In total, 120 subjects, attending an outpatient
clinic of vascular medicine, were grouped as follows: 40 were SyP
PAD patients, 40 were AsP patients and 40 were control subjects
(C). Syp and AsP, as well as C patients were carefully selected to
obtain a similar distribution of the main clinical parameters
between the groups in order to reduce the impact of certain
factors, such as diabetes, use of statins and smoking, on the serum
concentrations of N (Table I). To
diagnose PAD, the ankle brachial index (ABI) was taken as ≤0.9 and
patients that complained of pain in the lower limbs when walking
were considered Syp. Patients with ABI of ≤0.9 without experience
of pain when walking were considered AsP. Subjects with an ABI
measurement >0.9 and pain-free in the lower limbs were
considered C. The mean value of ABI was 0.70±0.9, 0.80±0.6 and
1.12±0.4 in SyP, AsP and C, respectively.
Venous blood samples were obtained from the patients
within 12 h of fasting. A Neopterin Elisa kit for quantitative
determination (IBL, Hamburg, Germany), as previously described by
Westermann et al(26) and
Smith et al(27), was used
to measure the N concentrations. N values were expressed as
nmol/litre (nmol/l). The within-coefficient of variability was
<3% in the 7.5 nmol/l range.
The results are expressed as the means ± standard
deviation (SD). The statistical analysis was performed using the
ANOVA test and the Student’s t-test to compare the values (mean ±
SD) identified in the three groups. A correlation between the N and
ABI values in patients with PAD was assessed by using the Pearson’s
Chi-Square test or Fisher’s exact test for categorical variables. A
computerized statistical package (SPSS 10.1) for Windows was used.
P<0.05 was considered to indicate a statistically significant
difference.
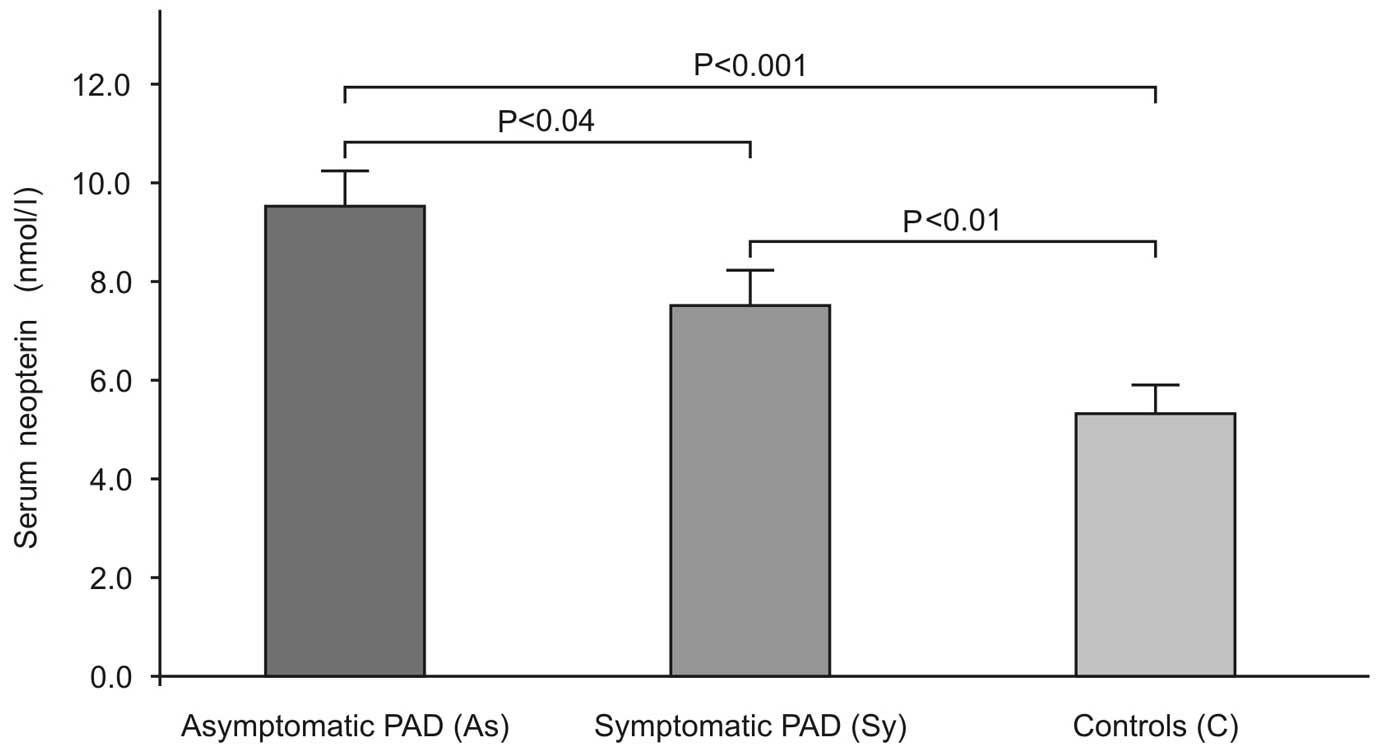
Mean plasma levels were higher in SyP (9.4±4.6
nmol/l) and AsP (7.4±4.0 nmol/l) patients as compared to C patients
(5.3±3.2 nmol/l) (Fig. 1).
Patients of the statistical analysis based on the ANOVA test
demonstrated significant differences in N levels between the groups
(P<0.0001). Moreover, an inverse correlation (Fig. 2) between the ABI values and the N
concentration (r=−0.266, P<0.003) was observed.
N is now considered a marker of macrophage activity
in atherosclerosis. High serum levels of N were found in patients
with chronic cardiac and coronary artery as well as acute coronary
