Introduction
Colorectal adenocarcinoma (CRA) is a common type of
malignant tumor. The 5-year survival rate of CRA is ~65%, and the
tumor stage, lymph node (LN) status, tumor grade and lymphatic and
venous invasion are critical morphological prognostic factors
(1,2). Although advances have been made while
studying the molecular basis of this disease, the spectrum of genes
that reveal altered expression in CRA, as well as their role in the
disease, remain unclear (3,4).
Therefore, more sensitive CRA biomarkers that are capable of
predicting prognosis and guiding effective targeting therapy are
required.
The ezrin gene is a member of the
ezrin-radixin-moesin (ERM) cytoskeleton-associated protein family
and is involved in a wide variety of cellular processes. It is one
of the components of cell-surface structures involved in cell
adhesion to the extracellular matrix, and has been implicated in
membrane-cytoskeleton interactions (5,6). The
ezrin protein correlates with tumor invasiveness, metastasis and
clinical prognosis in numerous types of human cancer, including CRA
(7–9). The molecular characteristics of the
ezrin protein may be important during tumor progression (10); however, the clinical significance
of these characteristics in human cancer requires clarification.
The aim of the present study was to investigate the correlation
between ezrin expression, clinicopathological parameters and the
prognosis of CRA patients. Our data suggested that cytoplasmic
ezrin expression is associated with tumor metastasis and poor
survival in CRA patients, and may be a useful marker for predicting
disease progression and prognosis in CRA patients.
Materials and methods
Samples
In total, 186 cases of routinely processed and
diagnosed CRA with strict follow-up were randomly selected from
patients who underwent surgery between 2004 and 2007 in the
Liaoning and Shanghai regions of China. Pathological parameters,
including age, gender, grade, the presence of nodal metastasis,
clinical stage and survival data, were carefully reviewed in all
cases. The ages of the patients ranged from 25–82 years, with a
mean age of 59.35 years. The male:female ratio was 119:67. Staging
was performed according to the TNM and the International Federation
of Gynecology and Obstetrics (FIGO) classifications of colonic and
rectal carcinomas; according to FIGO, 97 cases were classified as
stages I–IIA (early stage). According to the Union for
International Cancer Control (UICC) criteria 7th Edition and the
WHO classification (Pathology and Genetics of Tumors of the
Digestive System), 89 cases were classified as stages IIB–IV
(advanced stage). In addition, 99 cases were defined as
well-differentiated and 87 cases were defined as moderately or
poorly differentiated. All adjacent normal colorectal mucosal
tissues from the cancer resection margin were included and none of
the patients had received chemotherapy prior to surgery. All 186
patients were followed-up for 5 years or until they succumbed to
the disease. At the end of follow-up, 139 patients had survived.
Informed consent was obtained from each patient prior to commencing
the study and the research protocols were approved by the Ethics
Committee of the University Hospitals.
Immunohistochemistry for ezrin in
paraffin-embedded tissues
Tissue sections (4 μm) were prepared on
silane-coated slides (Sigma, St. Louis, MO, USA). Immunostaining
kits were purchased from DakoCytomaton Inc. (Glostrup, Denmark) and
Nichirei Inc. (Tokyo, Japan). Tissue sections were deparaffinized,
rehydrated and incubated with 3% H2O2 in
methanol for 15 min at room temperature in order to eliminate
endogenous peroxidase activity. The antigen was retrieved at 95ºC
for 20 min by placing the slides into a 0.01 M sodium citrate
buffer (pH 6.0). The slides were subsequently incubated with a
primary ezrin antibody (1:50, BD Biosciences Pharmingen, San Diego,
CA, USA) at 4°C overnight. Following incubation at room temperature
for 30 min with a biotinylated secondary antibody, the slides were
incubated with a streptavidin-peroxidase complex (BD Biosciences
Pharmingen) at room temperature for 30 min. Immunostaining was
developed using chromogen, 3,3′-diaminobenzidine and counterstained
with Mayer’s hematoxylin. We used mouse IgG isotope controls, which
demonstrated negative staining. Furthermore, the positive tissue
sections were processed omitting the primary antibody (mouse
anti-ezrin) as negative controls.
Analysis and interpretation of
staining
Immunoreactivity was independently evaluated by two
researchers who were blinded to the patient outcome. The evaluation
was based on the extent and intensity of the staining (12). The ezrin staining intensity was
scored as follows: 0, negative; 1, weak; 2, moderate; and 3,
strong. Staining extent was scored as follows: 0, 0%; 1, 1–25%; 2,
26–50%; 3, 51–75%; and 4, 76–100%, depending on the percentage of
positively stained cells. The sum of the staining intensity and the
staining extent scores was used as the final staining score. The
specimens were divided into three groups according to their 5-year
scores: 0–1, negative (−); 2–4, weakly positive (+); and 5–7,
strongly positive (++).
