Introduction
Diabetic cardiomyopathy (DCM) is a unique, chronic
and progressive heart disease with cardiac muscle dysfunction and a
long-term latent phase induced by diabetes. The pathological
alterations of DCM vary from other heart diseases, such as
hypertension and coronary artery disease, although the majority of
patients with DCM encounter earlier signs, including hypertension
and ischemic chest pain. DCM is characterized by ventricular
dilation, myocyte hypertrophy and interstitial fibrosis, and
combines with decreased systolic function and diastolic dysfunction
(1).
It is known that the complications in diabetes
derive from hyperglycemia. Several factors have been demonstrated
to be involved in the pathogenesis of DCM. Etiologically, there are
four primary factors that contribute towards heart failure in DCM:
i) Microangiopathy and related endothelial dysfunction; ii)
autonomic neuropathy; iii) metabolic alterations (abnormal
hyperglycemia and fatty acid hyperoxidation); and iv) the
accumulation of free radicals (reactive oxygen/nitrogen species,
ROS/RNS) (2).
Previously, the mechanism for DCM was established,
and was shown to be an acute reaction of cardiac myocytes, induced
by hyperglycemia, that gradually develops into chronic pathological
alterations, including abnormal gene expression, disorder of
signaling pathways and aggravated cellular apoptosis (3). Hyperglycemia, derived from metabolic
alteration, may lead to a cascade of endovascular reactions,
including the release of inflammatory cytokines, chemo-attractant
infiltration of monocytes and elevated angiotensin II levels. The
activation of angiotensin II receptors 1 and 2 (AT1 and AT2) by
angiotensin II provokes oxidative stress, induces cardiac
inflammation and produces a highly toxic peroxynitrite (RNS,
ONOO−) (4). Free
tyrosine and proteins with a tyrosine amino acid are rapidly
nitrated by ONOO−, resulting in the formation of the
end-product 3-nitrotyrosine (3-NT), which impairs cardiac
mitochondrial function (5).
The primary aim of this study was to investigate
whether 3-NT is a potential biomarker for DCM in cardiac tissue and
serum, and to establish the correlation between 3-NT and the
apoptosis of cardiomyocytes.
Materials and methods
Materials
Streptozotocin (STZ) was purchased from Sigma (St.
Louis, MO, USA). The citrate buffer vehicle (pH 4.2–4.5) was
prepared by mixing solution A (2.1% citric acid) and solution B
(2.94% sodium citrate) at a ratio of 1:1.32. An injective solution
of STZ was prepared by dissolving 1 g in 100 ml of the citrate
buffer. Anti-3-NT rat polyclonal antibodies were purchased from
Abcam (Cambridge, MA, USA) (6).
The TUNEL reagent kit was purchased from Roche (South San
Francisco, CA, USA). The rat 3-NT ELISA kit was purchased from
Wuhan USCN Sciences Co., Ltd. (Wuhan, China).
The color development reagent was purchased from Gui
Hai Bio-Tech Inc. (Nanning, China). Rat blood glucose levels were
measured using a VAC0037941 blood glucose meter (Taiwan Leader
Bio-Tech Inc., Taipei City, Taiwan, R.O.C.). Valsartan capsules
were provided by Tianda Pharmaceutical Inc. (Zhuhai, China).
Methods
Study design
A total of 70 male Sprague-Dawley (SD) rats (8 weeks
old; body weight, 180–220 g) were purchased from the Animal Breed
Center affiliated with Guangxi Medical University (Nanning, China).
The rats were housed individually in polycarbonate cages and the
animal room was conditioned according to National Standard
Regulations and The Association For Assessment and Accreditation of
Laboratory Animal Care International (AAALAC) requirements. The
room temperature was set to 23±3°C, the relative humidity at 50±2,
the air ventilation at 10–15 changes/h and a light cycle of 12 h
on, 12 h off. The rats were randomly allocated into four groups
based on recent body weight (n=15/group).
The N group
In this control group, 20 SD rats were given free
access to a standard pellet diet and distilled water. The rats were
injected intraperitoneally (i.p.) with citrate buffer solution. For
screening, 5 rats were used as a comparison with the DCM rats, and
15 were assigned to the end point of the study.
