Introduction
Traumatic injury to peripheral nerves results in
considerable loss of sensory and motor function, decreasing quality
of life in patients (1).
Peripheral nerve injuries substantially impact quality of life
through loss of function and increased risk of secondary
disabilities from falls, fractures and other injuries. Several
research groups have attempted to improve the regeneration of
traumatized nerves by developing favorable microsurgical
techniques. However, clinicians soon noted that despite advancement
in these techniques, complete recovery is rarely achieved (2,3).
Therefore, complete recovery remains an important clinical
challenge for the improvement of functional recovery following
peripheral nerve injury. In a discussion of future trends in the
management of brachial plexus injuries, Birch hypothesized that the
administration of nerve growth factor may represent a useful
treatment strategy (4).
Neurotrophic factors are important in a number of
biological processes, including survival, proliferation,
differentiation and apoptosis of neurons in the nervous system
(5–7). However, direct use of trophic factors
in clinical practice is extremely challenging as they are difficult
to administer and have severe side effects (8). However, molecules that easily diffuse
into nerve tissues, including ligands of the progesterone and
thyroid hormone receptors, and immunophilins, have beneficial
effects on peripheral nerves in various experimental lesion and
disease models (9–12). We hypothesize that clinically
established drugs may be suitable to elevate trophic factor levels
to improve the outcome of peripheral nerve injuries.
Previous studies have demonstrated that the drug,
etifoxine (2-ethylamino-6-chloro-4-methyl-4-phenyl-4H-3,
1-benzoxazine hydrochloride; Stresam; Biocodex, Moscow, Russia),
exerts anxiolytic effects by targeting GABAA receptors and
translocator protein (TSPO; 18 kDa) (8,13).
TSPO is mainly localized in the outer mitochondrial membrane and
has multiple functions (14).
Following peripheral nerve injury, TSPO expression is transiently
increased in a number of cells, including dorsal root ganglia (DRG)
neurons, Schwann cells and macrophages (15,16).
In addition, TSPO ligands have been identified to exert
neuroprotective effects and reduce neural inflammation in the CNS
(17,18). In rats, ligand binding stimulates
the cholesterol transfer function of TSPO. According to the concept
of neurosteroids (19), this
mechanism is likely to be responsible for the increase of
neurosteroid levels observed in the brain 0.5 h following
administration of 50 mg/kg etifoxine (13). Neurosteroids, including
pregnenolone (PREG), progesterone (PROG) and dehydroepiandrosterone
(DHEA) have been demonstrated to be regulated by the activation of
TSPO (20–23). Cholesterol and neurosteroids are
important for neuronal regeneration (24). For example, cholesterol deficiency
inhibits axonal branching and promotes axonal degeneration
(25,26). DHEA enhances functional recovery
and increases the number of nerve fibers in the sciatic nerve
following a crush injury (27) and
PROG has been reported to increase neurite outgrowth in
vitro and in vivo(28,29).
However, at present, it remains unknown whether administration of
etifoxine leads to enhanced neurite outgrowth and the underlying
mechanisms involved in this process are undefined.
Glial cell line-derived neurotrophic factor (GDNF)
was previously identified in conditioned media from a glial cell
line based on its ability to promote survival and increase cell
size and neurite length in mesencephalic dopaminergic neurons in
culture (30,31). As no effect was observed on
GABAergic neurons, GDNF was originally hypothesized to be a
selective survival factor for the nigrostriatal dopaminergic
neurons that degenerate in Parkinson’s disease (32). However, additional studies have
revealed that GDNF also supports the survival of spinal motor
neurons (33) and brain
noradrenergic neurons (34). GDNF
also regulates the survival, migration and differentiation of
several peripheral neurons (35).
The neuroprotective effects of GDNF on dopaminergic neurons has led
to studies on the effects of GDNF administration in animal models
of Parkinson’s disease (36,37).
Using various approaches for the administration of the trophic
factor, these studies demonstrated that the administration of GDNF
following lesion generation increases the number of dopaminergic
cell bodies in the substantia nigra, the density of dopaminergic
fibers and dopamine levels in the striatum. Administration of GDNF
also induces the recovery of motor impairments (38). Finally, GDNF has also been observed
to exhibit neuroprotective functions under conditions leading to
the death of other types of neurons. Since GDNF has been found to
be involved in a considerable number of effects in the nervous
system, the present study aimed to determine whether GDNF plays a
role in etifoxine-stimulated neurite outgrowth (8).
In the present study, a well-defined PC12 cell model
was used to test whether etifoxine is involved in axon
regeneration. Etifoxine was observed to lead to increased neurite
outgrowth, while GDNF expression increased following 3 days of
treatment and GDNF receptor inhibitor blocked the effects of
etifoxine on nerve regeneration. These results demonstrate a role
of etifoxine in GDNF-induced neurite outgrowth.
Materials and methods
Cell culture and measurement
Rat PC12 cells were cultured on collagen-coated
plates (5 μg/cm2) in DMEM supplemented with 5% horse
serum, 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin.
Cells were counted in 6 fields throughout the entire culture dish.
