Introduction
Liver fibrosis is considered to be a model of the
wound-healing response to chronic liver injury (1). Multiple etiologies of liver injury
may lead to fibrosis, such as viral infection, alcohol abuse, drug
toxicity and autoimmune hepatopathy (2). Liver fibrosis is characterized by the
over accumulation of extracellular matrix proteins (ECM),
particularly collagen types I and III (3). The activation of hepatic stellate
cells (HSCs) is crucial in the pathogenesis of liver fibrogenesis.
Transforming growth factor-β (TGF-β) is widely acknowledged as a
crucial factor in the acceleration of the process of liver
fibrosis, and is mostly activated by HSCs through the TGF-β/Smad
signaling pathway, thereby causing liver fibrosis (4–6).
Advanced liver fibrosis results in cirrhosis, portal hypertension
and liver failure, for which no effective medical treatments are
currently available (7). However,
liver fibrosis may be reversed in the early stages by the
intervention of external factors (8). Thus, there is a great need for
developing novel therapies to treat liver fibrosis.
In recent years, the majority of therapeutic
strategies for liver fibrosis have been focused on the TGF-β1/Smad
signaling pathway; however, the inhibition of TGF-β1 expression
signaling by various approaches, such as by using monoclonal
antibodies to target TGF-β1, soluble TGF-β receptors or an ALK5
(the TGF-β type I receptor) inhibitor only partially relieve liver
fibrosis (9,10). These results indicated that
blocking TGF-β1 alone may miss other potentially major therapeutic
targets for the treatment of liver fibrosis.
Wnt/β-catenin is an evolutionarily conserved
cellular signaling system essential for various biological
processes, such as embryonic development, homeostasis self-renewal
and the pathogenesis of a variety of human diseases (11). Studies have demonstrated that the
aberrant Wnt/β-catenin signaling pathway plays a vital role in the
development of liver fibrosis (12–14).
Our previous studies have suggested that β-catenin may aggravate
hepatic fibrosis induced by TGF-β1 in vitro(15). Furthermore, there is cross-talk
between Wnt/β-catenin signaling and TGF-β signaling in the fibrosis
process (16,17).
The histidine triad nucleotide-binding protein 1
(Hint1), a member of the evolutionary highly conserved HIT protein
superfamily, is a haplo-insufficient tumor suppressor (18,19).
A variety of studies have demonstrated that the Hint1 protein has
an inhibitory role in several pathways, such as the TGF-β/Smad and
Wnt/β-catenin pathways (20–23).
However, little data regarding the effects of Hint1 on liver
fibrosis exists. Herein, we hypothesize that Hint1 is a promising
therapeutic target for liver fibrosis. In this study, we used
genetic engineering technology to acquire the recombinant human
Hint1 protein (rhHint1), and subsequently investigated the effects
of rhHint1 on carbon tetrachloride (CCl4)-induced liver
fibrosis and the possible underlying mechanism.
Materials and methods
Purification of the rhHint1 protein
Human Hint1 cDNA was obtained from total RNA of LoVo
cells (China Center for Type Culture Collection, Wuhan, Hubei,
China) by reverse transcription-polymerase chain reaction (RT-PCR).
The recombinant PET28a-Hint1 expression vector was constructed and
transformed into E. coli (DE3). The activated engineering
strain PET28a-Hint1/(DE3) was subsequently inoculated into a
lysogeny broth (LB) medium (with kanamycin) at 37°C, whilst being
agitated at 200 rpm. After 12 h, isopropyl
β-D-1-thiogalactopyranoside (IPTG; Yuanye Biological Technology,
Shanghai, China) was added to induct for 16 h with a final IPTG
concentration of 0.1 mmol/l. Purification was accomplished using
immobilized metal (Ni2+) affinity column chromatography
(GE Healthcare, Madison, WI, USA). Sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie
brilliant blue staining confirmed the purity of the rhHint1.
Animals and treatment
In total, 55 adult male Sprague-Dawley (SD) rats
(200±20 g) were obtained from the Experimental Animal Center of
Tongji Medical College of Huazhong University of Science and
Technology (Wuhan, Hubei, China; certificate no. 4209800073). The
rats were provided with standard feed and water ad libitum
and individually housed at a constant temperature (18–20°C) and
humidity (60–70%) with a 12 h light/dark cycle. All experimental
procedures were approved by the Institutional Animal Ethics
Committee of Tongji Medical College, Huazhong University of Science
and Technology and also performed according to the Laboratory
Animal Care and Usage Manual of the university.
We used the liver fibrosis modeling method according
to Yao et al(24).