syndrome. A close correlation was observed between serum N
concentration and angiographic evidence of multiple stenoses of
coronary arteries in patients with stable angina (27). N is also considered a biomarker for
atherosclerotic plaque instability both in coronary and carotid
arteries (28). This biological
product released from activated macrophages acts as a pro-oxidant
(29,30). Consequently, N is crucial in the
inflammatory process and pathophysiology of the atheromatous
process as well as in cell death (28). Concerning the peripheral arterial
disorders, previously published data focused mainly on the high
level of N in command-line interface (CLI) (20) and as previously reported (31) patients suffering from CLI have a
poor diagnosis with regard to cardiovascular mortality. Those
findings noted in the ischemic patients must be considered as
relevant proof of the crucial pathophysiologic role played by the
activated monocyte-macrophage.
The present study has focused on the effective role
played by N in chronic AsP and SyP patients and the results showed
higher blood concentrations of this marker compared to the C group.
Our results must be considered useful in highlighting the activated
process of macrophage cells involved both in severe or progressive
stages of PAD as CLI, however, these cells are also deeply involved
in chronic and stable PAD. Thus, we hypothesize that N is useful in
identifying the activated process of macrophage cells in patients
with chronic PAD. It is known that different biomarkers of
inflammation, such as interleukins or metalloproteinases are
elevated both in chronic and more severe PAD patients (32–38).
Results of this study have shown the involvement of activated
macrophages in chronic arterial disease of the lower limbs, albeit
not symptomatic. This result may be associated with increased
oxidative stress caused by chronic low blood perfusion.
Furthermore, the inverse correlation between ABI values and levels
of N demonstrates that there is a close association between the
relative tissue ischemia as demonstrated by a lowered ABI value
with the inflammatory process as marked by a higher plasma level of
N. PAD patients suffer from relative ischemia as demonstrated by
muscular effort when walking (e.g., intermittent claudication).
Thus, a characteristic inflammatory pathway is present in the
atherosclerotic diseases of coronary arteries and carotids, and of
peripheral arteries (PAD).
