic pain research
and in the assessment of the effectiveness of analgesics by
observing the reaction to pain caused by heat. This test was
proposed by Eddy and Leimbach (11) and used in this study, with minor
modifications. Particularly, the mice were placed in glass funnels,
on a heated-face (55±0.5°C) and the time between placing the
animals and the beginning of paw-licking and jumping was evaluated
as the endpoint. Baseline was measured for 15 mins prior to drug
administration, and only those animals with a latency of 5–30 sec
were used for further investigation. The reaction time was recorded
in mice pretreated with OSC (10, 40 and 80 mg/kg) or morphine (20
mg/kg). Control mice were administered a similar volume of normal
saline (10 ml/kg). A lethal intravenous dose of OSC
(LD50) was 250.37 mg/kg in mice (12). The time of latency was determined
at 15, 30, 60, 90 and 120 min post-injection. A latency period
(cut-off) of 60 sec was evaluated as complete analgesia.
Percentage analgesia was calculated using the
formula: analgesia (%) = [(post-drug latency − pre-drug
latency)/(60 sec - pre-drug latency)] ×100.
Acetic acid-induced abdominal
constriction
Abdominal constriction was induced by i.p. injection
of acetic acid (0.6%), which consisted of abdominal muscle
constriction, together with stretching of hind limbs, and was
performed according to previously described methods (13). The animals were pretreated with OSC
(10, 40 and 80 mg/kg), morphine (50 mg/kg) or aspirin (400 mg/kg).
Control animals were administered a similar volume of 0.9% NaCl
solution (10 ml/kg). The drugs were administered 60 min prior to
acetic acid injection. After the injection, a pair of mice was
placed in separate boxes and the number of abdominal constrictions
was cumulatively counted during a period of 15 min following acetic
acid injection. Anti-nociceptive activity was expressed as the
reduction in the number of abdominal constrictions.
Formalin-induced pain test
This procedure was performed according to previously
described methods (14–16). Twenty microliters of 0.2% formalin
solution (0.92% formaldehyde) dissolved in saline solution were
injected under the paw surface of the right hind paw. The amount of
time spent licking the injected paw was considered to be indicative
of pain. The initial nociceptive time usually peaked 0–5 min (first
phase) and 10–60 min (second phase) after the formalin injection.
These peaks represented the tonic and inflammatory pain response,
respectively. The animals were treated with OSC (10, 40 and 80
mg/kg), morphine (5 mg/kg), or aspirin (400 mg/kg). Control animals
were administered a similar volume of 0.9% NaCl solution (10
ml/kg). The drugs were administered 60 min prior to formalin
injection. Following intraplantar injection of formalin, the
animals were immediately placed in a glass cylinder with a diameter
of 20 cm. The time spent licking the injected paw was considered to
be indicative of pain and was timed using a chronometer.
Intracerebroventricular injections
Animals were administered a unilateral
intracerebroventricular injection (2.0 mm caudal and 2.0 mm lateral
with respect to bregma and −2.5 mm ventral from the skull surface)
(17). OSC (0.25–4 mg/kg) and
morphine (2 mg/kg) were dissolved in saline (0.9% NaCl), in a
volume of 10 μl/mouse (for ~10 sec). Control mice were administered
the same volume of vehicle. Following the intracerebroventricular
injection, the animals were tested using the tail immersion test
described above.
Immunohistochemistry
Mice were treated i.p. with 80 mg/kg of OSC, and the
control mice were administered a similar volume of 0.9% NaCl
solution (10 ml/kg). The drugs were administered 45 min prior to
perfusion. Animals were deeply anesthetized i.p. in a volume of 0.1
ml/10 g pentobarbital sodium (1%) and were perfused with 40 ml of
saline for 2 min, followed by 100 ml of 4% paraformaldehyde (Sigma,
St. Louis, MO, USA) at 4°C for 20 min [0.01 mol/l
phosphate-buffered saline (PBS), pH 7.4]. Following fixation, the
brain was removed and post-fixed in the same fixation solution for
48 h. For immunohistochemical analysis, brain sections were mounted
onto slides, air-dried, dehydrated in alcohol (differentiation time
was evaluated under the microscope), cleared in xylenes and cover
slipped.
