Introduction
As an abundant and readily accessible source of
multipotent stem cells, adipose tissue-derived stem cells (ADSCs)
have been extensively investigated for their specific therapeutic
properties and are considered to be an ethical, practical and
biologically appropriate cell population for cell therapy (1). An important approach leading to
successful stem cell therapy is cell tracking in vivo, where
a nontoxic, biocompatible, efficient and highly sensitive exogenous
cell marker is required.
Green fluorescent protein (GFP) is capable of
exhibiting fluorescence in living cells and has been widely used as
a lineage marker for mammalian cells, predominantly as a
pathological tracer following sacrifice of the animal and
subsequent animal tissue slice culture (2). Although the fluorescent signal
emitted from GFP may be easily detected with optical imaging,
fluorescence microscopy or flow cytometry, there remains certain
disadvantages in the practical application of these techniques.
Magnetic resonance imaging (MRI), which may be used to track
transplanted stem cells labeled with superparamagnetic ironoxide
(SPIO), possesses the ability to non-invasively track and allow
real-time imaging of injected labeled cells (3). Therefore, the introduction of SPIO
into ADSCs labeled with lentiviral vectors encoding the gene for
emerald green fluorescent protein (lenti-eGFP) is hypothesized to
result in a non-pathological tracer in vivo, which may be
observed by MRI. However, results showed that no biological
characteristics of transplanted cells were detected by MRI and no
regenerative and differentiated cells from exogenous and endogenous
transplanted cells were distinguished.
In the current study, we propose that co-labeling
cells with eGFP and SPIO may play a synergetic role in cell
labeling. Whether this approach results in adverse effects on the
biophysical properties of cells remains unknown, thus further
investigations are required.
Molday ION Rhodamine B™ (MIRB) is an iron
oxide-based superparamagnetic contrast reagent of a colloidal size
of 50 nm. The colloidal particle may be visualized by MRI and
fluorescence due to the labeling of rhodamine B (4). The excitation and emission
wavelengths of this fluorescent dye are 555 and 565–620 nm,
respectively. In the present study, based on genetic modification
with the reporter genes eGFP by lenti-eGFP and physical labeling
with MIRB, a combined labeling strategy for ADSCs was established.
The feasibility of rat ADSCs co-labeled with lenti-eGFP and MIRB
and the biological characteristics of these labeled ADSCs were also
investigated.
Materials and methods
Preparation and characterization of
ADSCs
ADSCs were prepared following a method described by
Zuk et al(5) with
modifications. Subcutaneous adipose tissue (3–4 g) was obtained
from the abdominal and inguinal regions of adult male
Sprague-Dawley rats (~200 g each). Following removal of debris and
blood cells by washing with phosphate-buffered saline (PBS), the
excised adipose tissue was minced and digested with 2 mg/ml
collagenase I (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 30
min. Collagenase activity was neutralized by the addition of
Dulbecco’s modified Eagle’s medium with Ham’s F12 nutrient mixture
(Gibco, Carlsbad, CA, USA) containing 15% fetal bovine serum (FBS;
Thermo Fisher Scientific Inc., Rockford, IL, USA). The digested
adipose tissue was filtered with a 100-μm nylon membrane to
eliminate the undigested fragments. Following centrifugation at
1,000 × g for 5 min (X-12R Benchtop Centrifuge; Beckman Coulter,
Miami, FL, USA), cell pellets were resuspended in cell-culture
medium (CCM) and cultivated at 37°C in 5% CO2 for 24 h.
Following removal of unattached cells and debris, fresh CCM
containing 15% FBS was added and the adherent cells were cultured
at 37°C in 5% CO2. The medium was changed following two
days of incubation and every two days thereafter. Once attached
cells reached 80–90% confluency and cultures were trypsinized and
passaged at a ratio of 1:2. ADSCs from the third passage expansion
were washed with PBS and detached from the culture dish using 0.25%
trypsin-ethylenediamine tetra-acetic acid (Gibco). Cells were
incubated with phycoerythrin-conjugated anti-mouse antibodies
against CD34, CD44, CD45 and CD105 (eBioscience, San Diego, CA,
USA) for 30 min at 4°C in the dark. Isotype antibodies served as a
control. Cells were washed with PBS and analyzed by flow cytometery
(Beckman Coulter Inc., Brea, CA, USA). Animal care and procedures
were approved by the Animal Care and Use Committee of Southern
Medical University (Guangzhou, China) and all experiments were
conducted in accordance with the institutional guidelines.