At least 400 cells were counted per sample. Experiments were
repeated at least three times. Neuronal-like outgrowth was
determined on day 10 using the Olympus IX81 microscope and U-CMAD 3
camera (Olympus, Tokyo, Japan). Cell body size and axon length were
analyzed using the image analysis software version 3.2 (SIS,
Münster, Germany). PC12 cells with axons longer than the average
cell diameter were included for data collection. The two-tailed
Mann-Whitney U test (GraphPad Prism 4; GraphPad Software, San
Diego, CA, USA) was used for statistical assessment of GDNF release
and its bioactivity on the axonogenesis of PC12 cells.
RNA isolation
To collect total RNA, 2×106 cells were
seeded onto 10-cm diameter dishes in 8 ml growth medium. Cultures
were maintained at 37°C in a humidified atmosphere of 5%
CO2/95% air. Media were replaced with the drug of
interest. Following 6 days, total RNA was isolated using an Isogen
kit (Nippon Gene, Tokyo, Japan), according to the manufacturer’s
instructions. RNA quantity and purity were determined by
spectrophotometry (Beckman DU-65; Beckman Coulter, Miami, FL,
USA).
RT-PCR
RT-PCR was performed using an RNA PCR kit (AMV;
version 2.1) according to the manufacturer’s instructions and a PCR
Thermal Cycler (both Takara Bio, Inc., Shiga, Japan). First-strand
cDNA was synthesized from total RNA (1 mg) using AMV reverse
transcriptase XL primed by 50 pmol random 9-mers (Takara Bio,
Inc.). The first-strand reaction was performed as follows: 30°C for
10 min, 50°C for 30 min, 99°C for 5 min and 58°C for 5 min. RT
reaction products (10 ml) were utilized as templates in the PCR
with 0.2 mm each of the following primers:
5′-GGTCTACGGAGAGACCGATCCGAGGTGC-3′ and
5′-TCTCTGGAGCCAGGGTCAGATACATC-3′ for GDNF,
5′-TGAAGGTCGGTGTCAACGGATTTGGC-3′ and 5′-CATGTAGGCCATGAGGTCCACCAC-3′
for GAPDH. PCR products were separated by precast 2% agarose gel
(Daiichi Pure Chemicals, Tokyo, Japan) electrophoresis and
visualized by SYBR Green 2 (FMC Bio-Products, Rockland, ME, USA)
staining on a UV transilluminator. The signal intensity of the PCR
products was determined by ImageJ. The amounts of GDNF PCR product
were determined using calculations based on the intensity of the
product from the paired GAPDH reactions.
Western blot analysis
Western blot analysis was performed using antibodies
to detect total GDNF proteins (4G10; Upstate Biotechnology, Lake
Placid, NY, USA). PC12 cells were collected using ice-cold
phosphate-buffered saline and solubilized in the sample buffer [100
mM Tris-HCl (pH 6.8), 20% glycerol and 4% SDS]. Total protein in
each sample was adjusted to be the same amount for all samples.
Following the addition of 1,4-dithiothreitol, samples were boiled
for 5 min. Proteins were separated by SDS-polyacrylamide gel
electrophoresis and transblotted onto polyvinylidene difluoride
membranes. The blots were blocked with 10% skimmed milk for 2 h at
room temperature and then immunoblotted with rabbit anti-rat
antibodies against GDNF and GAPDH (1:100) overnight. After three
washes, the blots were subsequently incubated with a goat
anti-rabbit peroxidase conjugated secondary antibody (1:1,000) for
1 h at room temperature. Then the specific binding was detected
with the enhanced chemiluminescence system.
Statistical analysis
All numerical data are presented as the mean ± SE.
The results were subjected to statistical analysis using a student
version of SPSS 11.5 software for Windows. P<0.05 was considered
to indicate a statistically significant difference.
Results
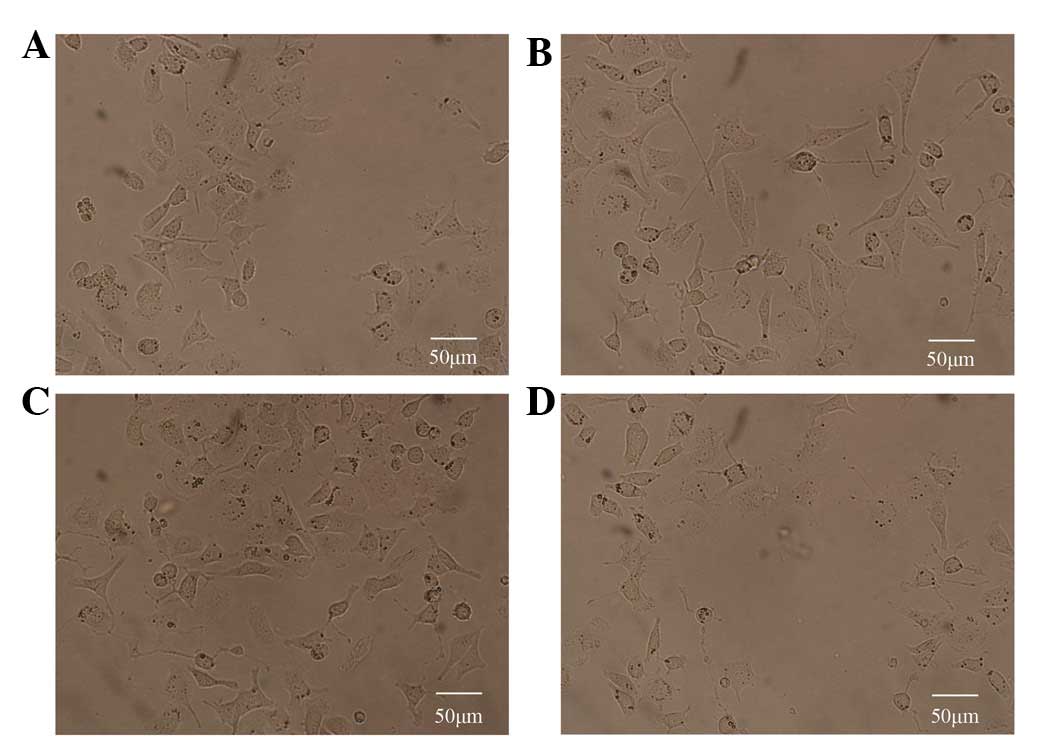
Etifoxine induces neuronal-like outgrowth
of PC12 cells
To detect the effect of etifoxine, the number of
PC12 cells demonstrating axonogenesis, their cell body sizes and
the overall length of outgrown fibers were determined. Fig. 1 presents the biological activity of
etifoxine; consistent with previous studies on the mechanism by
which etifoxine promotes neurite extension, a marked increase in
neuronal-like outgrowth was observed 10 days following the
application of etifoxine (Fig.