In conclusion, neopterin is representative of the
macrophage activation process and based on our findings, we
hypothesize that the plasma level of N is useful to elicit the
involvement of the activated macrophage, which, in turn, is able to
promote oxidative stress. The plasma level of N may also be
considered a new and diverse target for medical and interventional
procedures in PAD patients. Therefore, new and original medical
protocols are required to address this issue in order to protect
against the inflammatory process, with N serving as a novel marker
to monitor its efficacy.
|
1
|
Ross R: The pathogenesis of
atherosclerosis: a prospective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar
|
|
2
|
Libby P, Sukhova G, Lee RT and Galis ZS:
Cytokines regulate vascular functions related to stability of the
atherosclerotic plaque. J Cardiovasc Pharmacol. 25:S9–S12. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jacobs M, van Greevenbroek MM, van der
Kallen CJ, Ferreira I, Blaak EE, Feskens EJ, Jansen EH, Schalkwijk
CG and Stehouwer CD: Low-grade inflammation can partly explain the
association between the metabolic syndrome and either coronary
artery disease or severity of peripheral arterial disease: the
CODAM study. Eur J Clin Invest. 39:437–444. 2009. View Article : Google Scholar
|
|
4
|
Jenny NS, Yanez ND, Psaty BM, Kuller LH,
Hirsh CH and Tracy RP: Inflammation biomarkers and near-term death
in older man. Am J Epidemiol. 165:684–695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu H and Rifai N: High-sensitivity
C-reactive protein and atherosclerosis: from theory to therapy.
Clin Biochem. 33:601–610. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rifai N and Ridker PM: High-sensitivity
C-reactive protein: a novel and promising marker of coronary heart
disease. Clin Chem. 47:403–411. 2001.PubMed/NCBI
|
|
7
|
Jenny NS, Arnold AM, Kuller LH, Tracy RP
and Psaty BM: Serum amyloid P and cardiovascular disease in older
men and women. Results from the cardiovascular health study.
Arterioscler Thromb Vasc Biol. 27:352–358. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS,
Nicklas BJ, Snitker S, Horenstein RB, Hull K, Goldberg NH, Goldberg
AP, Shuldiner AR, Fried SK and Gong DW: Acute-phase serum amyloid
A: an inflammatory adipokine and potential link between obesity and
its metabolic complications. PloS Med. 3:e2872006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O’Brien KD and Chait A: Serum amyloid A:
the ‘other’ inflammatory protein. Curr Atheroscler Rep. 8:62–68.
2006.
|
|
10
|
O’Brien KD, McDonald TO, Kunjathoor V, Eng
K, Lewig K, Lopez R, Kirk EA, Chait A, Wight IN, de Beer FC and Le
Boeuf BC: Serum amyloid A and lipoprotein retention in murine
models of atherosclerosis. Arterioscler Thromb Vasc Biol.
25:785–790. 2005.PubMed/NCBI
|
|
11
|
Unlu Y, Karapolat S, Karaca Y and
Kiziltunc A: Comparison of levels of inflammatory markers and
haemostatic factors in the patients with and without peripheral
arterial disease. Thromb Res. 117:357–364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flugelman MY, Virmani R, Correa R, Yu ZX,
Farb A, Leon MB, Elami A, Fu YM, Casscells W and Epstein SE: Smooth
muscle cells abundance and fibroblast growth factors in coronary
lesions of patients with nonfatal unstable angina. A clue to the
mechanism of transformation from the stable to unstable state.
Circulation. 88:2493–2500. 1993. View Article : Google Scholar
|
|
13
|
Tzoulaki I, Murray GD, Lee AJ, Rumley A,
Lowe GD and Fowkes FG: C-reactive protein, interleukin-6, and
soluble adhesion molecules as predictors of progressive peripheral
atherosclerosis in the general population: Edinburgh Artery Study.