ADSC transduction and examination of GFP
fluorescence
Lenti-eGFP were produced with Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA) by cotransfection
of 293FT cells and expression of a packaging plasmid. Lenti-eGFP
viruses were transfected into 293T cells with Lipofectamine 2000
(Invitrogen Life Technologies) and then produced in 293T cells
Infection was performed at 37°C and 5% CO2 for 2 h. At
72 h after infection, cells were observed under a fluorescence
microscope (TE2000; Nikon, Tokyo, Japan) and five representative
high power fields were selected to analyze the fluorescence
intensities of GFP-positive cells. Infection efficiency of eGFP
expressed in ADSCs one week following transduction was examined
using the fluorescence-activated cell sorting (FACS) technique.
Cell labeling with MIRB
Medium (1 ml) containing passage 3 (P3) rat ADSCs
was plated in a 6-well plate (1×104
cells/cm2) and incubated at 37°C with 5% CO2
overnight. The MIRB reagent (BioPAL, Inc., Worcester, MA, USA)
containing 2 mg/ml Fe3+ was added, resulting in a final
Fe3+ concentration of 10 μg/ml. Following overnight
incubation, the MIRB-containing medium was removed by aspiration
and ADSCs were washed three times with PBS to remove extracellular
MIRB. MIRB-labeled ADSCs were incubated at 37°C with 5%
CO2 in CCM for subsequent experiments.
Cellular staining for intracellular
iron
Cell profiles and intracellular iron particles were
identified using prussian blue staining. Cells were continuously
incubated for 15 min with 2% potassium ferrocyanide in 6%
hydrochloric acid and counterstained with nuclear fast red for 3
min. Labeling efficiency was analyzed by light microscopy (Olympus
CK2; Olympus Optical Co. Ltd., Tokyo, Japan) and cells exhibiting
blue intracellular particles were considered to be prussian
blue-positive.
The distribution of the SPIO particles within the
cells was examined under transmission electron microscopy (TEM;
JEM1400; Jeol Ltd., Tokyo, Japan). Harvested labeled ADSCs were
immersed in 2.5% buffered glutaraldehyde at 4°C for 1 h and fixed
with 1% osmium tetroxide (Fluka, Sigma-Aldrich) for 2 h for
observation.
Groups
ADSCs were divided into four groups according to the
labeling treatment: Group 1, labeled with lenti-eGFP; group 2,
labeled with MIRB; group 3, labeled with lenti-eGFP and MIRB; and
group 4, control, with no labeling. ADSCs in the four groups were
incubated under the same conditions for further assessment.
Measurement of ADSC viability
Cell proliferation in the labeled and unlabeled
groups was monitored using a Trypan blue exclusion assay (Trypan
Blue Staining Cell Viability Assay Kit, Shanghai Biyuntian
Biological Co., Shanghai, China). The cell suspension (5 μl) was
mixed with 5 μl 0.4% Trypan blue solution for 1 min and 100 cells
were quantified using a hemocytometer (Neubauer cell counting
chamber, DRM-700, CELL-VU CBC; Millenium Sciences, Inc., New York,
NY, USA) for each assay. Cell viability was evaluated by the Trypan
blue exclusion rate calculation (unstained cells / total number of
cells × 100).
Flow cytometric analysis of
apoptosis
Apoptosis was evaluated by FACS analysis using the
Annexin V/PE Apoptosis Detection kit I (BD Biosciences, San Jose,
CA, USA). The lenti-eGFP and MIRB co-labeled ADSCs on 12
well-plates were harvested by trypsinization after 1, 2, 3 and 4
weeks, respectively. Following FBS containing culture media
neutralization and PBS washing, cells were resuspended in 100 μl
Annexin V binding buffer, and stained in the dark with 4 μl Annexin
V/PE (marker for apoptosis) and 4 μl 7-AAD (a necrosis marker) for
15 min at room temperature. Following the addition of 400 μl
Annexin V binding buffer, 10,000 cells/sample were measured using a
FACSCalibur flow-cytometer with CellQuest software
(Becton-Dickinson, Franklin Lakes, NJ, USA).