Statistical analysis
Statistical analyses were performed using SPSS 17.0
(SPSS Inc., Chiacgo, IL, USA). The correlation between ezrin
expression and clinicopathological characteristics was evaluated
using the χ2 and Fisher’s exact tests. The survival
rates following tumor removal were calculated using the
Kaplan-Meier method and the difference in survival curves was
analyzed using the log-rank test. Multivariate survival analysis
was performed on all significant characteristics and was measured
by univariate survival analysis with the Cox proportional hazard
regression model. P<0.05 was considered to indicate a
statistically significant difference.
Results
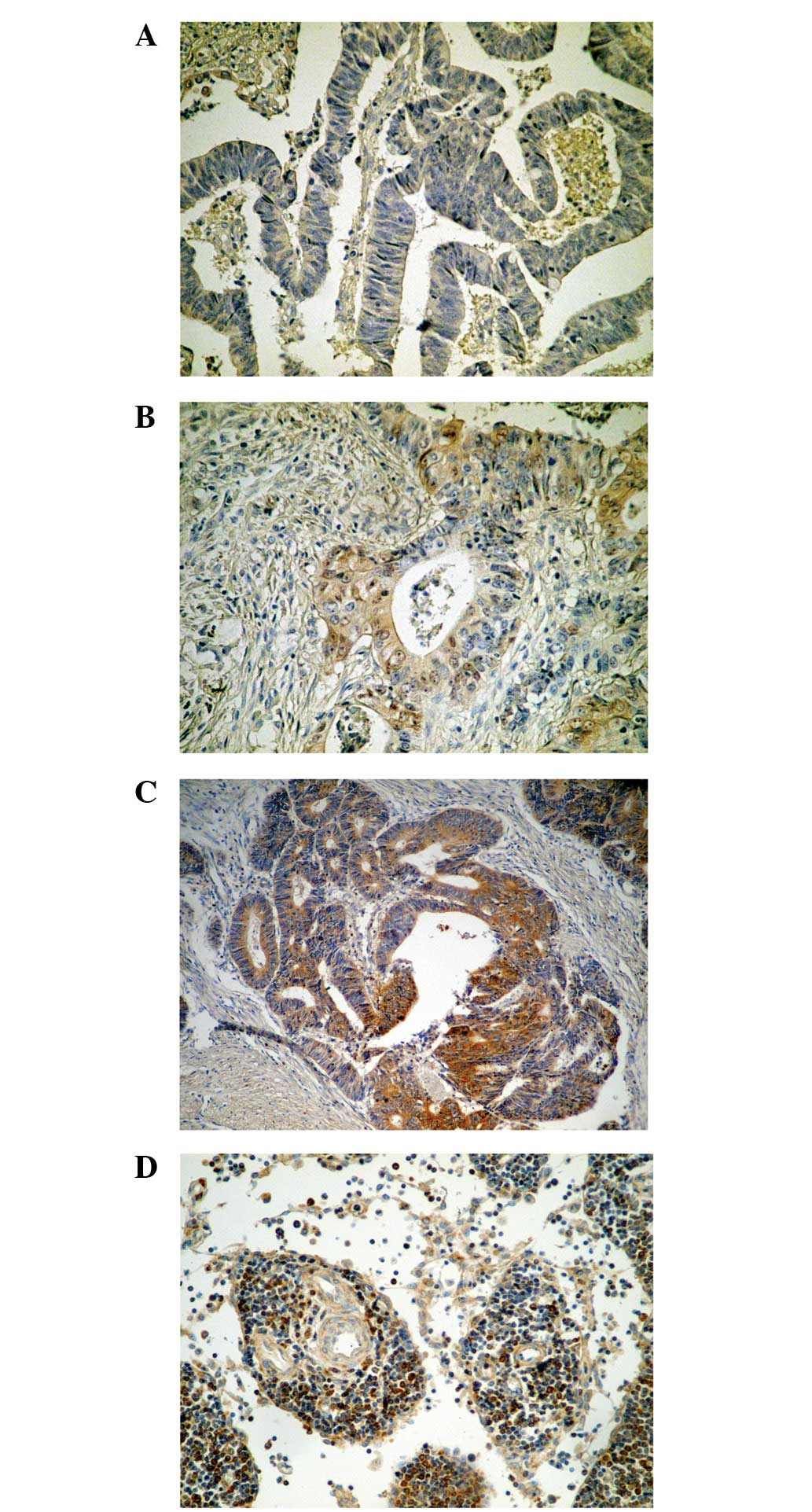
Expression of ezrin protein in CRA
The expression of ezrin protein revealed an
immunohistochemical cytoplasmic staining pattern in CRA. The
difference between the strongly positive rate (++) in the CRA
(15.6%, 29/186) and adjacent normal mucosal tissues (25.3%, 47/186)
was not deemed to be statistically significant (P=0.083); however,
the positive (+ and ++) rate in CRA (61.3%, 114/186) was
significantly lower than that found in the adjacent normal mucosal
tissues (91.9%, 171/186; P=0.000; Table I and Fig. 1)
 | Table IEzrin protein expression in CRA. |
Table I
Ezrin protein expression in CRA.