Following a week of acclimatizing to the standard conditions, the
rats (n=45, in addition, 10 rats were selected as the normal
control group) were subcutaneously administered 50% CCl4
(CCl4: olive oil, 1:1) at 0.3 ml/100 g of body weight,
twice a week for eight weeks. After four weeks, the model rats were
randomly divided into three experimental groups: i) fibrotic model
group (given CCl4 only); ii) CCl4 + low dose
rhHint1 (50 μg/kg); iii) CCl4 + high dose rhHint1 (100
μg/kg). The dose of rhHint1 was selected based on our previous
studies (25). All rats in the
rhHint1-treated groups were administered their respective doses by
an i.p. injection simultaneously with the CCl4 injection
for four continuous weeks. The rats of the normal control and model
groups were i.p. injected with the same dose of sterilized saline.
The rats were anesthetized using 10% chloral hydrate (0.4 ml/100 g)
24 h after the final treatment, and were subsequently sacrificed
and a section of the right hepatic lobe was removed. A section of
the liver was fixed in 10% buffered formalin and the remaining
tissues were stored at −80°C until required.
Histopathological analysis
The liver tissues were fixed and embedded in
paraffin, 5-μm sections were stained with hematoxylin & eosin
(HE) for routine histology and Masson’s trichrome stained to detect
collagen. Serial sections were examined under a microscope
(Olympus, Tokyo, Japan) and photographed. Quantitative analysis of
the fibrous area was calculated using Image-Pro plus 6.0 imaging
software using five microscopic fields for each specimen.
Immunohistochemistry
For immunohistochemical analysis, 5-μm sections were
deparaffinized in xylene and rehydrated in alcohol. Antigen
retrieval was achieved by a 500-W microwave, and the sections were
heated in citric saline for 15 min. After blocking endogenous
peroxidases with 3% hydrogen peroxide for 10 min, sections were
treated with 5% BSA in order to block the non-specific binding of
antibodies and subsequently incubated with the anti-α-SMA antibody,
1:100 dilution (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) overnight at 4°C. After washing in PBS, sections were
incubated with the appropriate peroxidase-conjugated secondary
antibody (goat anti-mouse IgG; Boster Biological Technology, Wuhan,
China) for 50 min at 4°C. Positive staining was visualized using a
DAB enhancer (Guge Biotechnology, Wuhan, Hubei, China) and washed
with water prior to counterstaining with hematoxylin. Quantitative
analysis of the immunopositive cell area was performed with the
Image-Pro plus 6.0 imaging software with five microscopic fields
for each specimen.
Detection of hydroxyproline in liver
tissue
Liver tissue (80 mg) samples were subjected to base
hydrolysis to determine the levels of hydroxyproline. It was
measured using the Hydroxyproline Alkaline Hydrolysis test kit
according to the manufacturer’s instructions (Jiancheng
Bioengineering Institute, Nanjing, Jiangsu, China). The
hydroxyproline levels were expressed as μg/g of wet liver
tissue.
Quantitative polymerase chain reaction
(qPCR) analysis
Total RNA of the liver tissues were extracted using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions. mRNA was converted
using a cDNA First Chain Synthesis kit (Fermentas, Foster City, CA,
USA). Quantitative assessment of cDNA was performed with a SYBR
Green Master Mix kit (Fermentas) using an ABI prism 7900 Sequence
detector (Applied Biosystems, Foster City, CA, USA) according to
the manufacturer’s instructions. β-actin was used as an internal
control. The following primers were used: TGF-β1:
5′-GTCAACTGTGGAGCAACACG-3′ (forward), 5′-ACTGAAGCGAAAGCCCTGTA-3′
(reverse); Smad3: 5′-TGATCCCTCCAATTCAGAGC-3′ (forward),
5′-GTTGGGAGACTGGACGAAAA-3′ (reverse); Smad7:
5′-TGTGTCCAAGAGCCCTCCCT-3′ (forward), 5′-CACGCCATCCACTTCCCTT-3′
(reverse); β-catenin: 5′-CGACTAAGCAGGAAGGGATG-3′ (forward),
5′-ATGGCAGGCTCGGTAATG-3′ (reverse); cyclin D1:
5′-TGCTGGCGAAGGTTTAGG-3′ (forward), 5′-GAGCGGCGGCAAGAATGT-3′
(reverse); β-actin: 5′-CACGATGGAGGGGCCGGACTCATC-3′ (forward),
5′-TAAAGACCTCTATGCCAACACAGT-3′ (reverse). The relative gene
expression levels were expressed as fold changes following
normalization with β-actin and calculated using the comparative Ct
method formula, 2−ΔΔCt.