Circulation. 112:976–983. 2005. View Article : Google Scholar
|
|
14
|
McDermott MM, Guralnik JM, Corsi A, Albay
M, Macchi C, Bandinelli S and Ferrucci L: Patterns of inflammation
associated with peripheral arterial disease: the InCHIANTI study.
Am Heart J. 150:276–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huber C, Batchelor JR, Fuchs D, Hausen A,
Lang A, Niederwieser D, Reibnegger G, Swettly P, Troppmair J and
Wacther H: Immune response-associated production of neopterin.
Release from macrophages primarily under control of
interferon-gamma. J Exp Med. 160:310–316. 1984. View Article : Google Scholar
|
|
16
|
Gupta S, Fredericks S, Scwartzman WA, Hot
DW and Kaski JC: Serum neopterin in acute coronary syndrome.
Lancet. 349:1252–1253. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Avanzas P, Domínguez-Rodríguez A,
Arroyo-Espliguero R and Kaski JC: Neopterin and coronary artery
disease. J Cardiol. 54:344–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fuchs D, Avanzas P, Arroyo-Espliguero R,
Jenny M, Consuegra-Sanchez L and Kaski JC: The role of neopterin in
atherogenesis and cardiovascular risk assessment. Curr Med Chem.
16:4644–4653. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nazer B, Ray KK, Sloan S, Scirica B,
Morrow DA, Cannon CP and Braunwald E: Prognostic utility of
neopterin and risk of heart failure hospitalization after an acute
coronary syndrome. Eur Heart J. 32:1390–1397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin M, Gottsäter A, Nilsson PM, Mollnes
TE, Lindblad B and Blom AM: Complement activation and plasma levels
of C4b-binding protein in critical limb ischemia patients. J Vasc
Surg. 50:100–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Haelst PL, Liem A, van Boven AJ,
Veegre NJ, van Veldhuisen DJ, Tervaert JW, Gans RO and Zijlstra F:
Usefulness of elevated neopterin and C-reactive protein levels in
predicting cardiovascular events in patients with non-Q-wave
myocardial infarction. Am J Cardiol. 92:1201–1203. 2003.PubMed/NCBI
|
|
22
|
Fowkes FG: Epidemiology of atherosclerotic
arterial disease of the lower limbs. Eur J Vasc Surg. 2:283–291.
1988. View Article : Google Scholar
|
|
23
|
Aronow WS and Ahn C: Prevalence of
coexistence of coronary artery disease, peripheral arterial disease
and atherotrombotic brain infarction in men and women > or =62
years of age. Am J Cardiol. 74:64–65. 1994.PubMed/NCBI
|
|
24
|
Agnelli G, Cimminiello C, Meneghetti G and
Urbinati S: The Polyvascular Atherothrombosis Observational Survey
(PATHOS) Investigators. Low ankle-brachial index predicts an
adverse 1-year outcome after acute coronary and cerebrovascular
events. J Thromb Haemost. 4:2599–2606. 2006.
|
|
25
|
Santo Signorelli S, Anzaldi M, Fiore V,
Catanzaro S, Simili M, Torrisi B and Neri S: Study on unrecognized
peripheral arterial disease (PAD) by ankle/brachial index and
arterial comorbidity in Catania, Sicily, Italy. Angiology.
61:524–529. 2010.PubMed/NCBI
|
|
26
|
Westermann J, Thiermann F, Gerstetner L,
Tatzber F, Kozak I, Bertsch T and Kruger C: Evaluation of a new
simple and rapid enzyme-like-immunosorbent assay kit for neopterin
determination. Clin Chem Lab Med. 38:345–353. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Avanzas P, Arroyo-Espliguerro R,
Coxin-Sales J, ldama G, Pizzi C, Quiles J and Kaski JC: Markers of
inflammation and multiple complex stenoses (pancoronary plaque
vulnerability) in patients with non-ST segment elevation acute
coronary syndrome. Heart. 90:847–852. 2004. View Article : Google Scholar
|
|
28
|
Sugioka K, Naruko T, Hozumi T, Nakagawa M,
Kitabayashi C, Ikura Y, Shirai N, Matsumura Y, Ehara S, Ujino K,
Itoh A, Haze K, Becker AE, Yoshiyama M and Ueda M: Elevated levels
of neopterin are associated with carotid plaques with complex
morphology in patients with stable angina pectoris.