| | Ezrin (n) | | |
|---|
| |
| | |
|---|
| Diagnosis | No. | − | + | ++ | Positive rate
(%) | Strongly positive
rate (%) |
|---|
| Carcinoma | 186 | 72 | 85 | 29 | 61.3a | 15.6 |
| Normal mucosa | 186 | 15 | 124 | 47 | 91.9 | 26.3 |
Clinicopathological and prognostic
significance of ezrin expression
To evaluate the role of ezrin protein in CRA
progression, we analyzed the correlation between the expression of
ezrin protein and major clinicopathological features. Table II and Fig. 3 demonstrate that the positive rate
of ezrin expression in cases with a large tumor was 52.1% (37/71),
which was significantly lower than in cases with a smaller tumor
(67.0%, 77/115; P=0.044). The positive rates of ezrin expression in
cases without serosal invasion (68.3%, 69/101) or LN metastasis
(70.3%, 78/111) were significantly higher than in CRA cases with
these factors (P=0.032 and P=0.002, respectively). The lymph node
ratio (LNR) refers to the examination of the positive LN rate, and
ezrin expression was identified to statistically correlate with the
LNR. Cases with a high LNR (≥0.7) had a lower positive rate of
ezrin expression (36.4%, 8/22) than cases with a low LNR (<0.7;
64.6%, 106/164; P=0.011). Finally, the positive rate of ezrin
expression was 47.2% (42/89) in late-stage CRA, which was
significantly lower than in early-stage cases (74.2%, 72/97;
P=0.000). However, the differences among ezrin expression, age,
gender and tumor grade were not statistically significant
(P>0.05, respectively).
 | Table IIUnivariate analysis of ezrin protein
expression and various risk factors in 186 patients with colorectal
adenocarcinoma. |
Table II
Univariate analysis of ezrin protein