1B). Next, to determine whether etifoxine affected the cells
through the GDNF receptors, GDNF family receptor α1 (GFRα1) and
rearranged during transfection (RET), cultures were treated with
specific compounds known to block GDNF signaling. In contrast to
the effects of etifoxine, the administration of phosphoinositide
phospholipase C (PI-PLC), which blocks signaling via GFRα1 or PRI-1
(both Calbiochem, La Jolla, CA, USA), a specific RET receptor
tyrosine kinase inhibitor, induced poor neuronal-like processes in
PC12 cells following 10 days of cultivation.
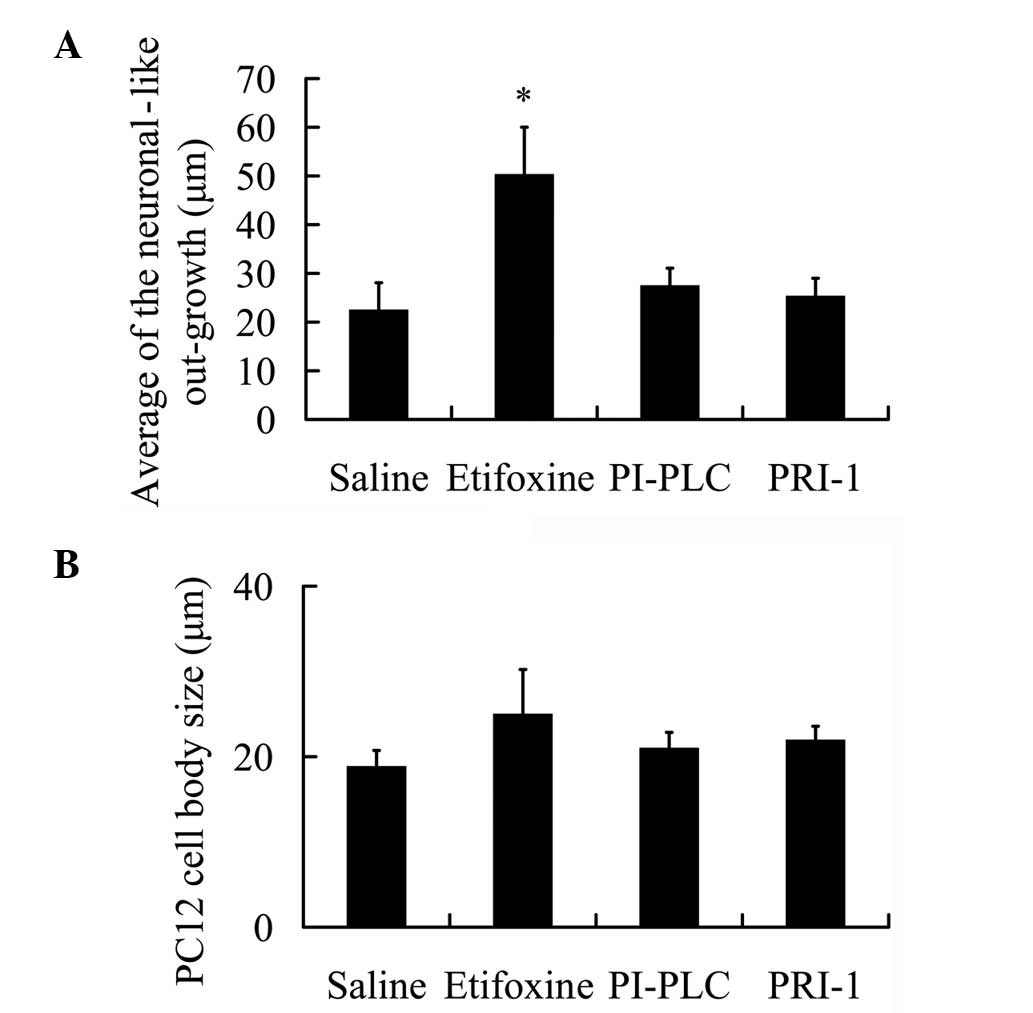
Statistical assessment of neuronal-like outgrowth of
PC12 cells revealed significant axonogenesis induction following
administration of GDNF (P<0.05; 50.29±9.73 μm) in comparison
with saline (22.46±5.62 μm; Fig.