Statistical analysis
Experiments were repeated a minimum of three times,
and the values were expressed as the means ± SD. Statistical
analysis was performed using one-way analysis of variance (ANOVA)
for multiple group comparisons or the Student’s t-test for two
group comparisons. The data were analyzed using SPSS 17.0 software.
P<0.05 was considered to indicate a statistically significant
difference.
Results
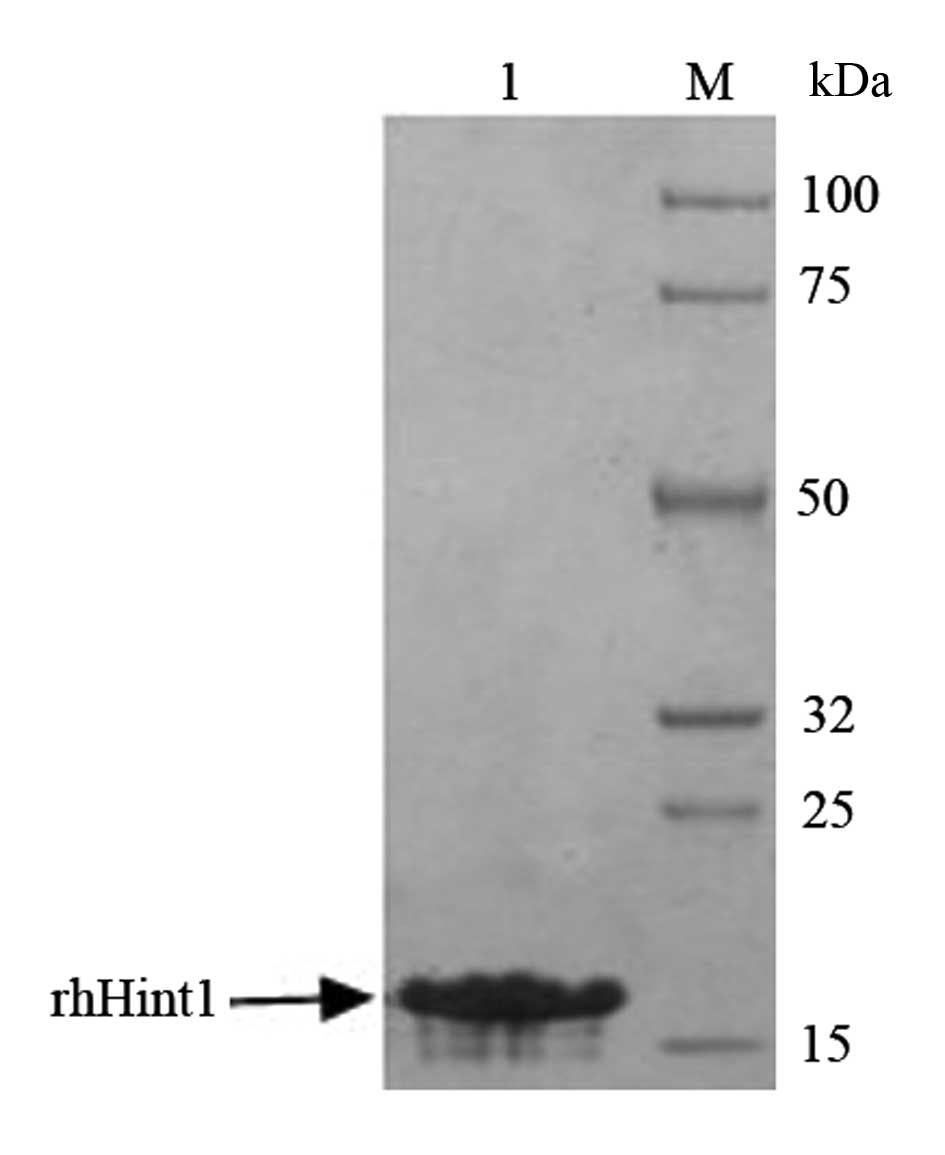
Identification of rhHint1
SDS-PAGE and Coomassie brilliant blue staining were
used to confirm and identify the purity of rhHint1. The protein was
>95% pure as judged by 12% SDS-PAGE with an expected molecular
mass of 14 kDa (Fig. 1).
Effect of rhHint1 on the histological
changes in the liver
In this study, routine histological analysis and
collagen fiber examination of the livers in SD rats used HE
staining and Masson’s trichrome staining, respectively.