expression and various risk factors in 186 patients with colorectal
adenocarcinoma.
| | Ezrin expression, n
(%) | | |
|---|
| |
| | |
|---|
| Characteristic | No. | − | + and ++ | OR (95% CI) | P-value |
|---|
| Age (years) | | | | 0.230
(0.680–2.227) | 0.297 |
| >59 | 82 | 34 (41.5) | 48 (58.5) | | |
| ≤59 | 104 | 38 (36.5) | 66 (63.5) | | |
| Gender | | | | 0.901
(0.488–1.663) | 0.429 |
| Male | 119 | 45 (37.8) | 74 (62.2) | | |
| Female | 67 | 27 (40.3) | 40 (59.7) | | |
| Tumor grade | | | | 0.585
(0.323–1.059) | 0.057 |
| Well | 99 | 32 (32.3) | 67 (67.7) | | |
| Moderate and
poor | 87 | 40 (46.0) | 47 (54.0) | | |
| Tumor size | | | | 0.537
(0.293–0.985) | 0.044a |
| ≤5 cm | 115 | 38 (33.0) | 77 (67.0) | | |
| >5 cm | 71 | 34 (47.9) | 37 (52.1) | | |
| Serosal invasion | | | | 1.917
(1.054–3.484) | 0.032a |
| Present | 85 | 40 (47.1) | 45 (52.9) | | |
| Absent | 101 | 32 (31.7) | 69 (68.3) | | |
| LN metastasis | | | | 2.561
(1.393–4.708) | 0.002b |
| Present | 75 | 39 (52.0) | 36 (48.0) | | |
| Absent | 111 | 33 (29.7) | 78 (70.3) | | |
| LNR | | | | 3.198
(1.267–8.072) | 0.011a |
| ≥0.7 | 22 | 14 (63.6) | 8 (36.4) | | |
| <0.7 | 164 | 58 (35.4) | 106 (64.6) | | |
| Clinical stage | | | | 3.223
(1.740–5.971) | 0.000b |
| I–II | 97 | 25 (25.8) | 72 (74.2) | | |
| IIIa–IV | 89 | 47 (52.8) | 42 (47.2) | | |
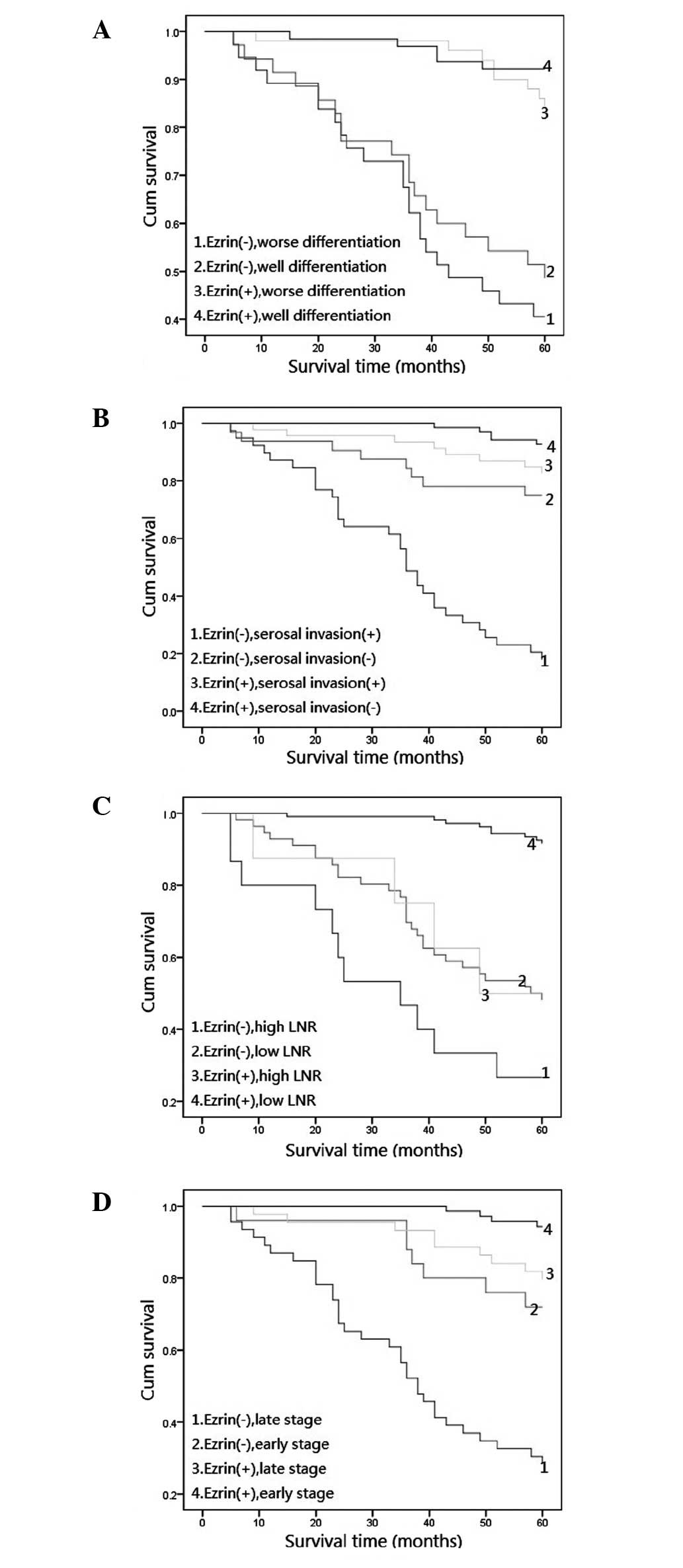
Ezrin expression level combined with
tumor grade, serosal invasion status and stage affects the
prognosis of CRA
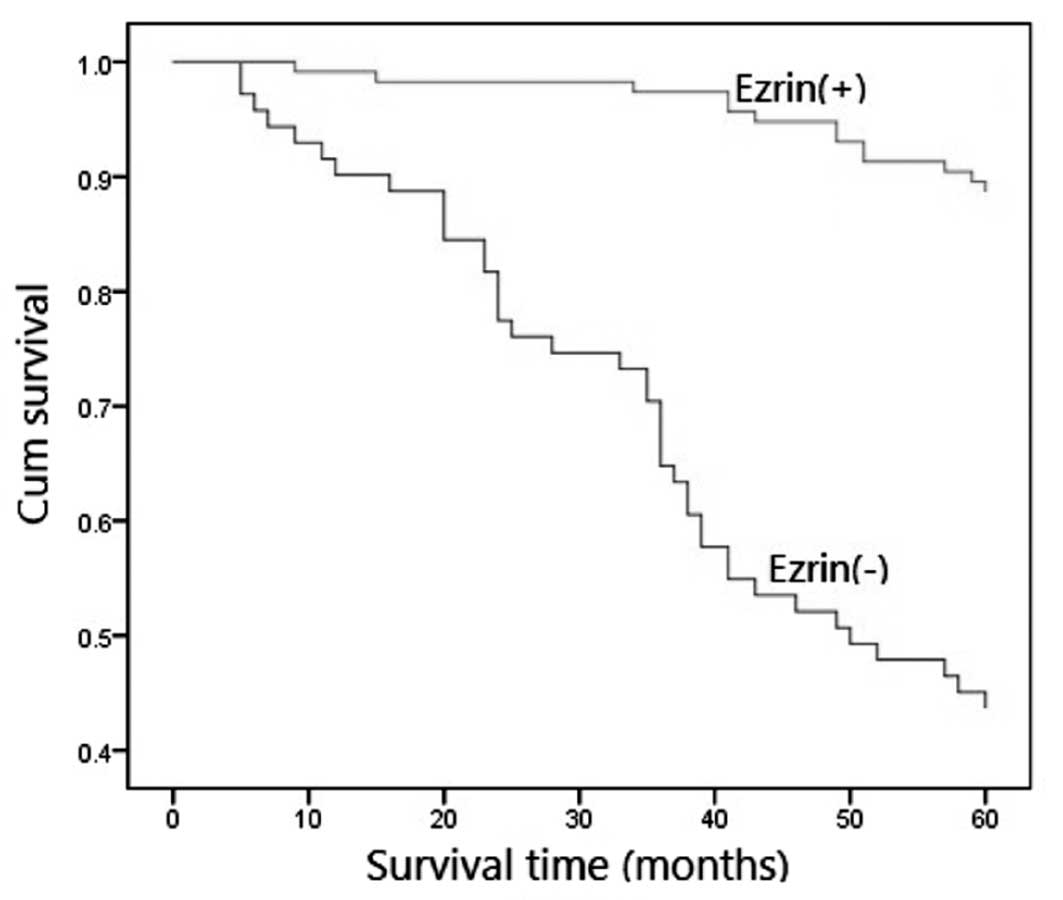
To confirm the role of ezrin expression in CRA
progression, we analyzed the 5-year survival rate of 186 CRA
patients using the Kaplan-Meier method and discovered that CRA
patients with no ezrin expression had a lower 5-year survival rate