A further 60 SD rats were used to establish the type
II diabetic animal model. These rats were fed a hyper-lipid and
hyperglycemia diet (consisting of 10% pork oil, 10% sucrose and 5%
egg yolk) for 4 weeks. Afterwards, the rats fasted overnight and
each rat was i.p. injected once with STZ at 40 mg/kg body weight to
establish a type II diabetic rat model. These rats were diagnosed
as type II diabetic rats, as determined by a higher blood glucose
level (≥16.7 mmol/l) measured via the tail vein.
The D+V group
In this group, 15 type II diabetic SD rats were
gavaged with a valsartan capsule at 40 mg/kg body weight every day
for 4 weeks, retaining free access to a standard rodent diet and
water.
A further 45 type II diabetic rats were fed
continuously on a hyper-lipid and hyperglycemia diet for a further
4 weeks. Afterwards, 5 rats were randomly selected and sacrificed
for comparison with 5 control rats. The absolute heart weight and
the ratio of heart/body weight were found to be higher than in the
control rats. Immunohistological staining of the heart slices
revealed swelling and necrosis of the cardiomyocytes, proliferation
of the interstitial fibroblast cells, over-growth of the collagen
fibers and disassembly of cellular organization. These rats were
diagnosed as DCM rats and used in the further study.
The DCM group
In this group, 15 DCM rats were selected from the
above process, assigned into the DCM group for the study and were
fed the hyper-lipid and hyperglycemia diet for another 4 weeks.
The DCM+V group
In this group, 15 DCM rats were fed a standard diet,
but were gavaged with a valsartan capsule at 40 mg/kg body weight
every day for a further 4 weeks.
After the 4 weeks, the rats in all four experimental
groups were sacrificed by CO2 inhalation to end the
in vivo study.
Measurement of animal heart
weight
Once the rats were euthanized, 3 ml of blood was
drawn from the aorta of each rat. Serum was isolated by
centrifugation for 10 min at 1500 × g at 4 °C, and frozen
immediately at −70 °C for the ELISA assay at a later time. The rat
heart was quickly removed and weighed after the blood had been
absorbed by filter paper. The ratio of heart/body weight was
calculated.
Histological staining and TUNEL
assay
After weighing the hearts, they were perfused with
4% paraformaldehyde for fixation. After fixation, the samples
underwent a series of gradient dehydrations, and were embedded in
paraffin and sliced for hemotoxylin and eosin (H&E)
staining.
The TUNEL assay was performed according to the
manufacturer’s instructions. Under a phase contrast microscope
(CHC-212 digital microscope; Olympus Optical, Co. Ltd., Tokyo,
Japan), cells with a yellow-brown nucleus were deemed to be positie
for apoptosis. Using ×400 magnification, 5 representative fields
were randomly selected. The apoptotic index (AI) was calculated
from the number of apoptotic cells per 100 cells. The amount of
3-NT in the myocardial tissue was determined by immunostaining, and
high definition images were captured using an Olympus CHC-212
digital microscope and statistically analyzed with Motic Images
Advanced 3.2 software (Motic China Group Co. Ltd., Xiamen, China).
The mean optical density (MOD) of positively stained apoptotic
nuclei was calculated and the percentage positive expression of
nucleus area was acquired by dividing the positively stained area
by the entire nucleus area. The expression index (EI) of 3-NT in
myocardial tissue was calculated as follows: MOD × positive area
(%) × 100 (7).
Blood concentration of 3-NT
The blood concentration of 3-NT was detected using
the ELISA method. The OD value of 3-NT via color development was
determined at 450 nm using a spectrometer. The concentration of
3-NT in the blood was determined using a standard curve.
Statistical analysis
All the data are expressed as the mean ± SD and
underwent statistical analysis with SPSS 13.0 software (IBM SPSS,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference in comparison with the
control.