Brain sections (5 μm) were treated with 3%
H2O2 to quench endogenous peroxidase
activity, then washed in PBS 3×5 min, and transferred to 0.1 M
citrate buffer in 5% normal bovine serum albumin (BSA) for 20 min
at room temperature. The sections were then incubated with
GABAARα1 receptor primary antibody at 4°C for 24 h,
which was an anti-rabbit polyclonal antiserum (1:100 in PBS; Santa
Cruz Biotechnology Inc., Santa Cruz, CA, USA). As a negative
control, additional sections were treated in a similar manner
although the primary antibody was omitted. Subsequent to incubation
with primary antibody, sections were washed in PBS 3×5 min. The
sections were incubated at 37°C for 2 h with the secondary antibody
(rabbit anti-goat IgG) and avidin-biotin complex (ABC). The
specimens were then washed as described above and visualized using
3,3′-diaminobenzidine (DAB; Wuhan Boster Biological Technology,
Ltd., Wuhan, China).
Image analysis and counting
Following immunostaining, brain specimens were
thoroughly rinsed in water and then examined under a light
microscope. Digital images of GABAAα1 receptor neurons
were captured using an Olympus BH-2 microscope (Olympus Optical
Co., Ltd., Tokyo, Japan), with an attached digital microscope
camera and a personal computer. Cells stained positive for
GABAAα1 receptors were marked on a computer screen
(magnification, ×400) and counted in every two random visual
fields/well, in the cerebral cortex and hippocampus of the brain
specimens. Up to four stained sections/mouse were obtained from the
segment and analyzed using the Motic Images Advanced 3.2 software
developed by Motic China Group Co., Ltd. (Xiamen, China). The
number of stained neurons/section were calculated [means ± standard
deviation (SD); one-way ANOVA]. P<0.05 was considered to
indicate statistically significant intergroup differences.
Statistical analysis
The data are presented as the means ± SD, except the
mean ED50 values, which were reported as geometric means
accompanied by their respective 95% confidence limits. Data were
analyzed using one-way analysis of variance (ANOVA) or t-test, with
between-group comparisons for drug treatment and within-group
comparisons for time. P<0.05 was considered to indicate a
statistically significant difference.
Results
Warm water tail-flick test
An injection of 80 mg/kg OSC (i.p.)increased the
tail-curling latency in the warm water tail-flick test and a
maximal inhibition of 25.46% was observed. This effect persisted
for up to 120 min. Under similar conditions, morphine 40 mg/kg
(i.p.) caused a significant increase (the maximal inhibition was
85.05% and persisted for ≥120 min) in the latency in the tail
immersion assay (Fig. 1).
Hot-plate test
Compared with the control group, i.p. injection of
OSC at doses of 40 and 80 mg/kg or morphine at a dose of 20 mg/kg
caused a significant increase in the response latency in the
hot-plate test (14.57±4.81 sec in the control group, 24.20±12.67
sec in the OSC-treated groups, and 60.00 sec in the
morphine-treated group). The analgesic effect persisted for ≥120
min post-injection (Fig. 2).
Acetic acid-induced abdominal
constriction
OSC administered i.p. at 10, 40 and 80 mg/kg 15 min
prior to the test, causing a significant dose-dependent inhibition
of acetic acid-induced abdominal constriction, with a maximal
inhibition of 47.02% at 80 mg/kg. Morphine (50 mg/kg) and aspirin
(400 mg/kg) induced an anti-nociceptive response with a maximal
inhibition of 99.56 and 93.38%, respectively (Fig. 3).