than those with positive ezrin expression (P=0.000; Fig. 2). Furthermore, we analyzed the
correlation between other factors (age, gender, tumor size, grade,
serosal invasion, LN, LNR and tumor stage) and the 5-year survival
rate in CRA and discovered that tumor grade, LNR, serosal invasion
and tumor stage were key factors associated with 5-year survival
rate (P=0.016, P=0.004, P=0.015 and P=0.006, respectively). Further
combination analysis revealed that CRA with poor differentiation
combined with no ezrin expression had the lowest 5-year survival
rate, and this was significantly lower than that in cases with
ezrin expression (Fig. 3A;
P=0.000). Furthermore, CRA without serosal invasion combined with
positive ezrin expression had the highest 5-year survival rate, and
CRA with serosal invasion combined with no ezrin expression had a
lower 5-year survival rate than cases with a positive level of
ezrin expression (Fig. 3B;
P=0.000). The 5-year survival rate of CRA patients with a high LNR
did not correlate with the cases, whether it was accompanied by
ezrin expression or not (Fig. 3C;
P=0.156). However, the 5-year survival rate of CRA patients with a
low LNR did correlate with the cases, whether it was accompanied by
ezrin expression or not (Fig. 3C;
P=0.008). Tumor stage is the most important histological prognostic
factor in CRA (11) and we
revealed that CRA patients with a high stage (IIIa–IV) and no ezrin
expression had a significantly lower 5-year survival rate than
those patients with ezrin expression (Fig. 3D; P=0.002). Therefore, the
non-expression of ezrin may be a poor prognostic marker for CRA
with poor differentiation, serosal invasion, high LNR and at a late
stage.