Results
Moribund and dead animals during the
study
A total of 7 animals died while the type II diabetic
model was being established and prior to entry to the DCM group;
three rats died while being medicated in the D+V group, two died
while being prepared for DCM, one rat died while being prepared for
the diabetes group and one rat died due to an accident during
valsartan capsule gavage. A further 10 rats were sacrificed to
measuere their heart weights. Of the DCM+V group, 3 rats were
observed not to have been induced into DCM, according to
hematoxylin and eosin-stained heart sections and the TUNEL assay
results of myocardial apoptosis. A total of 50 animals survived the
study, leaving 15 rats in the N group, 13 rats in the DCM group, 12
rats in the D+V group and 10 rats in the DCM+V group.
General physical observation
Diabetic rats were found to be thin or emaciated and
weak, and they walked at a slow and clumsy pace. They drank an
increased amount of water, resulting in an increase in urine
(approximately twice more than rats in the N group). Diabetic rats
had filthy depleted fur. Blood glucose was maintained at >16.7
mmol/l in diabetic rats, whereas in the control rats the blood
glucose levels were found to be <6.0 mmol/l.
Ratios of heart/body weight in the four groups were
analyzed statistically with ANOVA, and statistically significant
differences were found between the groups (F=18.63, P<0.01). The
ratios for the DCM group were higher than those in the N group, and
the ratios for the DCM+V group were higher than those in the D+V
group (P<0.01; Table I.)
 | Table IResults of heart/weight index. |
Table I
Results of heart/weight index.
| Group | n | Heart/body weight
ratio |
|---|
| N | 15 |
0.002824±0.000304 |
| DCM | 13 |
0.004504±0.001184a |
| D+V | 12 |
0.003271±0.000364 |
| DCM+V | 10 |
0.005181±0.001330a |
Effects of valsartan on cardiomyocyte
structure examined using histopathology
As shown in Fig. 1,
the cardiomyocytes were assembled neatly, and the sizes of the cell
bodies and nuclei were similar. H&E-stained plasma revealed
that the intracellular staining was even and smooth, and the plasma
membrane was intact. However, varied degradation and necrosis of
cardiomyocytes, proliferation of intercellular fibroblasts,
increased collagen levels and cell disarrangement were observed in
the DCM group.
Effects of valsartan on cardiomyocyte
apoptosis examined using histopathology
Cardiomyocyte apoptosis was examined using the TUNEL