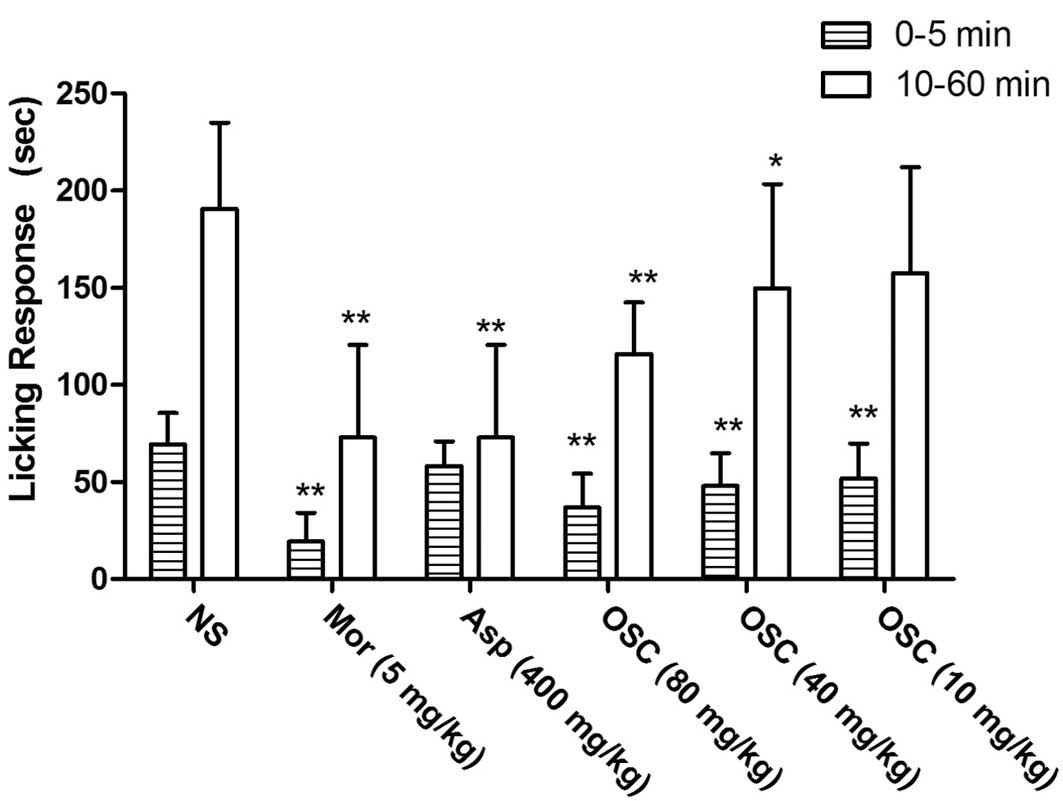
Formalin-induced pain test
In the formalin test, pretreatment (60 min) with
i.p. injection of different doses of OSC (10, 40 and 80 mg/kg) or
morphine (5 mg/kg) showed a significant dose-dependent inhibition
in the early (0–5 min) and late phases (10–60 min) of
formalin-induced licking (Fig. 4),
while aspirin caused inhibition only in the late phase. The results
showed that OSC was effective in the two phases of the test,
although it was more effective in the late phase. Thus, the effects
of OSC treatment were similar to those of morphine treatment.
Effect of i.c.v. treatment with OSC in
mice when assessed using the warm water tail-flick test
Compared with the controls or pre-test group, mice
were treated with OSC (0.25, 1 and 4 mg/kg, i.c.v., which was 1/10
of the dose of i.p. injection), which significantly increased the
tail-curling latencies with a maximal inhibition of 34.91% at 4
mg/kg in the warm water tail immersion test. The effect induced by
OSC treatment persisted for 90 min post-injection (Fig. 5)
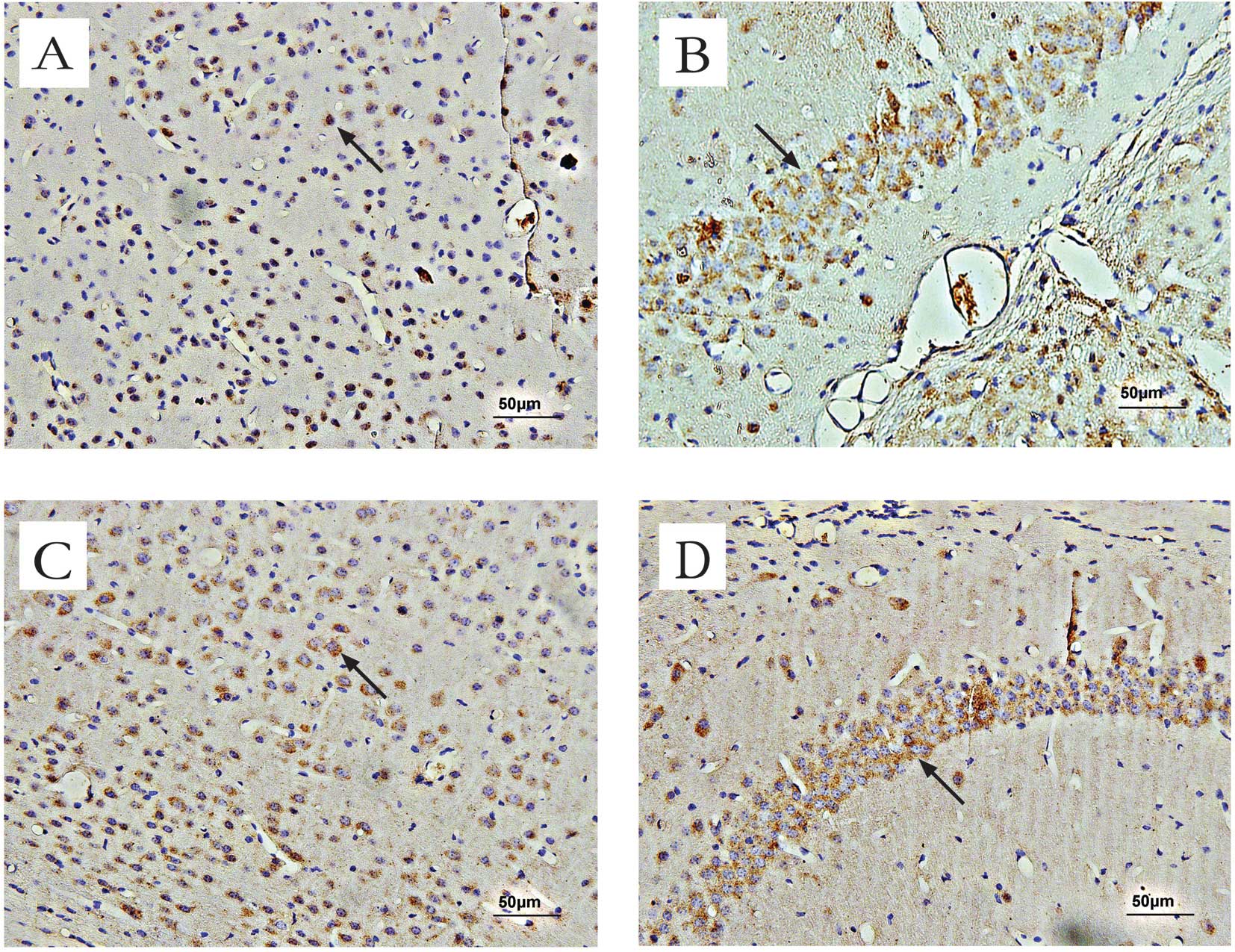
Effect of OSC treatment on
GABAAα1 receptor expression in mice
Immunohistochemical methods were used to assess the
expression and localization of deposited GABAAα1
receptors in mice. Positive staining for GABAAα1
receptors was brown-yellow. In the cerebral cortex and the
hippocampus of normal mice, moderate GABAAα1 receptor
immunoreactivity was observed in the cell membranes and neuronal
axons (Fig. 6). Average counts of
cells in the cerebral cortex and the hippocampal regions of mice
stained positive for GABAAα1 receptors are provided in
Table I.
 | Table IAverage counts of cells of each
region of interest in mice stained positive for GABAAα1
receptors. |
Table I
Average counts of cells of each
region of interest in mice stained positive for GABAAα1
receptors.
| Group | Cerebral
cortex | Hippocampus |
|---|
| Control | 85±13 | 87±13 |
| OSC-treated | 171±58a | 169±49a |
Discussion
The present study showed that i.p. treatment with
OSC induces a dose-dependent and significant anti-nociception in
models of chemical nociception, i.e., acetic acid-induced abdominal
constriction and formalin-induced licking response. Moreover, it
was found that OSC induces significant anti-nociceptive effects in
mice when assessed using the warm water tail-flick and hot-plate
tests.
