Introduction
Picrasma quassioides Benn. (of the
Simaroubaceae family) is a traditional Chinese medicinal plant
predominantly found in Southern China. The stems have been used for
the treatment of inflammation, gastroenteritis, eczema and
snakebites. However, the active constituents of this plant and
their mechanisms of action in treating these diseases remain to be
elucidated. Previous chemical investigation of this plant has led
to the isolation of several alkaloids and quassinoids (aglycones),
such as β-carbolines, canthin-6-ones and bis-β-carbolines (1–3).
Studies aiming to elucidate the active constituents of P.
quassioides have isolated and identified 48 compounds,
including 25 β-carboline alkaloids, six canthinone alkaloids, 11
bis-β-carboline alkaloids, three lignins, two quassinoids and a
flavonol (4–7). In the present study, the
anti-inflammatory effects and molecular mechanisms of the
neolignan, picrasmalignan A, were investigated.
Following inflammatory stimulation, macrophages
produce nitric oxide (NO) and proinflammatory cytokines, such as
tumor necrosis factor (TNF)-α and interleukin (IL)-6. These
mediators are highly expressed in macrophages in numerous
inflammatory diseases, including rheumatoid arthritis,
atherosclerosis and hepatitis (8–10).
NO, an important cellular second messenger, is produced via three
types of nitric oxide synthase (NOS). Small quantities of NO
produced by the constitutive NOS are essential for maintaining
normal cellular function. Inducible NOS (iNOS) sustainably produces
a high output of NO, which is one of the most important
inflammatory reactions in activated macrophages (11).
In addition, two isoforms of cyclooxygenase, COX-1
and COX-2, which are encoded by separate genes, have been
identified (12). The COX-1
isozyme is expressed at a constant level and does not fluctuate in
response to various stimuli. By contrast, COX-2 is induced by
lipopolysaccharide (LPS) and is the target enzyme for the
anti-inflammatory activity of nonsteroidal anti-inflammatory drugs.
Numerous studies have demonstrated that certain inducible enzymes
(COX and iNOS), cytokines and their reaction products are involved
in chronic inflammatory diseases (13–15).
Thus, the effect of picrasmalignan A on
LPS-stimulated proinflammatory mediators (including NO, TNF-α and
IL-6) in macrophages, and on the expression of iNOS and COX-2, was
also investigated. In addition, the inhibitory effect of
picrasmalignan A on iNOS and COX-2 enzymatic activity in
LPS-stimulated RAW 264.7 cells was demonstrated.
Materials and methods
Isolation and identification of
picrasmalignan A
Picrasmalignan A was isolated and identified as
previously demonstrated (4).
Briefly, the stems of P. quassioides (100 kg) were extracted
with 95% EtOH and the compounds were isolated from the ethanolic
extract. The dried ethanolic extract (200 g) was suspended in water
and partitioned with CHCl3, AcOEt and BuOH to yield
CHCl3-soluble (128 g), AcOEt-soluble (8.12 g) and BuOH
(20.8 g) fractions. The CHCl3-soluble fraction was
subjected to silica gel column chromatography eluted with
cyclohexane, cyclohexane/AcOEt, AcOEt and MeOH to produce 10
fractions. Fraction 7 (19.2 g) was eluted with cyclohexane/AcOEt
5:5 and then purified by silica gel column chromatography
(CHCl3/MeOH gradient) to yield seven subfractions. The
fourth subfraction was purified by silica gel column chromatography
and high-performance liquid chromatography (HPLC) to yield
picrasmalignan A (2.9 mg). Its chemical structure (Fig. 1) was identified by the chemical and
physical properties, and spectra data.
Picrasmalignan A: white powder, [α] −13.2°
(c0.5, MeOH); UV (MeOH) λmax (log ɛ) 208 (3.95), 283 (3.08),
337 (3.15) nm; IR (KBr) νmax 3404, 2942, 1661, 1597, 1497, 1482,
1383, 1330, 1278, 1213, 1138, 1031, 825 cm−1; 1H NMR
(400 MHz, DMSO-d6) and 13C NMR (100 MHz,
DMSO-d6), listed in Table
I; ESIMS (positive) m/z 557 [M+Na]+, ESIMS
(negative) m/z 569 [M+Cl]−; HRESIMS m/z
535.1941 [M+H]+ (calculated for
C30H31O9, 535.1963).
 | Table INMR data of picrasmalignan A. |
Table I
NMR data of picrasmalignan A.