Induction of adipogenic and osteogenic
differentiation of ADSCs
Following the aforementioned pretreatment, the
co-labeled ADSCs were induced by adipogenic and osteogenic
induction (Table I). For
adipocytic induction, the co-labeled ADSCs were cultured in DMEM
supplemented with 5 μg/ml streptomycin, 5 U/ml penicillin, 5% FBS,
1 μM dexamethasone (Sigma-Aldrich), 250 μM isobutyl-methylxanthine
(Sigma-Aldrich), 10 μM bovine insulin (Sigma-Aldrich), 17 mM
pantothenate (Sigma-Aldrich), 200 μM indometacin (Sigma-Aldrich)
and 33 mM biotin (Sigma-Aldrich). After 12 days of induction at
37°C in 5% CO2, cells were stained with Oil Red O
(Sigma-Aldrich). For osteogenic induction, the cells were cultured
in DMEM supplemented with 5 μg/ml streptomycin, 5 U/ml penicillin,
10% FBS, 10 nM 1,25-(OH)2 vitamin D3, 50 μM of
ascorbate-2-phosphate (Biojet, Guangzhou, China) and 10 mM
β-glycerolphosphate (Sigma-Aldrich). After 28 days of induction at
37°C in 5% CO2, staining was performed with Alizarin Red
(Sigma-Aldrich).
 | Table IInduction media. |
Table I
Induction media.
| Induction type | Induction
component | Induction period
(days) |
|---|
| Adipogenic | DMEM | 12 |
| Streptomycin (5
μg/ml) | |
| Penicillin (5
U/ml) | |
| FBS (5%) | |
| Biotin (33 mM) | |
| Pantothenate (17
mM) | |
| Bovine insulin (10
μM) | |
|
Isobutyl-methylxanthine (250 μM) | |
| Indomethacin (200
μM) | |
| Dexamethasone (1
μM)induction | |
| Osteogenic | DMEM | 28 |
| Streptomycin (5
μg/ml) | |
| Penicillin (5
U/ml) | |
| FBS (10%) | |
| β-glycerophosphate
(10 mM) | |
| Ascorbate-2-phosphate
(50 μM) | |
| 1,25-(OH)2 vitamin
D3 (10 nM) | |
Transdifferentiation potential of
co-labeled ADSCs
Adipogenic differentiation was evaluated by staining
cytoplasmic lipid deposits in the co-labeled ADSCs. Cells were
plated at 2.5×104 cells/cm2 and allowed to
reach 90% confluency, followed by incubation for three cycles in
induction/maintenance medium. ADSCs were cultured for seven days in
an adipogenic maintenance medium. Cells were fixed with 10%
buffered formalin and stained with Oil red O.
Osteogenic transdifferentiation was evaluated by
Alizarin red staining of the induced co-labeled ADSCs. Cells were
plated at 4.2×103 cells/cm2 and incubated for
24 h in CCM. Following incubation, cells were transferred to
osteogenic induction medium and cultured for 21 days and the medium
was changed every 3 days. Cells were fixed and stained with
Alizarin red staining to assess mineralization.
Statistical analysis
SPSS statistical package, version 11.0 (SPSS Inc.,
Chicago, IL, USA) was used for Student’s unpaired t-test
statistical analysis. Data are expressed as the mean ± SD. The
Kruskal-Wallis rank sum test was used to determine the difference
in absorbance of MTT for the comparison of labeled and unlabeled
ADSCs. P<0.05 was considered to indicate a statistically
significant difference.
Results
ADSC morphology and expression of surface
antigens
To determine ADSC features, morphological
observation and surface antigen identification was performed by
flow cytometry. Following primary culture, a small population of
single spindle-shaped cells was presented with adherent ADSCs. On
days 5–7, following initial plating, the cells began to form
colonies and became confluent (Fig.
1A). When ADSCs were passaged to P3, the purified ADSCs
retained the morphology of uniform fibroblast-like cells. Cell
surface phenotype analysis indicated that the ADSC population was
positive for CD44 and CD105, and negative for CD34 and CD45
(Fig. 1B).