The warm water tail-flick test is a model of
nociceptive pain produced via thermal stimuli of short duration
(i.e., phasic pain), which is commonly used to measure responses to
somatic stimulation and observe whether the drug has a central
anti-nociceptive effect, since the tail-flick is mainly a reflex to
the spinal cord. This test measures changes in the pain threshold
that produces a tail-flick response. The response is mainly a
spinal reflex, which is modulated by supraspinal mechanisms
(18). The results of the effect
on the warm water tail-flick test increased the response latency
time by 25.46%. The result was less active compared to that caused
by morphine in the same test, while the effectiveness of OSC in the
warm water tail-flick test showed that OSC acts on the central
nervous system, particularly on the spinal cord, which was
modulated by supraspinal mechanisms. However, further investigation
is needed to fully elucidate the mechanism of action.
The hot-plate test is a common sensorimotor task
that identifies drugs with a supraspinal analgesic effect, such as
opioid-derived analgesics (19).
In other words, analgesic agents play a primary role in the spinal
medulla and/or higher central nervous system levels or by an
indirect mechanism (20).
Therefore, analgesic drugs, which were known to be selectively
effective against painful thermal stimuli, act on the central
nervous system rather than the peripheral system (21). The present study demonstrated that
OSC (80 mg/kg, i.p.) was effective in the hot-plate test, where the
response latency time was increased by 26.60%. The effectiveness of
OSC in the hot-plate test showed that OSC acts on the central
nervous system, which was in agreement with the results obtained by
the warm water tail-flick test.
The anti-nociceptive effect of OSC is associated
with supraspinal components as shown by the warm water tail-flick
and the hot-plate tests, respectively. The results of these tests
indicated that OSC has a central anti-nociceptive effect as
demonstrated by the increased response time of the mice in the
hot-plate test, and also by the prolonged delay in reaction when
mice were subjected to a nociceptive stimulus during the warm water
tail-flick test.
Intracerebroventricular administration is a common
method to determine whether a drug has a central analgesic effect.
The mechanism by which OSC produces systemic, spinal or supraspinal
anti-nociception in mice has yet to be fully elucidated.
Nevertheless, OSC (1 and 4 mg/kg, i.c.v.; 1/10 of the dose used in
i.p. administration) produced anti-nociceptive effects in mice when
assessed using the warm water tail-flick test. These results
suggest that the anti-nociception effects of OSC occur through a
central mechanism.
The formalin-induced pain test is a valid and
reliable model of nociception and is sensitive to various classes
of analgesic drugs. It assesses the way an animal responds to
moderate, continuous, long-lasting pain generated by injured tissue
(20). The formalin-induced pain
test produced a distinct biphasic response, these two different
phases have different mechanisms of action in the test. The first
phase reflected direct chemical stimulation, which appeared to be
predominantly caused by the nociceptive afferent fibers, mainly
C-fibers, and the release of substance P (22). This phase may be inhibited by
centrally acting analgesic drugs. The second phase may be
associated with the release of inflammatory mediators locally, such
as prostaglandins, serotonin, histamine and bradykinin, and with
enhanced synaptic transmission in the spinal cord neurons (23). Therefore, this test could be used
to clarify the potential mechanism of a proposed analgesic. Drugs,
such as morphine, act primarily on the central nervous system and
inhibit the first and second phases equally, while peripherally
acting drugs such as aspirin inhibit only the second phase
(24). The i.p. pre-administration
of OSC (40 and 80 mg/kg) exerted dose-dependent and significant
anti-nociceptive effects when assessed against neurogenic pain
(first phase) and inflammatory pain (late phase) caused by
intraplantar injection of formalin in mice. Furthermore, the
results of this test were in agreement with those obtained in
thermal behavioral models of nociception, whereas OSC was also
demonstrated to have central anti-nociceptive effects.
The acetic acid-abdominal constriction test is
generally used for evaluating peripheral analgesic activity
(25,26). Previous studies have shown that
acetic acid acts indirectly by inducing the release of endogenous
mediators that stimulate the nociceptive neurons sensitive to
opioids and non-steroidal anti-inflammatory drugs (NSAIDs)
(27). Moreover, abdominal
constriction induced by acetic acid is usually used for screening
synthetic and natural compounds (11). In this behavioral model, an i.p.
injection of OSC (10, 40 and 80 mg/kg) caused a dose-dependent
inhibition of acetic acid-induced abdominal constriction. The
maximum inhibition observed was 47.02%. Based on these results,
this test indicated that OSC treatment has anti-nociceptive effects
on central mechanisms.