2A). However, the use of PI-PLC and PRI-1 following
administration of etifoxine did not significantly induce
neuronal-like processes in the PC12 cells (27.46±3.59 and
25.31±3.68 μm, respectively). These observations indicate that
following blockage of GDNF downstream, etifoxine exerts no effect
on neuronal-like outgrowth in PC12 cells.
The average cell body size of PC12 was also
determined during the culture period as changes in morphology and
size of the cells indicate higher metabolic activity induced by
external stimuli. Cell body sizes were measured in all the groups.
The average PC12 cell body size increased following exposure to
etifoxine for 10 days in comparison with the cell body size of
those incubated with saline (Fig.
2B). However, a decline in the PC12 cell body size was noted in
the PI-PLC and PRI-1 treatment groups compared with the etifoxine
group (Fig. 2B), however, these
slight changes were not significant.
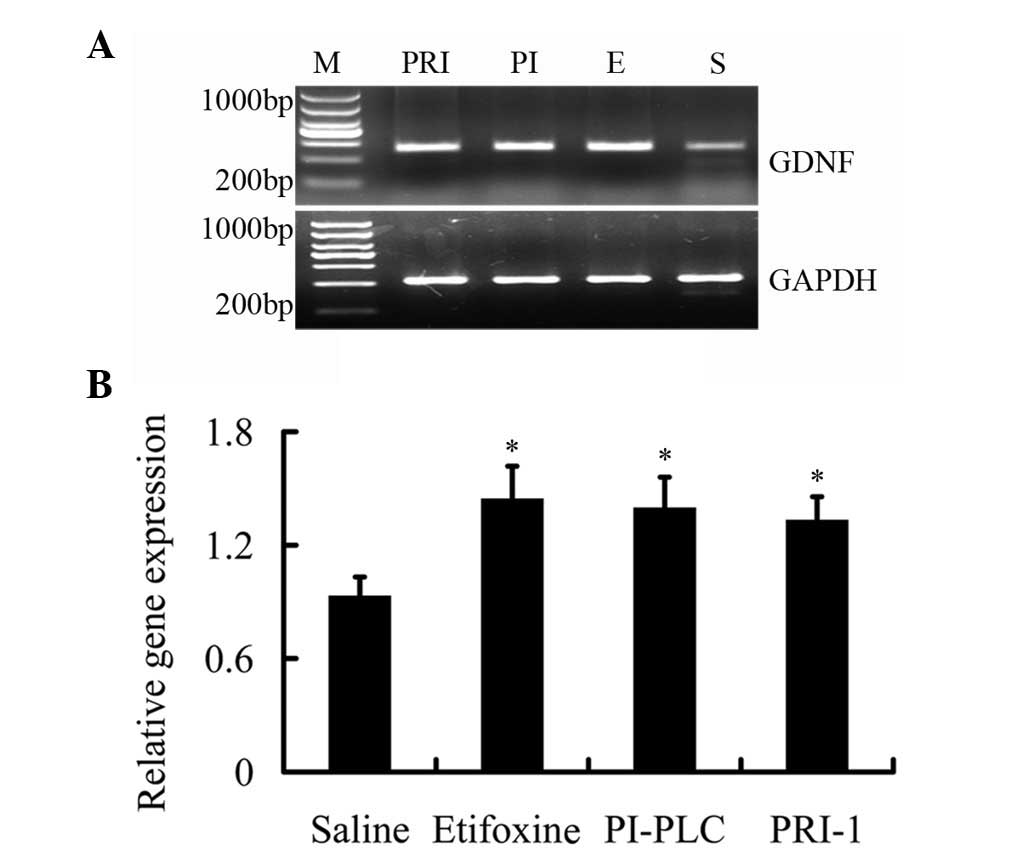
Expression of GDNF mRNA by drug
treatment
To address the mechanism of neuronal-like outgrowth
in PC12 cells induced by etifoxine, the expression of GDNF mRNA was
measured by RT-PCR using GDNF-specific primers. Following treatment
with etifoxine, GDNF mRNA expression levels increased 1.55-fold
compared with saline treatment; however, the difference compared
with etifoxine group was not identified to be significant (Fig. 3). These results were consistent
with the results of the outgrown fibers demonstrating that
etifoxine increased the neuronal-like outgrowth of PC12 cells.
Next, we determined whether treatment with PI-PLC and PRI-1 leads
to a sustained decrease in GDNF mRNA levels. Cells were treated
with etifoxine and fresh media were added with or without PI-PLC
and PRI-1. However, GDNF expression levels remained high following
the administration of PI-PLC and PRI-1 (1.50- and 1.43-fold,
respectively; P<0.05). These results demonstrate the opposite
result on the outgrown fibers (Fig.
3), which indicate that the use of PI-PLC and PRI-1 does not
change the expression levels of GDNF in PC12 cells.