Representative images of the liver morphology are shown in Fig. 2A and B. For the HE staining, when
compared with the normal control group, there were prominent
hepatic steatosis, necrosis, formation of regenerative nodules and
fibrotic septa caused by CCl4 treatment. However, in the
rhHint1 treatment group, the rats presented with hepatic steatosis,
fewer fibrotic septa and inflammatory infiltrate when compared with
the model group (Fig. 2A). With
Masson’s trichrome staining, there was a limited quantity of
visible collagen fiber (blue) in the normal control group, whereas,
it was found that a greater quantity of collagen fiber was present
with thickening of the partial compartments in the model group.
Furthermore, it was found that a medium quantity of collagen fiber
was present and extended to the peripheral region without distinct
compartment formation in the groups treated with rhHint1 and the
anti-fibrotic effect was more marked with an increase in the dose
(Fig. 2B). The quantitative
analysis of the fibrous area percentage showed that collagen
deposition was significantly lower in the rhHint1-treated group
compared with the model group (P<0.01; Fig. 2C).
Effect of rhHint1 on the level of
hydroxyproline in the liver
As the hydroxyproline level in the liver tissue was
parallel to the extent of fibrosis, next we detected the liver
hydroxyproline content in each group. In this study, we observed
that liver fibrosis induced by CCl4 caused a significant
rise in the levels of hydroxyproline (P<0.01), whereas treatment
with rhHint1 significantly decreased the level of the
hydroxyproline compared with the fibrotic model group (P<0.01),
and administration with rhHint1 (100 μg/kg) caused a more
significant decrease in the levels of hydroxyproline than rhHint1
(50 μg/kg; P<0.01; Fig.
2D).
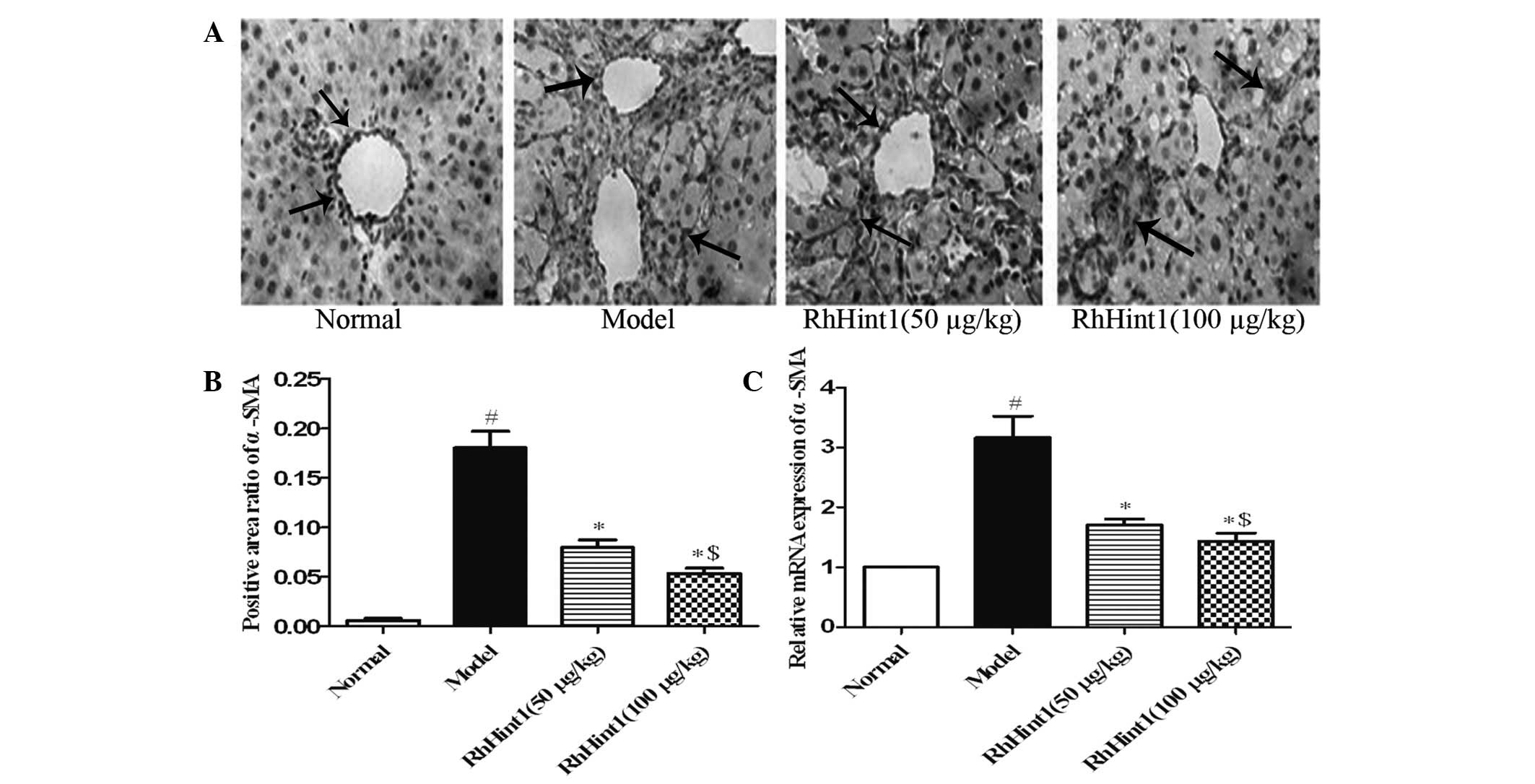
Effect of rhHint1 on the activation of
HSCs in the liver
To evaluate the effect of rhHint1 treatment on the
activation of HSCs in vivo, we detected the expression of
α-SMA in the liver by immunohistochemical staining and qPCR. As
shown in Fig. 3A and B, only a few