γ-aminobutyric acid (GABA) is the major inhibitory
transmitter in the adult mammalian central nervous system and has
been reported as the principle neurotransmitter of the circadian
system. It is also found in >50% of neurons in the central
nervous system (28). GABA neurons
and synapses are widely distributed throughout the peripheral and
central nervous systems, which are primarily located on the outer
layer of the cerebral cortex and the hippocampus (29). Moreover, accumulating evidence
suggest that the GABAergic system is important in the pre-synaptic
inhibition of primary afferents (primary afferent depolarization),
thus sensory transmission, motor activity and modulating
nociception motor activity on pre- and post-synaptic levels
(30).
The present study indicated that the
anti-nociceptive effect of OSC resulted from the activation of the
GABAA receptor. Most of the synaptic inhibitory action
of GABA is mediated by GABAA receptors, which constitute
hetero-oligomeric chloride channels encoded by a family of ≥16
known different subunit genes including 6α, 3β, 3γ, δ, ɛ, θ and π
subunits (31), among which GABA
affinity is mainly regulated by a subunit and plays an important
pharmacological role (32,33). Putative ligands and drugs are known
to interact at one of the major sites associated with the
GABAA receptors and to modulate GABA-gated chloride ion
conductance positively or negatively. The increased chloride
conductance regulates the membrane potential towards the reversal
potential of the chloride ion which inhibits the firing of new
action potentials and initiates inhibitory postsynaptic potential
(IPSPs) in order to produce an anti-nociceptive effect (34,35).
Molecular studies have identified the GABAA receptor as
a macromolecular complex consisting of ≥5 membrane-spanning
subunits. Five subunit types have been described, α, β, γ, δ and ρ
(33). A limited number of
subunits is present in the mammalian central nervous system, mainly
α1β2γ2. Different subunit isoforms (α1–6, β1–4, γ1–4, δ, ɛ, θ) give
rise to a considerable diversity of GABAA receptors
(36,37) that are differentially expressed in
the brain and localized in different cell types and subcellular
areas (38). Subtypes of
GABAA receptors are important in the development of
drugs which selectively influence this transmitter system (39). The differential expression of
GABAA receptor subtypes between the superficial layers
of the dorsal horn and projection neurons may be of particular
relevance within the framework of the ‘gate control’ theory
(40,41), according to which pain perception
has been suggested to be modulated in the substantial gelatinosa
(lamina II), which functions as a gate controlling impulse
transmission from primary afferents to projecting neurons.
Moreover, the α1 subunit acts particularly on the function of
GABAAα1 receptors (42), which are compared with controls in
the cerebral cortex and the hippocampal regions in mice.
In conclusion, OSC induces systemic anti-nociception
in mice when assessed using some classical behavioral tests, such
as thermal stimuli (hot-plate and warm water tail-flick tests) and
chemical stimuli (acetic acid- and formalin-induced pain). The
results obtained in the present study indicate that OSC has
significant analgesic effects that may be crucial in the central
and peripheral nervous systems. This study also showed that OSC
produced possible alterations in the expression of
GABAAα1 receptors in the central nervous system.
However, the mechanism by which OSC induces anti-nociception in the
models of nociception has yet to be fully elucidated.
Investigations on the potential involvement of GABA receptors in
the analgesic action of OSC are currently in progress.
Acknowledgements
The study was supported by the National Natural
Science Foundation of China (grant nos. 30960506 and 81160524), the
Key Scientific Research Projects of Ningxia Health Department
(2012059), the Scientific Research Starting Foundation for Special
Talent (XT2012015) and Ningxia Education Department (NGY2012055).
We are indebted to the staff in the Animal Center and the Science
and Technology Centre for poviding assistance during this study.
The authors would like to thank Dr Dingfeng Su, Professor Wannian
Zhang, Miss Jie Wang, Miss Shujing Wang and Yan Zhang who provided
technical support during the implementation of this study.
References
|
1
|
Millan MJ: Descending control of pain.