| Position | δC | δH |
1H-1H COSY | HMBC (H→C) |
|---|
| 1 | 127.7, qC | | | |
| 2 | 112.6, CH | 7.30 (1H, s) | | C-3, C-6, C-7,
C-7′ |
| 3 | 144.1, qC | | | |
| 4 | 150.6, qC | | | |
| 5 | 130.1, qC | | | |
| 6 | 118.5, CH | 7.30 (1H, s) | | C-2, C-4, C-7,
C-8′ |
| 7 | 153.9, CH | 7.64 (1H, d, J
= 15.3 Hz) | H-8 | C-2, C-6, C-9 |
| 8 | 126.1, CH | 6.76 (1H, dd,
J = 15.3, 7.8 Hz) | H-7, H-9 | C-1, C-7, C-9 |
| 9 | 193.9, CH | 9.59 (1H, d, J
= 7.8 Hz) | H-8 | C-8 |
| 1′ | 133.7, qC | | | |
| 2′ | 110.8, CH | 6.88 (1H, s) | | C-1′, C-4′, C-6′,
C-7′ |
| 3′ | 143.5, qC | | | |
| 4′ | 146.5, qC | | | |
| 5′ | 129.7, qC | | | |
| 6′ | 114.7, CH | 6.92 (1H, s) | | C-2′, C-4′, C-7′ |
| 7′ | 88.1, CH | 5.60 (1H, d, J
= 6.8 Hz) | H-8′ | C-4, C-1′, C-2′,
C-9′ |
| 8′ | 52.4, CH | 3.55 (1H, m) | H-7′, H-9′ | C-4, C-1′, C-5′,
C-7′ |
| 9′ | 62.6,
CH2 | 3.75 (1H, m) | H-8′ | C-5, C-7′ |
| 1″ | 132.1, qC | | | |
| 2″ | 118.9, CH | 6.74 (1H, d, J
= 1.0 Hz) | | C-4″, C-6″, C-7″ |
| 3″ | 147.5, qC | | | |
| 4″ | 147.5, qC | | | |
| 5″ | 115.3, CH | 6.75 (1H, d, J
= 8.0 Hz) | | C-1″, C-3″, C-6″ |
| 6″ | 110.4, CH | 6.90 (1H, dd,
J = 8.0, 1.0 Hz) | | C-1″, C-2″, C-7″ |
| 7″ | 87.2, CH | 5.44 (1H, d, J
= 6.9 Hz) | H-8″ | C-4′, C-1″, C-2″ |
| 8″ | 52.9, CH | 3.46 (1H, d, J
= 6.4 Hz) | H-7″, H-9″ | C-4′, C-5′, C-1″ |
| 9″ | 62.7,
CH2 | 3.64 (1H, t, J
= 6.4 Hz) | H-8″ | C-7″, C-8″ |
|
3-OCH3 | 55.9,
CH3 | 3.83 (3H, s) | | C-3 |
|
3′-OCH3 | 55.8,
CH3 | 3.76 (3H, s) | | C-3′ |
|
3″-OCH3 | 55.6,
CH3 | 3.73 (3H, s) | | C-3″ |
Reagents
RPMI-1640 medium and fetal bovine serum were
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
LPS, dimethylsulfoxide (DMSO), MTT and DAF-FM DA were obtained from
Sigma-Aldrich (St. Louis, MO, USA). Nicotinamide adenine
dinucleotide phosphate (NADPH) was purchased from Wako Pure
Chemical Industries, Ltd. (Tokyo, Japan). The
penicillin-streptomycin stock solution, mouse TNF-α ELISA, mouse
IL-6 ELISA, Bradford protein assay and nitric oxide synthase assay
kits were obtained from Yantai Science and Biotechnology Co., Ltd.
(Yantai, China). The colorimetric COX (ovine) inhibitor screening
assay kit, anti-murine iNOS polyclonal antibody and COX-2 (murine)
polyclonal antibody were obtained from Cayman Chemical (Ann Arbor,
MI, USA). β-actin antibody was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Hydrocortisone (H4001;
purity HPLC ≥98%; Sigma-Aldrich) was used as a positive control in
all experiments.
Cell culture
Mouse monocyte-macrophage RAW 264.7 cells (ATCC
TIB-71; American Type Culture Collection, Manassas, VA, USA) were
maintained in RPMI-1640 medium supplemented with 10%
heat-inactivated FBS at 37°C in a humidified incubator with 5%
CO2 and 95% air. The medium was replaced every 2 days.
RAW 264.7 cells were passaged by trypsinization until 80%
confluence was achieved.