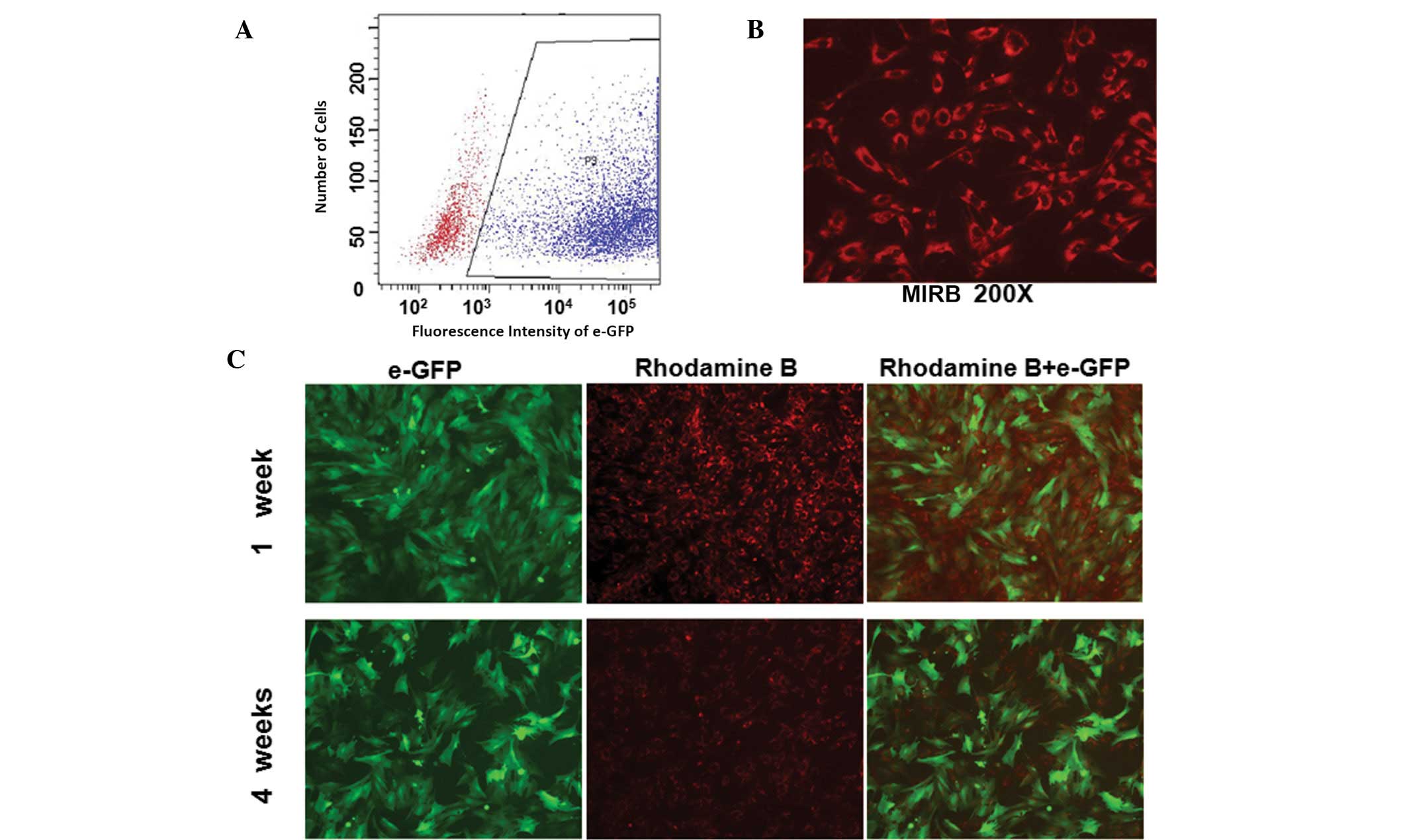
Labeling efficiency and fluorescence
intensity of labeled ADSCs
To characterize the co-labeled ADSCs, labeling
efficiency was determined by flow cytometry and fluorescence
intensity was examined via fluorescent and confocal microscopy (LSM
510 Confocal Laser Scanning Microscope, Carl Zeiss, Jena, Germany).
With an MOI of 400:1, >81.4% of ADSCs infected with lenti-eGFP
showed strong fluorescent signals for GFP expression on day 5
(Fig. 2A). Infected cells were
labeled with 20 g/ml−1 Fe3+ MIRB and the
percentage of rhodamine B-positive cells was >95% (Fig. 2B). Following culturing of the
co-labeled ADSCs for four weeks in vitro, the green
fluorescence expressed by eGFP remained stable while the red
fluorescence expressed by MIRB was significantly decreased
(Fig. 2C). These results indicate
that ADSCs may be labeled with GFP and MIRB successfully and
identified via specific colored fluorescence. The fluorescence
emitted by GFP was sustained and stably expressed, while
fluorescence with MIRB decreased with time.
Intracellular iron particle
distribution
To determine the intracellular iron particle
distribution of MIRB-labeled ADSCs, prussian blue staining was
observed by light microscopy. The labeling rate of ADSCs was ~100%,
according to the observation of blue-stained iron particles
(Fig. 3A). A similar proportion
was observed with rhodamine B staining. TEM results revealed that
the SPIO particles were located in the endosomal vesicles of
labeled ADSCs (Fig. 3B). These
results indicate that SPIO and rhodamine B were coupled together in
MIRB and their expression tended to be synchronized.