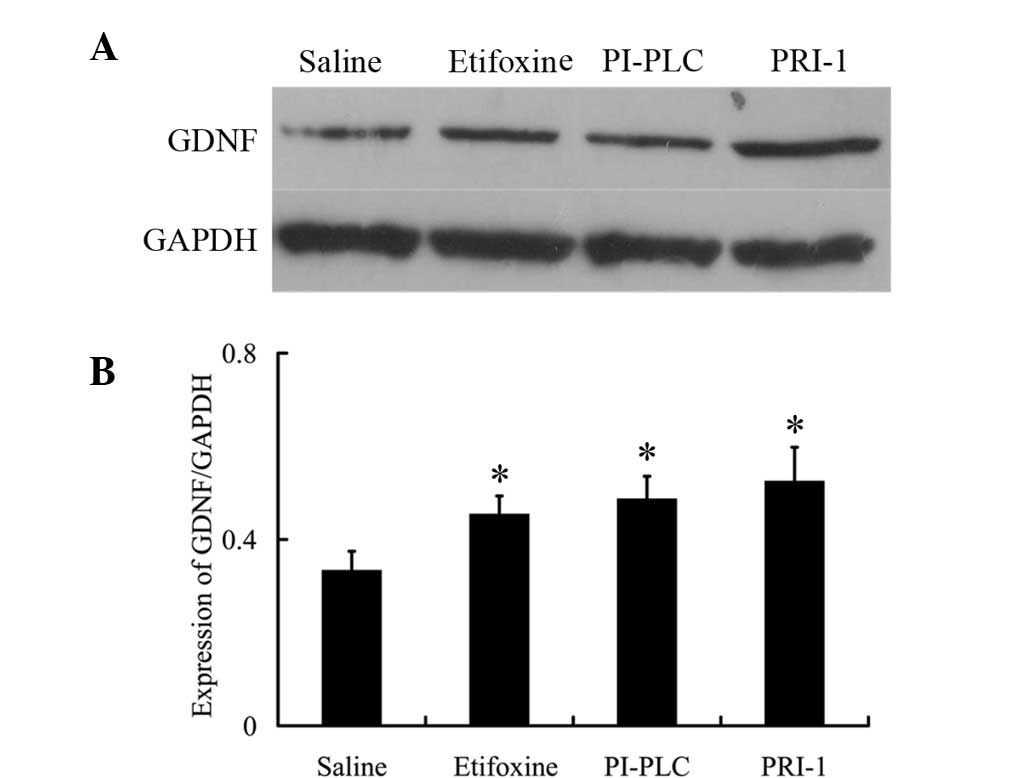
Expression of GDNF protein by drug
treatment
Finally, the effects of drug treatment on GDNF
protein expression were investigated. In addition, the effect of
etifoxine on GDNF expression was measured in PC12 cells by western
blot analysis. While the use of etifoxine increased GDNF levels in
PC12 cells (Fig. 4), this effect
was not inhibited by the use of PI-PLC and PRI-1. Following the
administration of etifoxine, GDNF protein expression increased by
~1.36-fold compared with the saline-treated group. Use of the GFRα1
inhibitor, PI-PLC, led to a higher expression of GDNF (1.46-fold
vs. saline). In addition, the RET inhibitor, PRI-1, was observed to
result in increased expression of GDNF (1.57-fold vs. saline);
however, the difference compared with etifoxine was not identified
to be significant (Fig. 4).
Discussion
Statistical analysis of the average length of fibers
in PC12 cells following exposure to etifoxine revealed
neuronal-like outgrowth increased up to 2.23-fold following day 10,
resulting in numerous partially arborescent axons. By contrast,
PC12 cultivation in supernatant with PI-PLC and PRI-1 induced poor
neuronal-like processes following 10 days of cultivation. These
observations indicate a marked in vitro bioactivity of
etifoxine, which is capable of inducing signaling in PC12 cells for
neuronal-like processes, indicating a potential clinical
application for etifoxine.
Previous studies have demonstrated that TSPO may
regulate outgrowth by increasing ATP availability, an essential
factor for neurite growth (39–41).
TSPO associates with the mitochondrial permeability transition pore
(42), which enables the
respiratory chain to create the transmembrane electrochemical
gradient that drives ATP synthesis. By this mechanism, TSPO may
regulate a number of biological functions in cells. In addition,
TSPO may also affect neurite outgrowth by controlling the rate of
neurosteroid formation (43).
Mitochondrial TSPO regulates the transport of cholesterol from the
outer to inner membrane, which is the rate-limiting step in steroid
production (19). The
neurosteroids, PREG, PROG and DHEA, play major roles in neuronal
regeneration. PROG increases neurite outgrowth of DRG explants,
promotes regeneration in cryolesioned sciatic nerves and
remyelination of regenerated nerve fibers (28,29).
PREG and PROG levels increase in injured sciatic nerves and have
neurotrophic effects (29,44). In addition, estradiol (a type of
steroid) leads to increased expression of GDNF. We hypothesize that
estradiol rapidly increases Ca2+ levels, which is
followed by CREB phosphorylation. CREB phosphorylation ultimately
leads to increased expression of GDNF (45). However, at present, the mechanism
by which TSPO activation leads to increased GDNF expression in PC12
cells remains unknown. One hypothesis is that TSPO activation leads
to increased steroidogenesis. Increased steroids may affect gene
expression through CREB phosphorylation. Thus, neurite outgrowth
increases, consistent with current observations in which treatment
with etifoxine led to an increase in GDNF expression, which
initiated its receptors resulting in poor regeneration without
alterations in GDNF expression.