method. As shown in Fig. 2, for
the DCM group, cellular nuclei stained brown or dark yellow were
considered to be positive for apoptosis when examined under a phase
contrast microscope. AI was deemed to be statistically significant
between the four groups (F=34.779; P<0.01; Table II).
 | Table IIComparison of apoptotic index (AI) in
each group. |
Table II
Comparison of apoptotic index (AI) in
each group.
| Group | n | AI |
|---|
| N | 15 | 0.272±0.155 |
| DCM | 13 | 63.705±26.341a |
| D+V | 12 | 23.876±18.385b |
| DCM+V | 10 | 14.077±11.140b |
Distribution of the EI for 3-NT among
different cardiomyocytes examined by histopathology
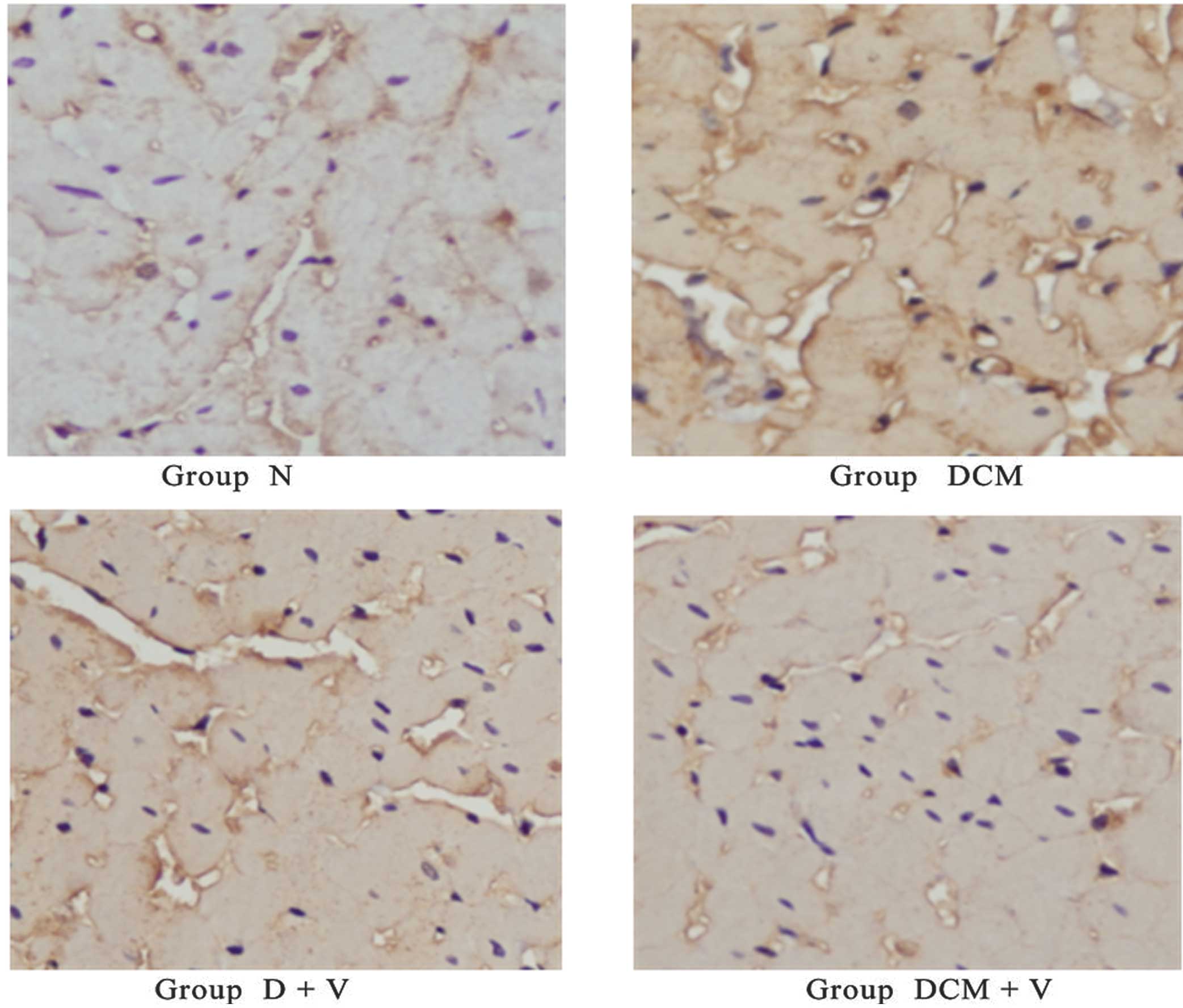
As shown in Fig. 3,
plasma area stained a yellow-brown color indicates a higher EI for
3-NT, which is a strong reaction. There was a high EI for 3-NT in
the DCM group, and a weak reaction or lower EI for 3-NT in the D+V
and DCM+V groups. However, there was a negative EI for 3-NT in the
N group (background color was light blue). As shown in Table III, the EIs for 3-NT correlated
positively with AI among all the tested groups. As shown in
Table IV, the EIs for 3-NT in the
DCM, D+V and DCM+V groups were markedly higher than in the N group.
The EI value for the DCM+V group was more than half that of the DCM
group.
 | Table IIICorrelation analysis of the expression
index (EI) of 3-NT in the heart and the apoptotic index (AI). |
Table III
Correlation analysis of the expression
index (EI) of 3-NT in the heart and the apoptotic index (AI).
| Group | n | EI | AI (r) |
|---|
| N | 15 | 46.749±30.295 | 0.272±0.155
(0.805a) |
| DCM | 13 | 966.204±555.885 | 63.705±26.341
(0.807a) |
| D+V | 12 | 370.173±297.776 | 23.876±18.385
(0.972a) |
| DCM+V | 10 | 342.508±227.516 | 14.077±11.140
(0.965a) |
 | Table IVComparison of the EI of 3-NT in the
heart. |
Table IV
Comparison of the EI of 3-NT in the
heart.
| Group | n | EI |
|---|
| N | 15 | 46.749±30.295 |
| DCM | 13 |
966.204±555.885a |
| D+V | 12 | 370.173±297.776 |
| DCM+V | 10 | 342.508±227.516 |
Correlation of blood 3-NT with the AI of
cardiomyocytes
In Table V, the
correlation factor r between blood 3-NT and AI was very small or
negative. In contrast to Table
III, it appears that blood 3-NT was unrelated to the AI in
cardiomyocytes.
 | Table V3-NT concentration and correlation
analysis of AI. |
Table V
3-NT concentration and correlation
analysis of AI.
| Group | n | 3-NT | AI (r) conc.