Prog Neurobiol. 66:355–474. 2002. View Article : Google Scholar
|
|
2
|
Malcangio M and Bowery NG: GABA and its
receptors in the spinal cord. Trends Pharmacol Sci. 17:457–462.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang JT, Wang W and Duan ZH: Progress of
research and application of matrine-type alkaloids. Prog Mod
Biomed. 7:541–544. 2007.(In Chinese).
|
|
4
|
Rodrigues AL, da Silva GL, Mateussi AS, et
al: Involvement of monoaminergic system in the antidepressant-like
effect of the hydroalcoholic extract of Siphocampylus
verticillatus. Life Sci. 70:1347–1358. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trentin AP, Santos AR, Miguel OG,
Pizzolatn MG, Yunes RA and Calixto JB: Mechanisms involved in the
antinociceptive effect in mice of the hydroalcoholic extract of
Siphocampylus verticillatus. J Pharm Pharmacol. 49:567–572.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li ZHR: The pharmaceutical effect and
clinical development of kushenin. West China J Pharm Sci.
18:435–437. 2003.(In Chinese).
|
|
7
|
Wang XH, Huang SK and Lin P:
Pharmacokinetics and pharmacodynamics of sophocarpine and
oxysophocarpine. J China Pharm Univ. 23:161–164. 1992.(In
Chinese).
|
|
8
|
Yan L and Jiang YX: Studies of analgesia
effect of oxysophoridien. Chin Tradit Herb Drugs. 37:1061–1062.
2006.(In Chinese).
|
|
9
|
Grant GJ, Vermenulen K, Zakowski MI,
Stener M, Turndorf H and Langerman L: Prolonged analgesia and
decreased toxicity with liposomal morphine in a mouse model. Anesth
Analg. 79:706–709. 1994.PubMed/NCBI
|
|
10
|
Goyal R and Anil K: Protective effect of
alprazolam in acute immobilization stress-induced certain
behavioral and biochemical alterations in mice. Pharmacol Rep.
59:284–290. 2007.PubMed/NCBI
|
|
11
|
Eddy NB and Leimbach D: Synthetic
analgesics. II Dithienylbutenyl- and dithienylbutylamines. J
Pharmacol Exp Ther. 107:385–393. 1953.PubMed/NCBI
|
|
12
|
Qian LW, Dai WH and Wang LL: Toxicity
study on sophocarpine and oxysophocarpine in mice. Chin J Exp
Tradit Med Formulae. 18:256–258. 2012.(In Chinese).
|
|
13
|
Dongmo AB, Nguelefack T and
Lacaille-Dubois MA: Antinociceptive and anti-inflammatory
activities of Acacia pennata wild (Mimosaceae). J
Ethnopharmacol. 98:201–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Corrêa CR and Calixto JB: Evidence for
participation of B1 and B2 kinin receptors in formalin-induced
nociceptive response in the mouse. Br J Pharmacol. 110:193–198.
1993.PubMed/NCBI
|
|
15
|
Vaz ZR, Filho VC, Yunes R and Calixto JB:
Antinociceptive action of 2-(4-bromobenzoyl)-3-methyl-4,6-dimethoxy
benzofuran, a novel xanthoxyline derivative on chemical and thermal
models of nociception in mice. J Phamacol Exp Ther. 278:304–312.
1996.PubMed/NCBI
|
|
16
|
Santos AR and Calixto JB: Further evidence
for the involvement of tachykinin receptor subtypes in formalin and
capsaicin models of pain in mice. Neuropeptides. 31:381–389. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu SY, Bian RL and Chen X: Methods of
pharmacological experiments. 3rd ed. The People’s Medical
Publishing House; Beijing: pp. 7022002
|
|
18
|
Le Bars D, Gozariu M and Cadden SW: Animal
models of nociception. Pharmacol Rev. 53:597–652. 2001.
|
|
19
|
Thomsen M, Wörtwein G, Olesen MV, et al:
Involvement of Y(5) receptors in neuropeptide Y agonist-induced
analgesic-like effect in the rat hot plate test. Brain Res.
1155:49–55. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tjølsen A, Berge OG, Hunskaar S, Rosland
JH and Hole K: The formalin test: an evaluation of the method.