cells in the liver section from the normal control group were
recognized by anti-α-SMA. The administration of CCl4
produced a marked increase in the number of cells recognized by
anti-α-SMA (P<0.01). rhHint1 treatment significantly reduced the
number of cells labeled with anti-α-SMA (P<0.01), but there was
no statistical significance between the rhHint1 (50 μg/kg) and
rhHint1 (100 μg/kg) groups (P>0.05). Meanwhile, rhHint1
treatment decreased the α-SMA mRNA expression compared with the
model group (P<0.01), and presented no statistical significance
difference with an increase in the dosage (P>0.05; Fig. 3C).
Effect of rhHint1 on the expression of
TGF-β1/Smad in the liver
To investigate the effect of rhHint1 treatment on
the TGF-β1/Smad signaling pathway, we examined TGF-β1, Smad3 and
Smad7 mRNA expression using qPCR. As shown in Fig. 4, compared with the normal control
group, TGF-β1 and Smad3 mRNA expression increased markedly, whereas
Smad7 mRNA levels decreased significantly (P<0.01). Compared
with the model group, TGF-β1 and Smad3 mRNA levels were markedly
reduced in the rhHint1-treated group, whereas Smad7 mRNA levels
were significantly elevated (P<0.01). Furthermore, there was no
statistically significant difference between rhHint1 (50 μg/kg) and
rhHint1 (100 μg/kg) treatment in TGF-β1, Smad3 mRNA expression
(P>0.05), but the high dose of rhHint1 treatment caused a
significant increase in Smad7 mRNA expression compared with the low
dose (P<0.01).
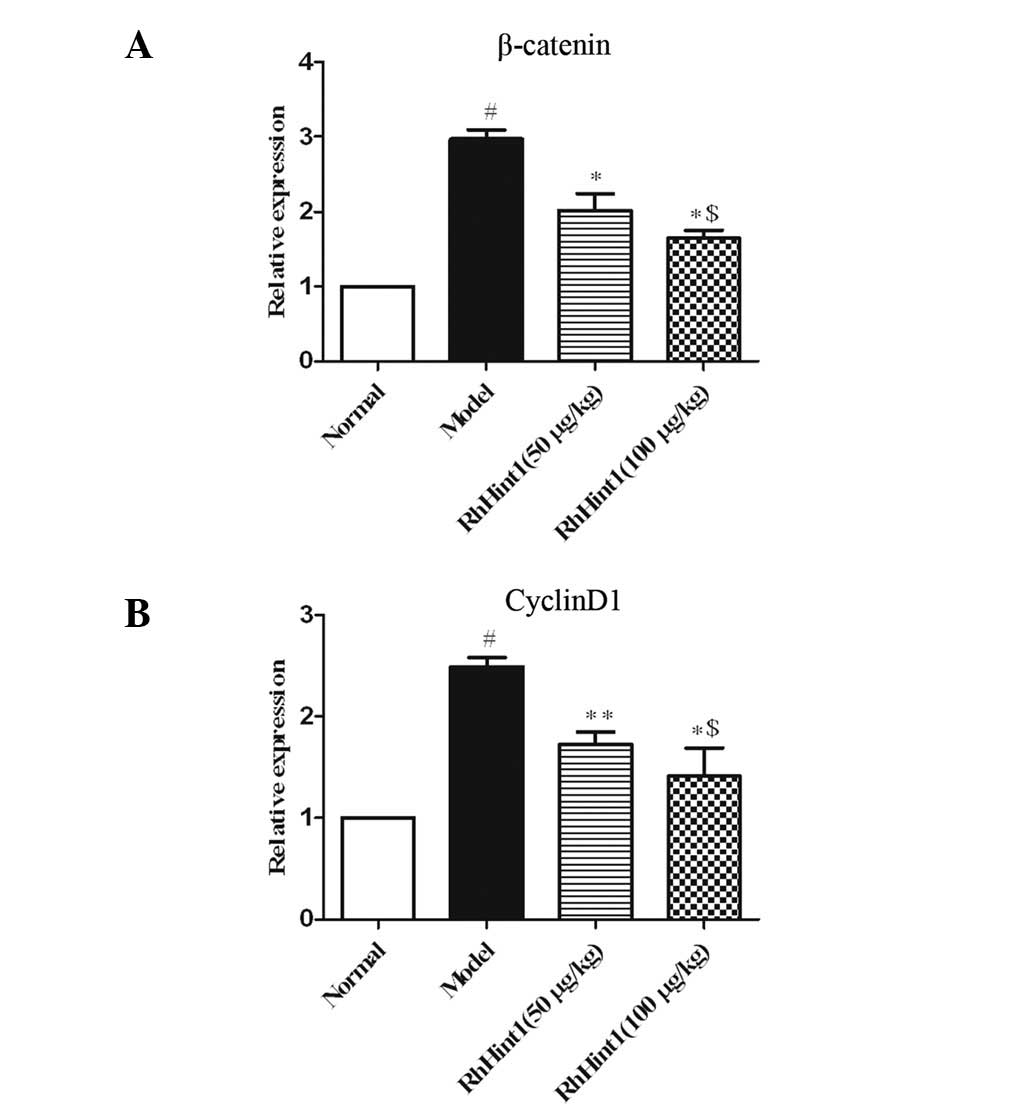
Effect of rhHint1 on the expression of
β-catenin/cyclin D1 in the liver
To further investigate the effects of rhHint1
treatment on the Wnt/β-catenin signaling pathway, we examined
β-catenin and cyclin D1 mRNA expression using qPCR (Fig. 5). The results demonstrated that
β-catenin and cyclin D1 mRNA expression were elevated in the
CCl4-treated group compared with the normal control
group (P<0.01), whereas β-catenin and cyclin D1 mRNA expression
were significantly reduced in the rhHint1-treated group compared
with the model group (P<0.05). Additionally, there was no
statistical significance between the rhHint1 (50 μg/kg) and the