Non-expression of ezrin is an independent
prognostic factor in CRA, as determined by the Cox proportional
hazard regression model
Table III shows
the univariate and multivariate analyses performed using the Cox
proportional hazards model. The LNR (HR, 0.589; 95% CI,
0.369–0.939; P=0.026) and tumor stage (HR, 0.655; 95% CI,
0.487–0.880; P=0.005) were demonstrated to be independent
prognostic factors for survival in CRA. Notably, the non-expression
of ezrin emerged as a significant independent prognostic factor in
CRA (HR, 0.562; 95% CI, 0.404–0.783; P=0.001).
 | Table IIIUnivariate and multivariate survival
analyses (Cox regression model) of various factors in 186 patients
with CRA. |
Table III
Univariate and multivariate survival
analyses (Cox regression model) of various factors in 186 patients
with CRA.
| Factor | B | SE | Wald | HR (95% CI) | P-value |
|---|
| Univariate survival
analyses |
| Age (years) | −0.016 | 0.148 | 0.011 | 0.984
(0.737–1.315) | 0.916 |
| Gender | 0.028 | 0.153 | 0.035 | 1.029
(0.763–1.388) | 0.852 |
| Tumor size
(cm) | −0.156 | 0.151 | 1.068 | 0.856
(0.636–1.150) | 0.301 |
| Tumor grade | −0.120 | 0.147 | 0.663 | 0.887
(0.665–1.183) | 0.416 |
| Serosal
invasion | −0.038 | 0.149 | 0.065 | 0.963
(0.719–1.289) | 0.799 |
| LN metastasis | −0.274 | 0.148 | 3.447 | 0.760
(0.569–1.015) | 0.063 |
| LNR | −0.622 | 0.228 | 7.464 | 0.537
(0.344–0.839) | 0.006b |
| Clinical
stage | −0.445 | 0.147 | 9.127 | 0.641
(0.480–0.855) | 0.003b |
| Ezrin | −0.605 | 0.152 | 15.901 | 0.546
(0.406–0.735) | 0.000b |
| Multivariate
survival analyses |
| Age (years) | 0.043 | 0.153 | 0.078 | 1.043
(0.774–1.408) | 0.781 |
| Gender | 0.078 | 0.166 | 0.221 | 1.081
(0.781–1.498) | 0.638 |
| Tumor size
(cm) | −0.076 | 0.165 | 0.213 | 0.927
(0.671–1.281) | 0.644 |
| Tumor grade | −0.115 | 0.150 | 0.580 | 0.892
(0.664–1.197) | 0.446 |
| Serosal
invasion | 0.246 | 0.164 | 2.230 | 1.278
(0.926–1.765) | 0.135 |
| LN metastasis | −0.276 | 0.154 | 3.232 | 0.759
(0.561–1.025) | 0.072 |
| LNR | −0.530 | 0.238 | 4.945 | 0.589
(0.369–0.939) | 0.026a |
| Clinical
stage | −0.424 | 0.151 | 7.862 | 0.655
(0.487–0.880) | 0.005b |
| Ezrin | −0.576 | 0.169 | 11.628 | 0.562
(0.404–0.783) | 0.001b |
Discussion
The ezrin gene is located on chromosome 6q25.2-q26.
The full length mRNA is 3166 bp, encoding a protein that consists
of 585 amino acids. In 1981, Fehon et al(5) separated and purified the protein in
the small intestinal epithelial cell brush border of a chicken. The
ezrin gene belongs to the ERM family and addresses all types of
epithelium, shares a homology with the amino terminal
membrane-binding domain of erythrocyte band 4.1, and is also
involved in membrane-cytoskeleton interactions (13). Ezrin is capable of interacting with
several membrane proteins, including CD44, CD43 (14), intercellular adhesion molecule-1,
intercellular adhesion molecule-2 and
phosphatidylinositol-bisphosphate (15). Ezrin, moesin and radixin are often
all expressed on the intramembrane. Ezrin may alter the
relationship between the cell membrane and the cytoskeleton and
promote the formation of microvilli and aphosphyses, which move
cells. The main function of ezrin is to interact with p85, the
regulatory subunit of PI3-kinase (PI3K), involved in determining
the survival of the epithelial cells by activating the PI3K/Akt
pathway (16). Subsequently, the
regulation of adhesion, migration and invasion were observed and
shown to be important to tumor development and progression
(17).