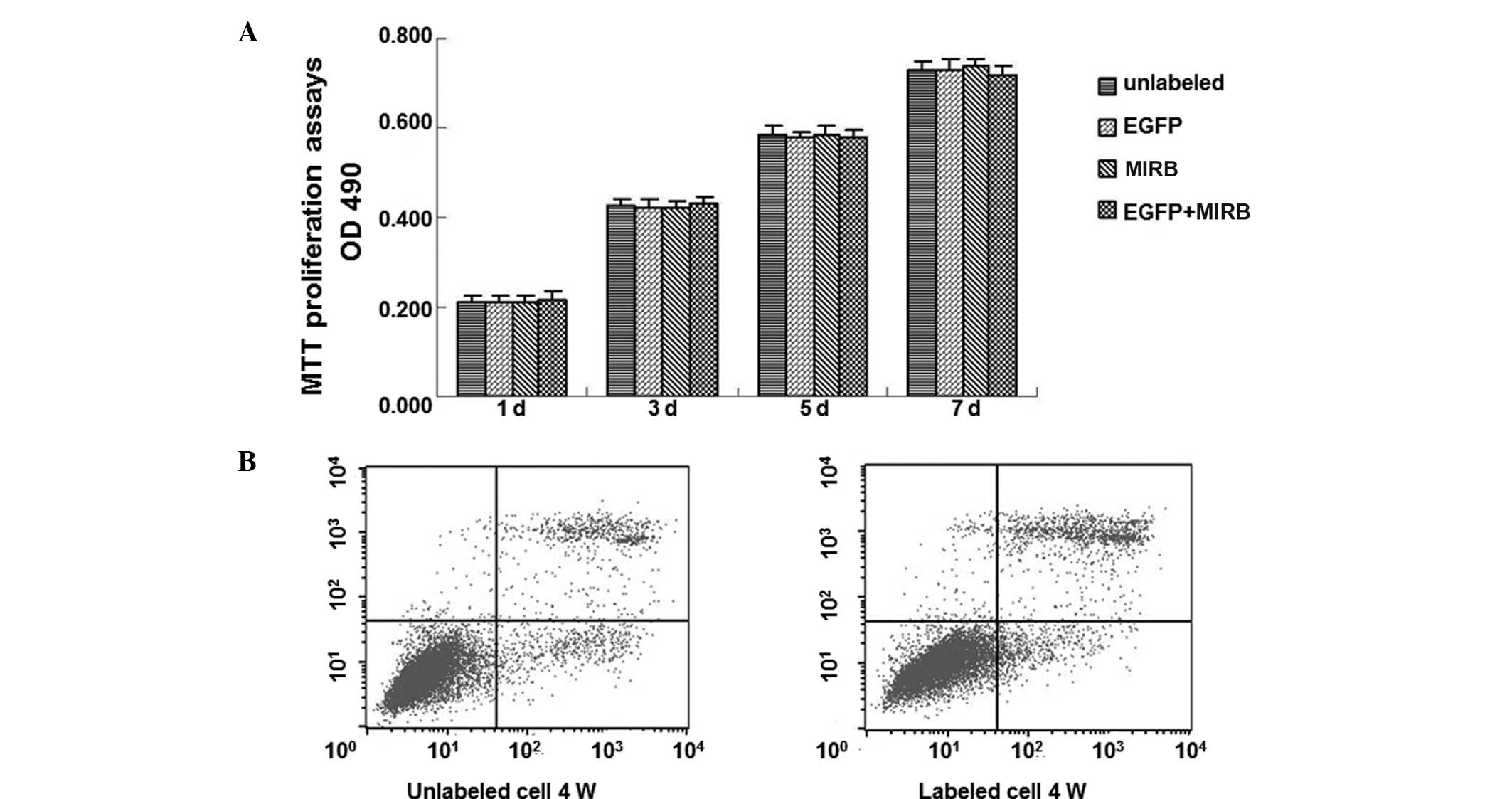
Lenti-eGFP and MIRB effect on the
viability, proliferation and apoptosis of ADSCs
To investigate the effect of the markers on the
proliferation and the toxicity of markers to ADSCs, cell viability
and proliferation were determined by the trypan blue exclusion test
and MTT assay, while the cell apoptosis was observed by flow
cytometry. Trypan blue exclusion testing showed a mean cell
viability of 98.12±1.26% for the GFP-labeled ADSCs and 98.72±1.65%
for the MIRB-labeled ADSCs, respectively. The mean viability of the
unlabeled ADSCs was 98.63±1.41% and 99.06±1.08%, respectively.
However, no significant differences on the exclusion rate were
identified between labeled and unlabeled cells (P>0.05) at all
time points. MTT proliferation assays showed no marked differences
among the four groups on day 1, 3, 5 and 7, respectively (Fig. 4A). Low rates of apoptosis and
necrosis in co-labeled and control groups were observed following
1, 2, 3 and 4 weeks, respectively (Fig. 4B). These results indicate that
co-labeling of ADSCs with lenti-eGFP and MIRB is applicable and
exhibited no significant adverse effect on ADSC viability,
proliferation and apoptosis.