rhHint1 (100 μg/kg) groups (P>0.05).
Discussion
Liver fibrosis is the progressive accumulation of
ECM proteins that occurs in the majority of chronic liver diseases.
Advanced liver fibrosis is associated with high morbidity and
mortality (26). However, there
are currently no effective anti-fibrotic therapies available for
clinical use. Thus, it is essential to develop new therapeutic
strategies to counteract liver fibrosis.
The increased deposition of collegen is a
characteristic feature of liver fibrosis. As shown in Fig. 2, the fibrosis model group exhibited
a pattern of fibrosis with the formation of regenerative nodules
and fibrotic septa upon histopathological analysis. Thus,
CCl4-induced liver fibrosis in SD rats is a useful
animal model for studying the anti-fibrotic effect of rhHint1. In
this study, HE and Masson’s trichrome staining indicated that
fibrogenesis could be reduced following treatment with rhHint1. In
addition, the hydroxyproline content of the livers of the
rhHint1-treated group was significantly lower compared with that of
the fibrotic model group. These results suggested that rhHint1
exhibited a suppressive effect on the progression of liver fibrosis
induced by CCl4 in rats.
Several studies have theorized that HSCs play a
central role in the pathogenesis of liver fibrosis, and α-SMA
expression is a reliable marker of the activation of HSCs in
vivo. Furthermore, the expression of α-SMA is commonly used to
quantitate the number of activated HSCs (27,28).
In this study, we found that α-SMA expression was markedly reduced
in the rhHint1 treatment group compared with the model group. This
suggested that rhHint1 may inhibit the activation of HSCs.
The TGF-β/Smad signaling pathway is central to the
development of liver fibrosis. Smad7, an inhibitory Smad, acts in a
negative feedback loop to inhibit TGF-β1 activity by preventing the
phosphorylation of Smad2/3 (29).
The results of this study indicate that rhHint1 may decrease TGF-β1
and Smad3 gene expression by increasing Smad7 gene expression in
CCl4-induced rat liver fibrosis, therefore activating
the negative feedback effects of inhibiting liver fibrosis.