Two theories exist with regard to the role of ezrin
in tumor invasion and metastasis. One states that ezrin is the
promotion factor for tumor invasion and metastasis and is
overexpressed in a variety of invasive carcinoma tissues. In 2004,
Yu et al(18) and Khanna
et al(19) reported that
ezrin is crucial for the metastasis of rhabdomyosarcoma and bone
sarcoma in children; they transferred the Vil2 gene (ezrin coding
gene) into low-transfer ability cell lines and discovered that the
transfer capability of the tumor cell was greatly improved, and the
creation of lung metastases was simple. This demonstrates that the
overexpression of ezrin in tumor cells may provide the cell with a
high transfer activity. Contrary to the upregulation of ezrin in
tumor cells, another hypothesis is that ezrin is downregulated in
tumor cells and inhibits tumor invasion and metastasis. Karmakar
and Das (20) observed that in the
human chorioepithelioma cell line JEG-3 cultivated by IL-IB, ezrin,
E-cadherin and β2 serial protein expression levels were
downregulated, CD44 expression was enhanced, adhesion between the
JEG-3 cells and the matrix was increased, cell-cell adhesion abated
and aggressiveness was enhanced. Hiscox and Jiang (21) cultured the colon cancer cell line
in vitro and observed that a lack of ezrin decreased tumor
cell aggregation, increased the separation phenomenon, broadened
the intercellular space, formatted pseudopodia, increased the
ability to move, decreased intercellular adhesion, increased matrix
adhesion and enhanced aggressiveness. Our study suggests that ezrin
expression is deregulated in CRA and that the non-expression of
ezrin exerts a profound effect on cellular proliferation, cell
adhesion, invasion and aggressiveness during cancer growth and
metastasis.
Tumor staging is an important histological feature
of CRA prognosis, but knowledge of its associated molecules is very
limited. In this study, no ezrin expression was identified in a
third of CRA cases, which correlated with the tumor size, serosal
invasion status, LNR and the pathological stage. Additionally, the
non-expression of ezrin in early-stage CRA was three times lower
than in the late-stage cases. Furthermore, the cases had a lower
5-year survival rate, and more notably, late-stage CRA accompanied
by ezrin non-expression resulted in a poorer survival rate than
that in late-stage CRA only. These results indicate that tumor
stage and the non-expression of ezrin may indicate the survival of
patients. Additionally, tumor staging was found to be an
independent prognostic factor, and we demonstrated that the
non-expression of ezrin is associated with the poor prognosis of
late-stage CRA.
According to the recommendations of the American
Joint Committee on Cancer (AJCC) and the National Cancer Institute,
≥12 LNs should be examined in order to confirm the absence of nodal
involvement in colorectal cancer patients (1,3), due
to the fact that the number of LNs examined is a reflection of the
aggressiveness of the surgical dissection and positive pathological
identification (22).
Additionally, the number of positive LNs examined in colorectal
surgery may be associated with the outcome for the patient. Telian
and Bilchik (23) reported that
the LNR, rather than LN number, is an important positive prognostic
factor for colorectal cancer. Wang et al(24) concluded that LNR is an independent
survival predictor after adjusting for the age of the patient,
tumor size, tumor grade, ethnicity, number of positive LNs and
total number of LNs collected. Therefore, LNR ≥12 may be a better
prognostic indicator than the number of positive LNs. Consistent
with other reports, we confirmed that a high LNR (≥12) is an
independent prognostic factor for patient survival in CRA.
Moreover, CRA with a low LNR had 3-fold ezrin expression compared
with a high LNR in CRA. Additionally, CRA with a decreased LNR
without ezrin expression had the lowest 5-year survival rate, even
though it was not statistically lower than a high LNR with ezrin
expression.
In conclusion, we identified ezrin as a potential
biomarker to evaluate the tumor progression and prognosis of CRA.
The non-expression of ezrin was more commonly observed in cases
with poor CRA prognostic factors, leading to late-stage tumors and
short survival times. Ezrin has been suggested as a novel
therapeutic method for selectively targeting cancer cells,
particularly for late-stage CRA.
References
|
1
|
Zlobec I and Lugli A: Prognostic and
predictive factors in colorectal cancer. J Clin Pathol. 61:561–569.