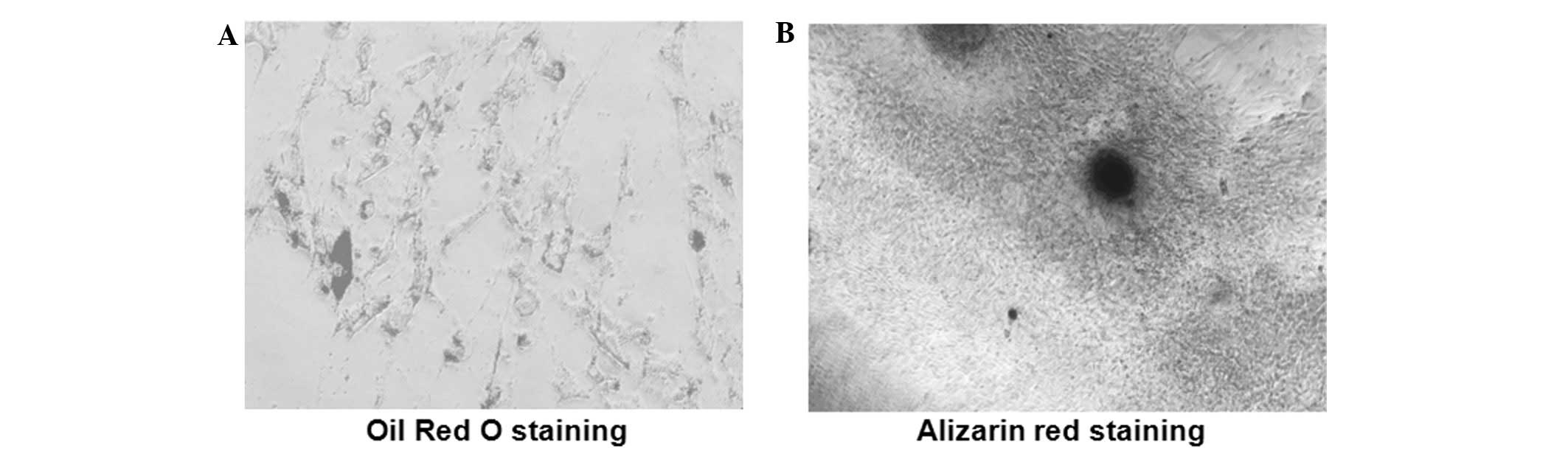
Transdifferentiation potential of
co-labeled ADSCs
To assess the effect of co-labeling on ADSCs
trans-differentiation potency, the multipotency of labeled and
unlabeled ADSCs was detected with osteogenic and adipogenic
induction, respectively. In adipogenic differentiation, Oil Red O
staining demonstrated that the formation of lipid vacuoles occurred
inside co-labeled cells (Fig. 5A)
and the mineralization in the osteogenic differentiation was
confirmed using alizarin red staining (Fig. 5B). These results indicate that
ADSCs labeled with lenti-eGFP and MIRB have the stem cell potential
for multi-directional differentiation.
Discussion
The monitoring of the migration and homing of
transplanted cells, as well as the engraftment efficiency and
functional capability in vivo has become a critical issue
with the rise of stem cell-based therapies. Stem cells have
therapeutic effects in refractory diseases (6,7).
However, the mechanisms underlying cell therapy remain unclear and
their actions in vivo require further investigation. Thus,
the approach of transplanted stem cell imaging has become a focus
of stem cell studies and is widely accepted as a preclinical tool
to evaluate novel therapeutic strategies. At present, to improve
the monitoring of cellular dynamics of the transplanted stem cells
in vivo, a reliable technique for tracking grafted stem
cells is required.
A model cell tracer requires the following features:
i) a sufficiently high signal for detection, ii) is not eluted,
iii) is not metabolized and iv) is specific to labeled cells. As a
labeling protein technology, GFP gene-tagging is most extensively
applied due to its high efficiency, non-toxicity, stabilization and
capability for long-term follow-up testing (8,9).
Although GFP is frequently used as an animal pathological tracer,
disadvantages are occasionally encountered in the practical
application as fluorescent labeling technology is difficult to
assess by dynamic and noninvasive monitoring. Previously, it has
been shown that MRI is effective in tracking the distribution of
transplanted stem cells in vivo by labeling with SPIO
particles. SPIO effectively labels numerous cell types and the
labeled cells are able to be monitored for a number of weeks
(10,11). However, the technology also has
disadvantages of short tracer time and low spatial resolution.