A number of studies have suggested that the
reactivation of the Wnt/β-catenin pathway is linked to liver
fibrosis (12–14). Furthermore, Wnt/β-catenin signaling
functions in a combinational manner with TGF-β signaling in liver
fibrosis. Cheon et al(30)
found that the wound phenotype imparted by a Smad3 deficiency and
by the injection of TGF-β prior to inflicting the wound is mediated
in part by β-catenin in cutaneous healing, and TGF-β was unable to
regulate the proliferation in β-catenin null fibroblasts. Medici
et al(31) determined that
there is a unified signaling mechanism driven by the convergence of
multiple TGF-β and β-catenin-TCF signaling molecules in promoting
EMT. Furthermore, Cheng et al(12) investigated the role of
Wnt/β-catenin in HSCs and verified that nuclear β-catenin was
markedly increased in culture-activated HSCs compared with
quiescent HSCs. Cyclin D1 is a major regulator of the progression
of cells into the proliferative stage of the cell cycle, and is
also a direct downstream target gene of the Wnt/β-catenin pathway
(32). In this study, we examined
the effect of rhHint1 on the Wnt/β-catenin pathway and observed a
significant downregulation of β-catenin and cyclin D1, suggesting
that the anti-fibrotic effect of rhHint1 is mediated through the
suppression of the Wnt/β-catenin pathway.
Hint1, as a novel tumor suppressor, is a member of
the Hint branch of the evolutionary conserved histidine triad (HIT)
protein family, which is characterized by a common
His-X-His-X-His-XX motif (X, hydrophobic amino acid). The
identification of the Hint1 interaction partners suggested that it
might be involved in the regulation of transcription processes
(33). Weiske et
al(21) reported that Hint1
was a negative regulator of the TCF-β-catenin transcriptional
activity, and repressed the expression of its downstream
fibrosis-related target gene cyclin D1. Wang et al(23) found that the increased expression
of HINT1 in HepG2 cells markedly inhibited the transcriptional
activities of β-catenin, and inhibited the expression of endogenous
cyclin D1 and TGF-β. To date, no treatment has been capable of
completely inhibiting the progress of liver fibrosis. Herein, we
present a new treatment that is a promising therapeutic strategy
that may inhibit TGF-β and β-catenin.
In conclusion, the results in this study showed that
rhHint1 treatment may markedly attenuate CCl4-induced
fibrosis. The primary mechanisms of this anti-fibrotic effect were
mediated by suppressing collagen deposition, inhibiting the
activation of HSCs and regulating the TGF-β1/Smad and Wnt/β-catenin
pathways simultaneously. Furthermore, it may provide us a with new
therapeutic strategy for liver fibrosis.
Acknowledgements
This study was supported by the National Nature
Science Fund of China (no. 30972607).
References
|
1
|
Albanis E and Friedman SL: Hepatic
fibrosis: Pathogenesis and principles of therapy. Clin Liver Dis.
5:315–334. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gutierrez-Reyes G, Gutierrez-Ruiz MC and
Kershenobich D: Liver fibrosis and chronic viral hepatitis. Arch
Med Res. 38:644–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedman SL: Liver fibrosis - from bench
to bedside. J Hepatol. 38:S38–S53. 2003. View Article : Google Scholar
|
|
4
|
Parsons CJ, Takashima M and Rippe RA:
Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol
Hepatol. 22:S79–S84. 2007. View Article : Google Scholar
|
|
5
|
Gressner AM and Weiskirchen R: Modern
pathogenetic concepts of liver fibrosis suggest stellate cells and
TGF-beta as major players and therapeutic targets. J Cell Mol Med.
10:76–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inagaki Y and Okazaki I: Emerging insights
into transforming growth factor beta Smad signal in hepatic
fibrogenesis. Gut. 56:284–292. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View
Article : Google Scholar
|
|
8
|
Sohrabpour AA, Mohamadnejad M and
Malekzadeh R: Review article: the reversibility of cirrhosis.
Aliment Pharmacol Ther. 36:824–832. 2012.PubMed/NCBI
|
|
9
|
Varga J and Pasche B: Antitransforming
growth factor-beta therapy in fibrosis: recent progress and
implications for systemic sclerosis. Curr Opin Rheumatol.
20:720–728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Gouville AC, Boullay V, Krysa G, et al:
Inhibition of TGF-beta signaling by an ALK5 inhibitor protects rats
from dimethylnitrosamine-induced liver fibrosis. Br J Pharmacol.