2008.PubMed/NCBI
|
|
2
|
Berman BP, Weisenberger DJ, Aman JF, et
al: Regions of focal DNA hypermethylation and long-range
hypomethylation in colorectal cancer coincide with nuclear
lamina-associated domains. Nat Genet. 44:40–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carlisle J, Swart M, Dawe EJ and Chadwick
M: Factors associated with survival after resection of colorectal
adenocarcinoma in 314 patients. Br J Anaesth. 108:430–435. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li D, Yan D, Tang H, Zhou C, et al: IMP3
is a novel prognostic marker that correlates with colon cancer
progression and pathogenesis. Ann Surg Oncol. 16:3499–3506. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fehon RG, McClatchey AI and Bretscher A:
Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol
Cell Biol. 11:276–287. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neisch AL and Fehon RG: Ezrin, radixin and
moesin: key regulators of membrane-cortex interactions and
signaling. Curr Opin Cell Biol. 23:377–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie JJ, Xu LY, Wu ZY, et al: Prognostic
implication of ezrin expression in esophageal squamous cell
carcinoma. J Surg Oncol. 104:538–543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Korkeila EA, Sundström J, Pyrhönen S and
Syrjänen K: Carbonic anhydrase IX, hypoxia-inducible factor-1α,
ezrin and glucose transporter-1 as predictors of disease outcome in
rectal cancer: multivariate Cox survival models following data
reduction by principal component analysis of the
clinicopathological predictors. Anticancer Res. 31:4529–4535.
2011.
|
|
9
|
Zhang XQ, Chen GP, Wu T, Yan JP and Zhou
JY: Expression and clinical significance of ezrin in non-small-cell
lung cancer. Clin Lung Cancer. 13:196–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schlecht NF, Brandwein-Gensler M, Smith
RV, et al: Cytoplasmic ezrin and moesin correlate with poor
survival in head and neck squamous cell carcinoma. Head Neck
Pathol. 6:232–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O’Connell JB, Maggard MA and Ko CY: Colon
cancer survival rates with the new American Joint Committee on
Cancer sixth edition staging. J Natl Cancer Inst. 96:1420–1425.
2004.
|
|
12
|
Wu Q, Li Z, Lin H, et al: DEK
overexpression in uterine cervical cancers. Pathol Int. 58:378–382.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takeuchi K, Kawashima A, Nagafuchi A and
Tsukita S: Structural diversity of band 4.1 superfamily members. J
Cell Sci. 107:1921–1928. 1994.
|
|
14
|
Yonemura S, Hirao M, Doi Y, et al:
Ezrin/radixin/moesin (ERM) proteins bind to a positively charged
amino acid cluster in the juxta-membrane cytoplasmic domain of
CD44, CD43, and ICAM-2. J Cell Biol. 140:885–895. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heiska L, Alfthan K, Grönholm M, et al:
Association of ezrin with intercellular adhesion molecule-1 and -2
(ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4,
5-bisphosphate. J Biol Chem. 273:21893–21900. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gautreau A, Poullet P, Louvard D and Arpin
M: Ezrin, a plasma membrane-microfilament linker, signals cell
survival through the phosphatidylinositol 3-kinase/Akt pathway.
Proc Natl Acad Sci USA. 96:7300–7305. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou B, Leng J, Hu M, et al: Ezrin is a
key molecule in the metastasis of MOLT4 cells induced by
CCL25/CCR9. Leuk Res. 34:769–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Y, Khan J, Khanna C, et al: Expression
profiling identifies the cytoskeletal organizer ezrin and the
developmental homeoprotein Six-1 as key metastatic regulators. Nat
Med. 2:175–181. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khanna C, Wan X, Bose S, et al: The
membrane-cytoskeleton linker ezrin is necessary for osteosarcoma
metastasis. Nat Med. 10:182–186. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karmakar S and Das C: Modulation of ezrin
and E-cadherin expression by IL-1beta and TGF-beta1 in human
trophoblasts. J Reprod Immunol. 64:9–29. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hiscox S and Jiang WG: Ezrin regulates
cell-cell and cell-matrix adhesion, a possible role with
E-cadherin/beta-catenin. J Cell Sci. 112:3081–3090. 1999.PubMed/NCBI
|
|
22
|
Gao P, Song YX, Wang ZN, et al: Integrated
ratio of metastatic to examined lymph nodes and number of
metastatic lymph nodes into the AJCC staging system for colon
cancer. PLoS One. 7:e350212012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Telian SH and Bilchik AJ: Significance of
the lymph node ratio in stage III colon cancer. Ann Surg Oncol.
15:1557–1578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Kulaylat M, Rockette H, et al:
Should total number of lymph nodes be used as a quality of care
measure for stage III colon cancer? Ann Surg. 249:559–563. 2009.
View Article : Google Scholar : PubMed/NCBI
|