(nmol/l) |
|---|
| N | 10 | 40.175±6.403 | 0.272±0.155
(−0.071)a |
| DCM | 10 | 38.667±4.939 | 63.705±26.341
(−0.604) |
| D+V | 10 | 35.661±5.743 | 23.876±18.385
(0.133) |
| DCM+V | 10 | 37.665±6.256 | 14.077±11.140
(0.367) |
Discussion
In this study, 3-NT in the myocardial tissue was
deemed to be a successful biomarker for predicting cardiomyocyte
apoptosis in SD rats. 3-NT levels were increased and were closely
related to cardiomyocyte apoptosis. Post-treatment with valsartan
reversed the increase in 3-NT levels and apoptosis in diabetic
cardiomyopathic cells.
It was demonstrated that the hearts of rats in the
DCM group were larger than those in the N group. After receiving
valsartan treatment, the rat hearts remained enlarged and the
ratios of heart/body weight increased through the development of
the DCM rat model. As the development period for DCM was short (12
weeks), neither myocardial ischemia due to severe narrowing of the
coronary arteries nor heart congestion due to ventricle overload
were observed in the DCM rats. Under the microscope, cardiomyocytes
were assembled neatly, nucleus size was similar, plasma staining
was even and plasma membranes appeared to be intact for the hearts
of rats without DCM. However, in the hearts of rats with DCM,
degeneration and necrosis were observed to some extent in the
myocardial cells, with the proliferation of fibroblast cells
between the inter-cardiomyocytes, an increase in collagen fibers
and disordered cell assembly. The above pathological alterations
led to dilated congested hearts and an increase in the heart ratios
(heart/body weight), which is a comprehensive early response of
cardiomyocytes to diabetes, leading to the development of heart
failure in the late stages of DCM (8). Hyperglycemia was thought to trigger
the above pathological process, but how to initiate and develop the
degeneration and necrosis of cardiomyocytes and intercellular
fibration was unknown; the data from the present study may provide
the answer. Results of this study indicate that the AI of
cardiomyocytes in the DCM, D+V and DCM+V groups were significantly
higher than in the N group, and revealed associations between AI
and the degeneration and necrosis of cardiomyocytes and
intercellular fibration.
In 1972, Rubler et al(9) observed a special cardiomyopathy in
diabetic patients without significant coronary atherosclerosis.
Later, Hamby et al(10)
proposed a new concept of DCM through further pathological
diagnosis, and were the first to link DCM to the metabolism of
diabetics. Up until 2000, studies reported necrosis in a great
number of myocardial cells in diabetic patients, and the necrosis
of the myocardial cells was more severe and significant if the
diabetic patient also suffered from hypertension.
Previously, Ceriello (11) observed that under hyperglycemic
conditions, overabundant ROS were provoked into bursting out from
the endothelial cells, and were accompanied by higher levels of
3-NT and increases in collagen and fibronectin, which activated
PKC-β, NAD(P)H oxidase and Bax. Hyperglycemia in the diabetic rats
was well controlled in a normal range, but if it relapsed for two
months, the levels of ROS and nitrogen and nitric oxide were
inhibited by half and the levels of nitrotyrosine were 80% higher
than in the control group. Our data agree with the above
observations. In this study, the overexpression of 3-NT in
myocardial tissue (EI) was a unique characteristic, and was
observed to be significantly higher in the DCM, D+V and DCM+V
groups than in the N group. Furthermore, the profile of 3-NT EI was
correlated positively to the profile of AI, where an increase in
3-NT was likely to provoke apoptosis in cardiomyocytes. Oxidative
stress paved the way for apoptosis, cardiomyocyte necrosis and
fibration. Hyperglycemia may exacerbate the damage caused by
oxidative stress induced by angiotensin II. The oxidative
product-3-NT in diabetic rats with DCM triggers a dysfunctional
pathway of the mitochondrial and membrane receptors, which
participate in the activation of the caspase-cascade pathway and
Bcl-2 expression in apoptosis (12). 3-NT was a post-translational
protein modification, and increased when accompanied by ROS and
RNS. Turko and Murad (13)
proposed that the production pathways of 3-NT may be both
ONOO−- and non-ONOO−-dependent. 3-NT
production from these pathways caused the tyrosine residues of
proteins or free tyrosine residues to nitrate, and feedback to
enhance 3-NT production. The nitration of proteins may deactivate
the protein enzymes and induce cellular necrosis and apoptosis.