Pain. 51:5–17. 1992.
|
|
21
|
Chau T: Pharmacological methods in the
control of inflammation. Modern Methods in Pharmacology. Chang JY
and Lewis AJ: Alan R. Liss; New York: pp. 195–212. 1989
|
|
22
|
Walwyn WM, Matsuka Y, Arai D, et al:
HSV-1-mediated NGF delivery delays nociceptive deficits in a
genetic model of diabetic neuropathy. Exp Neurol. 198:260–270.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rujjanawate C, Kanjanapothi D and Panthony
A: Pharmacological effect and toxicity of alkaloids from
Gelsemium elegans Benth. J Ethnopharmacol. 89:91–95. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hunskaar S and Hole K: The formalin test
in mice: dissociation between inflammatory and non-inflammatory
pain. Pain. 30:103–114. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koster R, Anderson M and De Beer EJ:
Acetic acid for analgesic screening. Fed Proc. 18:412–416.
1959.
|
|
26
|
Hendershot LC and Forsaith J: Antagonism
of the frequency of phenylquinone-induced writhing in the mouse by
weak analgesics and nonanalgesics. J Pharmacol Exp Ther.
125:237–240. 1959.PubMed/NCBI
|
|
27
|
Collier HO, Dinneen LC, Johnson CA and
Schneider C: The abdominal constriction response and its
suppression by analgesic drugs in the mouse. Br J Pharmacol
Chemother. 32:295–310. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martire M, Castaldo P, D’Amico M, Preziosi
P, Annuzito L and Taglialatela M: M channels containing KCNQ2
subunits modulate norepinephrine, aspartate, and GABA release from
hippocampal nerve terminals. J Neurosci. 24:592–597. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lujan R: Subcellular regulation of
metabotropic GABA receptors in the developing cerebellum.
Cerebellum. 6:123–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Condés-Lara M, Rojas-Piloni G,
Martínez-Lorenzana G, López-Hidalgo M and Rodríguez-Jiménez J:
Hypothalamospinal oxytocinergic antinociception is mediated by
GABAergic and opiate neurons that reduce A-delta and C fiber
primary afferent excitation of spinal cord cells. Brain Res.
1247:38–49. 2009.
|
|
31
|
Barnard EA, Skolnick P, Olsen RW, et al:
International Union of Pharmacology. XV Subtypes of γ-aminobutyric
acidA receptors: classification on the basis of subunit structure
and receptor function. Pharmacol Rev. 50:291–313. 1998.PubMed/NCBI
|
|
32
|
Knabl J, Witschi R, Hösl K, et al:
Reversal of pathological pain through specific spinal
GABAA receptors subtypes. Nature. 451:330–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Whiting PJ, Bonnert TP, McKernan RM, et
al: Molecular and functional diversity of the expanding
GABAA receptor gene family. Ann N Y Acad Sci.
868:645–653. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sieghart W: Structure, pharmacology, and
function of GABAA receptor subtypes. Adv Pharmacol.
54:231–263. 2006. View Article : Google Scholar
|
|
35
|
Nutt D: GABAA receptors:
subtypes, regional distribution, and function. J Clin Sleep Med.
2:S7–S11. 2006.
|
|
36
|
Möhler H, Fritschy JM and Rudolph U: A new
benzodiazepine pharmacology. J Pharmacol Exp Ther. 300:2–8.
2002.
|
|
37
|
Sieghart W and Sperk G: Subunit
composition, distribution and function of GABA(A) receptor
subtypes. Curr Top Med Chem. 2:795–816. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pirker S, Schwarzer C, Wieselthaler A,
Sieghart W and Sperk G: GABA(A) receptors: immunocytochemical
distribution of 13 subunits in the adult rat brain. Neuroscience.
101:815–850. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Crestani F, Martin JR, Möhler H and
Rudolph U: Mechanism of action of the hypnotic zolpidem in vivo. Br
J Pharmacol. 131:1251–1254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Melzack R and Wall PD: Pain mechanism: a
new theory. Science. 150:971–979. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wall PD: The substantia gelatinosa. A gate
control mechanism set across sensory pathway. Trends Neurosci.
3:221–224. 1980. View Article : Google Scholar
|
|
42
|
Redecker C, Luhmann HJ, Hagemann G,
Fritschy JM and Witte OW: Differential downregulation of
GABAA receptor subunits in widespread brain regions in
the freeze-lesion model of local cortical malformations. J
Neurosci. 20:5045–5053. 2000.PubMed/NCBI
|