Atherosclerosis. 208:524–530. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weiss G, Fuchs D, Hausen A, Reibnegger G,
Werner ER, Werner-Felmayer G, Semenitz E, Dierich MP and Wachter H:
Neopterin modulates toxicity mediated by reactive oxygen and
chloride species. FEBS Lett. 321:89–92. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murr C, fuchs D, Gossler W, Hausen A,
Reibnegger G, Werner ER, Werner-Felmayer G, Esterbauer H and
Wachter H: Ehnancement of hydrogen peroxide induced
luminol-dependent chemiluminescence by neopterin depends on the
presence of iron chaltor complexes. FEBS Lett. 338:223–226. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Resnick HE, Linday RS, Mc Dermott MM,
Deveraux RB, Jones KL, Fabsitz RR and Howard RR: Relationship
between high and low ankle brachial index to all-cause and to
cardiovascular disease mortality: the Strong Heart Study.
Circulation. 109:733–739. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fiotti N, Giansante C, Ponte E, Delbello
C, Calabrese S, Zacchi T, Dobrina A and Guarnieri G:
Atherosclerosis and inflammation. Patterns of cytokine regulation
in patients with peripheral arterial disease. Atherosclerosis.
145:51–60. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brevetti G, Silvestro A, Di Giacomo S,
Bucur R, Di Donato A, Schiano V and Scopacasa F: Endothelial
dysfunction in peripheral arterial disease is related to increase
in plasma markers of inflammation and severity of peripheral
circulatory impairment but not to classic risk factors and
atherosclerotic burden. J Vasc Surg. 38:374–379. 2003. View Article : Google Scholar
|
|
34
|
Schnabel RB, Schulz A, Messow CM, Lubos E,
Wild PS, Zeller T, Sinning CR, Rupprecht HJ, Bickel C, Peetz D,
Cambien F, Kempf T, Wollert KC, Benjamin EJ, Lackner KJ, Münzel TF,
Tiret L, Vasan RS and Blankenberg S: Multiple marker approach to
risk stratification in patients with stable coronary artery
disease. Eur Heart J. 31:3024–3031. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Rosa S, Cirillo P, Pacileo M, Petrillo
G, D’Ascoli GL, Maresca F, Ziviello F and Chiariello M: Neopterin:
from forgotten biomarker to leading actor in cardiovascular
pathophysiology. Curr Vasc Pharmacol. 9:188–199. 2011.PubMed/NCBI
|
|
36
|
Signorelli SS, Malaponte G, Libra M, Di
Pino L, Celotta G, Bevelacqua V, Petrina M, Nicotra GS, Indelicato
M, Navolanic PM, Pennini G and Mazzarino MC: Plasma levels and
zymographic activities of matrix metalloproteinases 2 and 9 in type
II diabetics with peripheral arterial disease. Vasc Med. 10:1–6.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Libra M, Signorelli SS, Bevelacqua Y,
Navolanic PM, Bevelacqua V, Polesel J, Talamini R, Stivala F,
Mazzarino MC and Malaponte G: Analysis of G(-174)C IL-6
polymorphism and plasma concentrations of inflammatory markers in
patients with type 2 diabetes and peripheral arterial disease. J
Clin Pathol. 59:211–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Signorelli SS, Anzaldi M and Fiore V:
Inflammation in peripheral arterial disease (PAD). Curr Pharm Des.
18:4350–4357. 2012. View Article : Google Scholar : PubMed/NCBI
|