145:166–177. 2005.PubMed/NCBI
|
|
11
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng JH, She HY, Han YP, et al: Wnt
antagonism inhibits hepatic stellate cell activation and liver
fibrosis. Am J Physiol Gastrointest Liver Physiol. 294:G39–G49.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li W, Zhu C, Chen X, Li Y, Gao R and Wu Q:
Pokeweed antiviral protein down-regulates Wnt/beta-catenin
signalling to attenuate liver fibrogenesis in vitro and in vivo.
Dig Liver Dis. 43:559–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thompson MD and Monga SP: WNT/beta-catenin
signaling in liver health and disease. Hepatology. 45:1298–1305.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li WT, He YW, Xiao ZH and Ma YB: Effect of
beta-catenin on the activation of hepatic stellate cells induced by
transforming growth factor-beta1. Zhonghua Gan Zang Bing Za Zhi.
17:188–192. 2009.(In Chinese).
|
|
16
|
Baarsma HA, Spanjer AI, Haitsma G, et al:
Activation of WNT/beta-catenin signaling in pulmonary fibroblasts
by TGF-beta1 is increased in chronic obstructive
pulmonary disease. PLoS ONE. 6:e254502011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian X, Zhang J, Tan TK, et al:
Association of beta-catenin with P-Smad3 but not LEF-1
differentiates in vitro profibrotic and anti-inflammatory effects
of TGF-beta1. J Cell Sci. 2012.PubMed/NCBI
|
|
18
|
Li H, Zhang Y, Su T, Santella RM and
Weinstein IB: Hint1 is a haplo-insufficient tumor suppressor in
mice. Oncogene. 25:713–721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weiske J and Huber O: The histidine triad
protein Hint1 triggers apoptosis independent of its enzymatic
activity. J Biol Chem. 281:27356–27366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huber O and Weiske J: Beta-catenin takes a
HIT. Cell Cycle. 7:1326–1331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weiske J and Huber O: The histidine triad
protein Hint1 interacts with Pontin and Reptin and inhibits
TCF-beta-catenin-mediated transcription. J Cell Sci. 118:3117–3129.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Genovese G, Ghosh P, Li H, et al: The
tumor suppressor HINT1 regulates MITF and beta-catenin
transcriptional activity in melanoma cells. Cell Cycle.
11:2206–2215. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Li H, Zhang Y, Santella RM and
Weinstein IB: HINT1 inhibits beta-catenin/TCF4, USF2 and NFκB
activity in human hepatoma cells. Int J Cancer. 124:1526–1534.
2009.PubMed/NCBI
|
|
24
|
Yao H, Pan J, Qian Y, et al: Enhanced
effect of soluble transforming growth factor-beta receptor II and
IFN-gamma fusion protein in reversing hepatic fibrosis. Eur J Med
Res. 15:152–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu T and He YW: Protective effect of
recombinant HMGB1 A box protein in mouse with acute hepatic
failure. Zhonghua Gan Zang Bing Za Zhi. 18:222–226. 2010.(In
Chinese).
|
|
26
|
Schuppan D and Afdhal NH: Liver cirrhosis.
Lancet. 371:838–851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carpino G, Morini S, Ginanni Corradini S,
et al: Alpha-SMA expression in hepatic stellate cells and
quantitative analysis of hepatic fibrosis in cirrhosis and in
recurrent chronic hepatitis after liver transplantation. Dig Liver
Dis. 37:349–356. 2005. View Article : Google Scholar
|
|
28
|
Safadi R and Friedman SL: Hepatic
fibrosis-role of hepatic stellate cell activation. MedGenMed.
4:272002.PubMed/NCBI
|
|
29
|
Shek FW and Benyon RC: How can
transforming growth factor beta be targeted usefully to combat
liver fibrosis? Eur J Gastroenterol Hepatol. 16:123–126. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheon SS, Wei Q, Gurung A, et al:
Beta-catenin regulates wound size and mediates the effect of
TGF-beta in cutaneous healing. FASEB J. 20:692–701. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Medici D, Hay ED and Olsen BR: Snail and
Slug promote epithelial-mesenchymal transition through
beta-catenin-T-cell factor-4-dependent expression of transforming
growth factor-beta3. Mol Biol Cell. 19:4875–4887. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shtutman M, Zhurinsky J, Simcha I, et al:
The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway.
Proc Nat Acad Sci USA. 96:5522–5527. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Razin E, Zhang ZC, Nechushtan H, et al:
Suppression of microphthalmia transcriptional activity by its
association with protein kinase C-interacting protein 1 in mast
cells. J Biol Chem. 274:34272–34276. 1999. View Article : Google Scholar : PubMed/NCBI
|