GDNF is a glycosylated, disulfide-bonded homodimer
with a molecular weight of 33–45 kDa. The monomer has a molecular
weight of 16 kDa following deglycosylation (31) and it regulates cellular activity
through interaction with glycosylphosphatidylinositol-anchored cell
surface receptors. GFRα1, which may signal through the
transmembrane RET receptor or neural cell adhesion molecule (NCAM),
promotes cell survival, neurite outgrowth and synaptogenesis
(32). GFRα1 lacks transmembrane
and intracellular domains (46)
and therefore, GFRα1 functions only as a binding receptor,
requiring a transduction receptor for signaling. GFRα1 signals
through transmembrane RET tyrosine kinase, which in turn may
activate several intracellular signaling cascades, including
Ras/mitogen-activated protein kinase, phosphatidylinositol
3-kinase/Akt and PLCg pathways (47).
An additional signaling receptor for GFRα1 is NCAM
(48), which mediates neurite
outgrowth induced by GDNF in cultured hippocampal neurons (49). In the absence of GDNF, GFRα1 binds
NCAM, inhibiting cell adhesion mediated by homophilic NCAM
interaction. However, in the presence of GDNF, the GFRα1/GDNF/NCAM
complex mediates cell adhesion by a mechanism involving Fyn kinase
and focal adhesion kinase (46).
Considering the discussed observations, the physiological
significance of the functions and roles mediated through the
GDNF-GFRα1-RET pathway was reinforced by the observation that
RET-independent GFRα1 is dispensable for organogenesis and nerve
regeneration in vivo, indicating that trans- and
GFRα1-dependent NCAM signaling plays a minor physiological role
(50). Thus, RET-mediated
signaling in the nervous system is important for the survival and
differentiation of cancer, but also in human forms of cancer where
excessive activation of RET has been observed.
The current study demonstrates that treatment with
etifoxine in PC12 cells stimulated GDNF expression, which
correlated with neurite outgrowth. In addition, the results
indicate that following inhibition of the GDNF receptor, GFRα1-RET,
the increase in neurite outgrowth was lost. These results indicate
that in PC12 cells, the GDNF-GFRα1-RET pathway was dominant for
etifoxine-induced neurite outgrowth. As observed in this study, RET
blockage resulted in total abolition of neurite growth in
etifoxine-treated PC12 cells, demonstrating that for
etifoxine-induced neurite outgrowth, this pathway is important.
We hypothesize that the bioactivity of GDNF is
represented by PC12 cell body size since changes in morphology and
size of the cells indicate higher metabolic activity induced by
external stimuli (51). A slight,
but not significant, increase in the average cell body size was
demonstrated following 3 and 10 days of GDNF cultivation compared
with PC12 cells cultivated in medium with extremely low GDNF
content, indicating that GDNF is capable of inducing internal
signaling pathways in PC12 cells. However, cell body size may not
be an appropriate marker for GDNF or, in general, neurotrophic
factor (NTF) bioactivity.
In conclusion, the results of the present study
indicate that etifoxine markedly enhances neurite outgrowth by
increasing GDNF expression. Etifoxine fulfills the criteria of a
drug that is clinically useful for the treatment of altered
peripheral axons: i) easy diffusion into nerve tissues; ii)
selective modulation of inflammatory responses to injury; iii) able
to increase the expression of neurotrophic factors; iv) suitable
for long-term use (52,53) and (v) convenient administration.
Considering the important benefits of etifoxine, etifoxine
treatment may represent a promising strategy for the treatment of
peripheral nerve injury.
Acknowledgements
The authors would like to thank Dr Weihong Yang for
technical assistance. The current study was supported by grants
from the National High Technology Research and Development Program
of China (no. 2012AA020507), the National Nature Science Grant of
China (no. 30700847), Medical Scientific Research Foundation of
Guangdong Province, China (B2011176), the Key Project of Nature
Science Grant of Guangdong China (no. 9251008901000017) and the
China Postdoctoral Science Foundation (no. 20110490929).
References
|
1
|
IJkema-Paassen J, Jansen K, Gramsbergen A
and Meek MF: Transection of peripheral nerves, bridging strategies
and effect evaluation. Biomaterials. 25:1583–1592. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kline DG and Hudson AR: Vertebral artery
compression. J Neurosurg. 83:7591995.PubMed/NCBI
|
|
3
|
Lundborg G: Intraneural microcirculation.