There is increasing evidence (14,15)
that AT1 and AT2 produce excess oxygen or nitrogen radicals by
reducing the nicotinamide adenine dinucleotide phosphate oxidase
pathway, thereby resulting in oxidative stress, myocardial cell
death and inflammation. In 2006, Cai et al(16) demonstrated, using a transgenic
diabetic mouse model, that in the early stages of diabetes, high
blood glucose levels are able to cause the heart to produce
overabundant free oxygen or nitrogen radicals. ROS and RNS exhaust
the capacity of myocardial inherent antioxidants, tipping the
balance from antioxidant to oxidant, thereby leading to
mitochondrial dysfunction and myocardial cell death via oxidative
damage. When the number of myocardial cell deaths reaches this
threshold, heart function cannot be replenished or compensated,
resulting in heart failure.
In this study, after intervention with valsartan, an
antagonist of the angiotensin II receptor, the EIs of 3-NT in the
cardiomyocyte tissue of the D+V and DCM+V groups decreased compared
with the DCM group. The number of cardiomyocyte apoptotic cells
decreased, indicating that the expression of 3-NT in cardiac tissue
was inhibited, whereas cardiomyocyte apoptosis was induced by 3-NT.
It was demonstrated that 3-NT played a key role in oxidative damage
in the development of DCM. The angiotensin II receptor pathway was
inhibited by valsartan, whereby the inhibition of oxidative stress
induced by hyperglycemia interrupted the production of 3-NT, and
diminished cardiomyocyte apoptosis. Although the EI of 3-NT and
apoptosis in cardiomyocytes were inhibited by valsartan, rat heart
ventricles remained expanded and dilated, and the heart/weight
ratios were increased. This demonstrated that cardiomyocyte
necrosis was not the only mechanism producing dilated rat heart
ventricles, as the inhibition of 3-NT expression diminished
cardiomyocyte apoptosis but did not halt the ventricular dilation.
In the diabetic state, the abnormal proliferation of myocardial
extracellular matrix composed of collagen, non-collagenous
glycoproteins and proteoglycans also contributed to the myocardial
contractility, diastolic dysfunction and heart dilation (17). Further investigation into the
effect of 3-NT on myocardial extracellular fibrosis and myocardial
interstitial fibrosis is required to reveal the mechanism for
DCM.
Ceriello et al(18) demonstrated that the nitro-tyrosine
concentration in the plasma correlated positively with the blood
glucose concentration in patients with type II diabetes.
Angiotensin II receptor antagonists, such as irbesartan, may
decrease 3-NT levels in diabetic patients, and may effectively
counteract the formation of 3-NT in a state of acute hyperglycemia
(19). However, in this study, no
significant difference in 3-NT levels was found among the four
different groups, and no correlation was found between the 3-NT
levels in blood and the AI in myocardial tissue in the DCM group.
After treatment with valsartan, there was no correlation between
the 3-NT in the serum and the AIs in the DCM group.
This study demonstrated that overexpression of 3-NT
in the myocardial tissues of rats in the DCM group was not
associated with cardiomyocyte apoptosis; however, valsartan
alleviated myocardial apoptosis in rats of the DCM group by
inhibiting the expression of 3-NT in myocardial tissue. Blood 3-NT
in the peripheral circulation did not reflect the actual content of
3-NT in myocardial tissue. The EI of 3-NT in myocardial tissue (but
not blood 3-NT) was able to predict cardiomyocyte apoptosis.