Moreover, cell death is observed in a significant number of
SPIO-labeled stem cells shortly following transplantation due to
the resulting proapoptotic and cytotoxic microenvironment (12). The SPIO nanoparticles were observed
to remain in the interstitial compartment for a period of time or
were taken up by macrophages and were not distinguished by prussian
blue staining. Therefore, the signal voids observed from MRI may
not necessarily represent living stem cells (13). Normal cellular uptake mechanisms
are inadequate in loading cells with a tracking label long-term,
but the multimodality imaging of cell tracking techniques permits
analysis of transplantation efficiency and the potential migration,
distribution and turnover of transplanted cells in vivo.
ADSCs have similar surface markers and gene
profiling to bone marrow stem cells and are readily available with
clonogenic potency and a robust proliferative capacity (14,15).
The aforementioned advantages have resulted in this population
becoming a predominant candidate for cell therapy and selection for
the current study. As previously demonstrated, SPIO showed no
effect on ADSC viability, transdifferentiation potential or
cell-factor secretion (16), while
MIRB was observed to exhibit a high physical labeling efficiency
without any transfection agent and was less toxic (17). Therefore, a novel SPIO contrast
agent, MIRB, which may be visualized by MRI and fluorescence
microscopy was used in the current study. To obtain an accurate
assessment of the migration and turnover of the transplanted ADSCs
in vivo, the detection, tracking and multimodal image
acquisition were conducted for a number of months. In the present
study, a co-labeled method was used, with lenti-eGFP infection of
P3 ADSCs followed by a direct transfection of the GFP-ADSCs with
MIRB.
Present results showed that the GFP protein was
steadily expressed with the GFP-labeled cell proliferation. A CCK-8
assay was used to determine cell viability and flow cytometric
analysis of cell apoptosis and cycles suggested that co-labeling of
lenti-eGFP and MIRB was noncytotoxic, indicating its potential use
in cell therapy. The multipotency of labeled and unlabeled ADSCs
was confirmed by osteogenic and adipogenic induction with specific
differentiation media. Osteogenic and adipogenic differentiation
assessments showed that no significant difference occurred in cell
viability, proliferation and multiple differentiation capability
between the labeled and unlabeled ADSCs, demonstrating that
lenti-eGFP and MIRB did not affect ADSC viability, which is similar
to the results of a previous study concerning SPIO (18).
After four weeks, a marginal decrease of MIRB red
fluorescence intensity was identified in the co-labeled ADSCs;
however, the GFP green fluorescence was observed to be sustained.
The differences in stability demonstrated that MIRB allows the
observation of grafted cell migration and distribution for a
short-time period only, while GFP allows a longer period of
observation. Due to the biological degradation of MIRB, the number
of surviving labeled cells in vivo may be underestimated by
MRI. Considering the drawbacks of label leakage and false positive
signals with macrophage engulfed debris in this direct cell
labeling (19,20), SPIO is therefore, inappropriate for
use in long-term observation in vivo. By contrast, GFP may
verify the presence of living stem cells at target sites and may
eliminate adverse factors, including hemorrhage and cell death
(21) and its application for
implanted cell monitoring in vivo remains an option.
Moreover, co-labeling of lenti-eGFP and MIRB may be improved by
obtaining cell migration data via noninvasive methods, including
MRI in early stages combined with a pathological tracer
technique-like labeling immunofluorescent staining and laser
scanning confocal microscopy methods in later stages.
In conclusion, the biological characteristics of
ADSCs labeled with MIRB and GFP did not exhibit any significant
differences in cell viability, proliferation, membrane-bound
antigens and multiple differentiation ability compared with
unlabeled ADSCs in vitro and the co-labeling technique may
be employed for cell observation under a number of conditions,
without any significant adverse effects. Real-time tracking for
transplanted cells by MRI and pathological tracer techniques using
immunofluorescent staining and laser scanning confocal microscopy
may aid in understanding the mechanism of stem cell transplantation
therapy. Dynamic tracking MRI results require further investigation
via GFP expressed green fluorescence. The technology developed in
the current study improves the accuracy for observation of the
existence and functional status of transplanted cells in
vivo and provides a novel approach for curative cell therapy
evaluation.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81171831).
References
|
1
|
De Ugarte DA, Morizono K, Elbarbary A, et
al: Comparison of multi-lineage cells from human adipose tissue and
bone marrow. Cells Tissues Organs. 174:101–109. 2003.PubMed/NCBI
|
|
2
|
Bauer G, Dao MA, Case SS, et al: In vivo
biosafety model to assess the risk of adverse events from
retroviral and lentiviral vectors. Mol Ther. 16:1308–1315. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bulte JW and Kraitchman DL: Iron oxide MR
contrast agents for molecular and cellular imaging. NMR Biomed.