Orthop Clin North Am. 19:1–12. 1988.PubMed/NCBI
|
|
4
|
Birch R: Surgery for brachial plexus
injuries. J Bone Joint Surg Br. 75:346–348. 1993.PubMed/NCBI
|
|
5
|
Airaksinen MS and Saarma M: The GDNF
family: signalling, biological functions and therapeutic value. Nat
Rev Neurosci. 3:383–394. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baloh RH, Enomoto H, Johnson EJ and
Milbrandt J: The GDNF family ligands and receptors - implications
for neural development. Curr Opin Neurobiol. 10:103–110. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chu TH and Wu W: Neurotrophic factor
treatment after spinal root avulsion injury. Cent Nerv Syst Agents
Med Chem. 9:40–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Girard C, Liu S, Cadepond F, et al:
Etifoxine improves peripheral nerve regeneration and functional
recovery. Proc Natl Acad Sci USA. 105:20505–20510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barakat-Walter I: Role of thyroid hormones
and their receptors in peripheral nerve regeneration. J Neurobiol.
40:541–559. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gold BG, Udina E, Bourdette D and Navarro
X: Neuroregenerative and neuroprotective actions of
neuroimmunophilin compounds in traumatic and inflammatory
neuropathies. Neurol Res. 26:371–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melcangi RC and Garcia-Segura LM:
Therapeutic approaches to peripheral neuropathy based on
neuroactive steroids. Expert Rev Neurother. 6:1121–1125. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schumacher M, Guennoun R, Mercier G, et
al: Progesterone synthesis and myelin formation in peripheral
nerves. Brain Res Brain Res Rev. 37:343–359. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verleye M, Akwa Y, Liere P, et al: The
anxiolytic etifoxine activates the peripheral benzodiazepine
receptor and increases the neurosteroid levels in rat brain.
Pharmacol Biochem Behav. 82:712–720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Papadopoulos V, Baraldi M, Guilarte TR, et
al: Translocator protein (18kDa): new nomenclature for the
peripheral-type benzodiazepine receptor based on its structure and
molecular function. Trends Pharmacol Sci. 27:402–409. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karchewski LA, Bloechlinger S and Woolf
CJ: Axonal injury-dependent induction of the peripheral
benzodiazepine receptor in small-diameter adult rat primary sensory
neurons. Eur J Neurosci. 20:671–683. 2004. View Article : Google Scholar
|
|
16
|
Lacor P, Benavides J and Ferzaz B:
Enhanced expression of the peripheral benzodiazepine receptor (PBR)
and its endogenous ligand octadecaneuropeptide (ODN) in the
regenerating adult rat sciatic nerve. Neurosci Lett. 220:61–65.
1996. View Article : Google Scholar
|
|
17
|
Torres SR, Frode TS, Nardi GM, et al:
Anti-inflammatory effects of peripheral benzodiazepine receptor
ligands in two mouse models of inflammation. Eur J Pharmacol.
408:199–211. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Veiga S, Azcoitia I and Garcia-Segura LM:
Ro5-4864, a peripheral benzodiazepine receptor ligand, reduces
reactive gliosis and protects hippocampal hilar neurons from kainic
acid excitotoxicity. J Neurosci Res. 80:129–137. 2005. View Article : Google Scholar
|
|
19
|
Baulieu EE: Neurosteroids: of the nervous
system, by the nervous system, for the nervous system. Recent Prog
Horm Res. 52:1–32. 1997.PubMed/NCBI
|
|
20
|
Ferzaz B, Brault E, Bourliaud G, et al:
SSR180575 (7-chloro-N,
5-trimethyl-4-oxo-3-phenyl-3,5-dihydro-4H-pyridazino[4,5-b]in
dole-1-acetamide), a peripheral benzodiazepine receptor ligand,
promotes neuronal survival and repair. J Pharmacol Exp Ther.
301:1067–1078. 2002.
|
|
21
|
Korneyev A, Pan BS, Polo A, Romeo E,
Guidotti A and Costa E: Stimulation of brain pregnenolone synthesis
by mitochondrial diazepam binding inhibitor receptor ligands in
vivo. J Neurochem. 61:1515–1524. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lacor P, Gandolfo P, Tonon MC, et al:
Regulation of the expression of peripheral benzodiazepine receptors
and their endogenous ligands during rat sciatic nerve degeneration
and regeneration: a role for PBR in neurosteroidogenesis. Brain
Res. 815:70–80. 1999. View Article : Google Scholar
|
|
23
|
Papadopoulos V, Amri H, Boujrad N, et al:
Peripheral benzodiazepine receptor in cholesterol transport and
steroidogenesis. Steroids. 62:21–28. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schumacher M, Robel P and Baulieu EE:
Development and regeneration of the nervous system: a role for
neurosteroids. Dev Neurosci. 18:6–21. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan QW, Yu W, Gong JS, et al:
Cholesterol-dependent modulation of dendrite outgrowth and
microtubule stability in cultured neurons. J Neurochem. 80:178–190.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan QW, Yu W, Senda T, Yanagisawa K and
Michikawa M: Cholesterol-dependent modulation of tau
phosphorylation in cultured neurons. J Neurochem. 76:391–400. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gudemez E, Ozer K, Cunningham B, Siemionow
K, Browne E and Siemionow M: Dehydroepiandrosterone as an enhancer
of functional recovery following crush injury to rat sciatic nerve.
Microsurgery. 22:234–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koenig HL, Gong WH and Pelissier P: Role
of progesterone in peripheral nerve repair. Rev Reprod. 5:189–199.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koenig HL, Schumacher M, Ferzaz B, et al:
Progesterone synthesis and myelin formation by Schwann cells.