Cardiomyocyte apoptosis induced by 3-NT was alleviated by treatment
with valsartan, but had no effect on the ventricular dilation in
the hearts of rats with DCM, implying that cardiomyocyte apoptosis
was not the only pathway leading to ventricular dilation in
DCM.
Acknowledgements
The authors would like to express their gratitude to
all the authors who reviewed this manuscript. This study was not
supported by any grant sponsors.
References
|
1
|
Bell DS: Diabetic cardiomyopathy. Diabetes
Care. 26:2949–2951. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dokken BB: The Pathophysiology of
Cardiovascular Disease and Diabetes: Beyond Blood Pressure and
Lipids. Diabetes Spectr. 21:160–165. 2008. View Article : Google Scholar
|
|
3
|
Cai L and Kang YJ: Oxidative stress and
diabetic cardimyopathy: a brief review. Cardiovasc Toxicol.
1:181–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aksakal E, Akaras N, Kurt M, et al: The
role of oxidative stress in diabetic cardiomyopathy: an
experimental study. Eur Rev Med Pharmacol Sci. 15:1241–1246.
2011.PubMed/NCBI
|
|
5
|
Cai L and Kang YJ: Cell death and diabetic
cardimyopathy. Cardiovasc Toxicol. 3:219–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srinivasan K, Viswanad B, Asrat L, et al:
Combination of high-fat diet-fed and low-dose
streptozotocin-treated rat: a model for type 2 diabetes and
pharmacological screening. Pharmacol Res. 52:313–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raviv G, Kiss R, Vanegas JP, et al:
Objective measurement of the different collagen types in the corpus
cavernosum of potent and impotent men: an immunohistochemical
staining with computerized-image analysis. World J Urol. 15:50–55.
1997. View Article : Google Scholar
|
|
8
|
Tillquist MN and Maddox TM: Update on
diabetic cardiomyopathy: inches forward, miles to go. Curr Diab
Rep. 12:305–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rubler S, Dlugash J, Yuceoglu YZ, et al:
New type of cardiomyopathy associated with diabetic
glomerulosclerosis. Am J Cardiol. 30:595–602. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamby RI, Zoneraich S and Sherman L:
Diabetic cardiomyopathy. JAMA. 229:1749–1754. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ceriello A: The hyperglycemia-induced
metabolic memory: the new challenge for the prevention of CVD in
diabetes. Rev Esp Cardiol. 8(Suppl C): 11–17. 2008.
|
|
12
|
Kowluru RA: Effect of reinstitution of
good glycemic control on retinal oxidative stress and nitrative
stress in diabetic rats. Diabetes. 52:818–823. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Turko IV and Murad F: Protein nitration in
cardiovascular diseases. Pharmacol Rev. 54:619–634. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh VP, Le B, Khode R, et al:
Intracellular angiotensin II production in diabetic rats is
correlated with cardiomyocyte apoptosis, oxidative stress, and
cardiac fibrosis. Diabetes. 57:3297–3306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou G, Li X, Hein DW, et al:
Metallothionein suppresses angiotensin II-induced nicotinamide
adenine dinucleotide phosphate oxidase activation, nitrosative
stress, apoptosis, and pathological remodeling in the diabetic
heart. J Am Coll Cardiol. 52:655–666. 2008. View Article : Google Scholar
|
|
16
|
Cai L, Wang Y, Zhou G, et al: Attenuation
by metallothionein of early cardiac cell death via suppression of
mitochondrial oxidative stress results in a prevention of diabetic
cardiomyopathy. J Am Coll Cardiol. 48:1688–1697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mason RM and Wahab NA: Extracellular
matrix metabolism in diabetic nephropathy. J Am Soc Nephrol.
14:1358–1373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ceriello A, Mercuri F, Quagliaro L, et al:
Detection of nitrotyrosine in the diabetic plasma: evidence of
oxidative stress. Diabetologia. 44:834–838. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ceriello A, Assaloni R, Da Ros R, et al:
Effect of irbesartan on nitrotyrosine generation in
non-hypertensive diabetic patients. Diabetologia. 47:1535–1540.
2004. View Article : Google Scholar : PubMed/NCBI
|