17:484–499. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Addicott B, Willman M, Rodriguez J,
Padgett K, Han D, Berman D, Hare JM and Kenyon NS: Mesenchymal stem
cell labeling and in vitro MR characterization at 1.5T of new SPIO
contrast agent: Molday ION Rhodamine-B™. Contrast Media Mol
Imaging. 6:7–18. 2011.PubMed/NCBI
|
|
5
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Caplan AI: Review: mesenchymal stem cells:
cell-based reconstructive therapy in orthopedics (Review). Tissue
Eng. 11:1198–1211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dyson SC and Barker RA: Cell-based
therapies for Parkinson’s disease. Expert Rev Neurother.
11:831–844. 2011.
|
|
8
|
Ward TH and Lippincott-Schwartz J: The
uses of green fluorescent protein in mammalian cells. Methods
Biochem Anal. 47:305–337. 2006.PubMed/NCBI
|
|
9
|
Serganova I, Mayer-Kukuck P, Huang R and
Blasberg R: Molecular imaging: reporter gene imaging. Handb Exp
Pharmacol. 185:167–223. 2008. View Article : Google Scholar
|
|
10
|
Zhu XM, Wang YX, Leung KC, Lee SF, Zhao F,
Wang DW, Lai JM, Wan C, Cheng CH and Ahuja AT: Enhanced cellular
uptake of aminosilane-coated superparamagnetic iron oxide
nanoparticles in mammalian cell lines. Int J Nanomedicine.
7:953–964. 2012.PubMed/NCBI
|
|
11
|
Lee ES, Chan J, Shuter B, et al: Microgel
iron oxide nanoparticles for tracking human fetal mesenchymal stem
cells through magnetic resonance imaging. Stem Cells. 27:1921–1931.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Domen J and Weissman IL: Self-renewal,
differentiation or death: regulation and manipulation of
hematopoietic stem cell fate. Mol Med Today. 5:201–208. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kraitchman DL, Heldman AW, Atalar E, Amado
LC, Martin BJ, Pittenger MF, Hare JM and Bulte JW: In vivo magnetic
resonance imaging of mesenchymal stem cells in myocardial
infarction. Circulation. 107:2290–2293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu Y, Liu T, Song K, Fan X, Ma X and Cui
Z: Adipose-derived stem cell: a better stem cell than BMSC. Cell
Biochem Funct. 26:664–675. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taléns-Visconti R, Bonora A, Jover R,
Mirabet V, Carbonell F, Castell JV and Gómez-Lechón MJ: Hepatogenic
differentiation of human mesenchymal stem cells from adipose tissue
in comparison with bone marrow mesenchymal stem cells. World J
Gastroenterol. 12:5834–5845. 2006.PubMed/NCBI
|
|
16
|
Wang L, Deng J, Wang J, et al:
Superparamagnetic iron oxide does not affect the viability and
function of adipose-derived stem cells, and superparamagnetic iron
oxide-enhanced magnetic resonance imaging identifies viable cells.
Magn Reson Imaging. 27:108–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren Z, Wang J, Zou C, Guan Y and Zhang YA:
Labeling of cynomolgus monkey bone marrow-derived mesenchymal stem
cells for cell tracking by multimodality imaging. Sci China Life
Sci. 54:981–987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arbab AS, Yocum GT, Rad AM, Khakoo AY,
Fellowes V, Read EJ and Frank JA: Labeling of cells with
ferumoxides-protamine sulfate complexes does not inhibit function
or differentiation capacity of hematopoietic or mesenchymal stem
cells. NMR Biomed. 18:553–559. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Acton PD and Zhou R: Imaging reporter
genes for cell tracking with PET and SPECT. Q J Nucl Med Mol
Imaging. 49:349–360. 2005.PubMed/NCBI
|
|
20
|
Higuchi T, Anton M, Dumler K, et al:
Combined reporter gene PET and iron oxide MRI for monitoring
survival and localization of transplanted cells in the rat heart. J
Nucl Med. 50:1088–1094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu B, Treuting P, Zhan X, Xie D, Frevert
CW and Yang X: Dual transfer of GFP gene and MGd into
stem-progenitor cells: toward in vivo MRI of stem cell-mediated
gene therapy of atherosclerosis. Acad Radiol. 17:547–552. 2010.
View Article : Google Scholar : PubMed/NCBI
|