Science. 268:1500–1503. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin LF, Doherty DH, Lile JD, Bektesh S and
Collins F: GDNF: a glial cell line-derived neurotrophic factor for
midbrain dopaminergic neurons. Science. 260:1130–1132. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin LF, Zhang TJ, Collins F and Armes LG:
Purification and initial characterization of rat B49 glial cell
line-derived neurotrophic factor. J Neurochem. 63:758–768. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duarte EP, Curcio M, Canzoniero LM and
Duarte CB: Neuroprotection by GDNF in the ischemic brain. Growth
Factors. 30:242–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Henderson CE, Phillips HS, Pollock RA, et
al: GDNF: a potent survival factor for motoneurons present in
peripheral nerve and muscle. Science. 266:1062–1064. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arenas E, Trupp M, Akerud P and Ibanez CF:
GDNF prevents degeneration and promotes the phenotype of brain
noradrenergic neurons in vivo. Neuron. 15:1465–1473. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trupp M, Ryden M, Jornvall H, Funakoshi H,
Timmusk T, Arenas E and Ibanez CF: Peripheral expression and
biological activities of GDNF, a new neurotrophic factor for avian
and mammalian peripheral neurons. J Cell Biol. 130:137–148. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Georgievska B, Kirik D, Rosenblad C,
Lundberg C and Bjorklund A: Neuroprotection in the rat Parkinson
model by intrastriatal GDNF gene transfer using a lentiviral
vector. Neuroreport. 13:75–82. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun B, Hui GZ, Guo LH and Reiser J:
Dopaminergic trophism after intrastriatal injection of
lentivirus-transferred GDNF in Parkinson rat model. Sheng Wu Hua
Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 35:937–940.
2003.PubMed/NCBI
|
|
38
|
Saavedra A, Baltazar G and Duarte EP:
Driving GDNF expression: the green and the red traffic lights. Prog
Neurobiol. 86:186–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Behrsing HP and Vulliet PR: Purinergic and
calcium-mediated enhancement of NGF-induced neurite expression in
PC12 cells. Proc West Pharmacol Soc. 42:59–62. 1999.PubMed/NCBI
|
|
40
|
Behrsing HP and Vulliet PR:
Mitogen-activated protein kinase mediates purinergic-enhanced nerve
growth factor-induced neurite outgrowth in PC12 cells. J Neurosci
Res. 78:64–74. 2004. View Article : Google Scholar
|
|
41
|
D’Ambrosi N, Murra B, Cavaliere F, Amadio
S, Bernardi G, Burnstock G and Volonte C: Interaction between ATP
and nerve growth factor signalling in the survival and neuritic
outgrowth from PC12 cells. Neuroscience. 108:527–534. 2001.
|
|
42
|
Soustiel JF, Zaaroor M, Vlodavsky E,
Veenman L, Weizman A and Gavish M: Neuroprotective effect of
Ro5-4864 following brain injury. Exp Neurol. 214:201–208. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mills CD, Bitler JL and Woolf CJ: Role of
the peripheral benzodiazepine receptor in sensory neuron
regeneration. Mol Cell Neurosci. 30:228–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Akwa Y, Schumacher M, Jung-Testas I and
Baulieu EE: Neurosteroids in rat sciatic nerves and Schwann cells.
C R Acad Sci III. 316:410–414. 1993.PubMed/NCBI
|
|
45
|
Ivanova T, Karolczak M and Beyer C:
Estradiol stimulates GDNF expression in developing hypothalamic
neurons. Endocrinology. 143:3175–3178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Paratcha G and Ledda F: GDNF and GFRalpha:
a versatile molecular complex for developing neurons. Trends
Neurosci. 31:384–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sariola H and Saarma M: Novel functions
and signalling pathways for GDNF. J Cell Sci. 116:3855–3862. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Paratcha G, Ibanez CF and Ledda F: GDNF is
a chemoattractant factor for neuronal precursor cells in the
rostral migratory stream. Mol Cell Neurosci. 31:505–514. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nielsen J, Gotfryd K, Li S, et al: Role of
glial cell line-derived neurotrophic factor (GDNF)-neural cell
adhesion molecule (NCAM) interactions in induction of neurite
outgrowth and identification of a binding site for NCAM in the heel
region of GDNF. J Neurosci. 29:11360–11376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Enomoto H: Regulation of neural
development by glial cell line-derived neurotrophic factor family
ligands. Anat Sci Int. 80:42–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wissel K, Stöver T, Hofmann NS, et al:
Fibroblast-mediated delivery of GDNF induces neuronal-like
outgrowth in PC12 cells. Otol Neurotol. 29:475–481. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Micallef J, Soubrouillard C, Guet F, Le
Guern ME, Alquier C, Bruguerolle B and Blin O: A double blind
parallel group placebo controlled comparison of sedative and mnesic
effects of etifoxine and lorazepam in healthy subjects [corrected].
Fundam Clin Pharmacol. 15:209–216. 2001.PubMed/NCBI
|
|
53
|
Nguyen N, Fakra E, Pradel V, et al:
Efficacy of etifoxine compared to lorazepam monotherapy in the
treatment of patients with adjustment disorders with anxiety: a
double-blind controlled study in general practice. Hum
Psychopharmacol. 21:139–149. 2006. View Article : Google Scholar
|