Cell viability assay
RAW 264.7 cells were treated with picrasmalignan A
at concentrations of 1–100 μM. The mitochondrial-dependent
reduction of MTT to formazan was used to measure cell respiration
as an indicator of cell viability. Briefly, following 24 h
incubation, an MTT solution (final concentration, 200 μg/ml) was
added and the cells were incubated for another 4 h at 37°C.
Following the removal of the supernatant, 100 μl DMSO was added to
the cells to dissolve the formazan. The absorbance of each group
was measured by a microplate reader at a wavelength of 570 nm
(Biotek Synergy HT; BioTek Instruments, Inc., Winooski, VT, USA).
The control group consisted of untreated cells, which were
considered to be 100% viable. Results are expressed as the
percentage of viable cells when compared with that of the control
group.
NO analysis
NO levels were determined by measuring the quantity
of nitrite in the cell culture supernatant using Griess reagent.
RAW 264.7 cells were treated with LPS (1 μg/ml) with or without
picrasmalignan A (10–100 μM) for 24 h. Cell culture supernatant
(100 μl) was mixed with 100 μl Griess reagent and absorbance was
measured at 540 nm. The nitrite concentrations were calculated
using a standard calibration curve prepared from different
concentrations of sodium nitrite.
Measurement of TNF-α and IL-6
RAW 264.7 cells were treated with LPS (1 μg/ml) with
or without picrasmalignan A (10–100 μM) for 6 h. The culture
supernatant (100 μl) was removed to determine the level of TNF-α
and IL-6 using the respective mouse TNF-α and mouse IL-6 ELISA
kits, according to the manufacturer’s instructions.
Detection of iNOS, COX-2 and β-actin
expression
RAW 264.7 cells were washed with cold
phosphate-buffered saline (PBS) and lysed in western cell lysis
buffer 24 h following treatment. Cell debris was removed by
centrifugation. Following the determination of the protein
concentration of each aliquot by a commercial Bradford protein
assay kit, 30 μg total protein boiled in sodium dodecyl
sulfate-polyacrylamide gel electrophoresis loading buffer was
subjected to gel electrophoresis and electrophoretically
transferred onto nitrocellulose membranes. The membranes were
blocked with 5% non-fat dried milk in Tris-buffered saline with
Tween 20 (TBST) at room temperature for 1 h. Following washing, the
membranes were incubated in the respective primary antibody
solution (anti-iNOS, anti-COX-2 and anti-β-actin antibodies)
overnight at 4°C. The membranes were washed with TBST and incubated
with horseradish peroxidase-conjugated secondary antibody solution
for 1 h at room temperature. The blots were washed again three
times in TBST, detected using enhanced chemiluminescence (ECL) and
exposed to photographic films. Images were collected and the bands
corresponding to iNOS, COX-2 and β-actin were quantitated by
densitometric analysis using DigDoc100 program (Genetic
Technologies, Inc., Glencoe, MO, USA). Data regarding iNOS and
COX-2 were normalized on the basis of the β-actin levels.
Assay of iNOS enzymatic activity
RAW 264.7 cells were treated with LPS (1 μg/ml) and
picrasmalignan A (3–100 μM) for 2 h at 3°C, the culture supernatant
was removed and 100 μl NOS assay buffer were added to each well.
Then 100 μl NOS assay reaction solution (50% NOS assay buffer,
39.8% MilliQ water, 5% L-Arginine solution, 5% 0.1 mM NADPH, 0.2%
DAF-FM DA) was added to each well and incubated for 2 h at 37°C.
Fluorescence was measured with a fluorescence plate reader (Biotek
Synergy HT; BioTek Instruments, Inc.) at excitation 485 nm and
emission 528 nm.
Assay of COX-2 enzymatic activity
COX-2 activity was determined by a colorimetric COX
inhibitor screening assay kit according to the manufacturer’s
instructions. Briefly, 160 μl assay buffer and 10 μl heme were
added to the background wells, while 150 μl assay buffer, 10 μl
heme and 10 μl COX-2 enzyme were added to the 100% initial activity
wells. Picrasmalignan A (10 ml; final concentrations, 1, 2.5 and 5
mM) was added to the sample wells and 10 μl DMSO was added to the
background wells. The plate was carefully shaken for a few seconds
and incubated for five min at 25°C. The colorimetric substrate
solution (20 μl) followed by arachidonic acid (20 μl) were added to
each well. The plate was again shaken carefully for a few seconds
and incubated for 5 min at 25°C. The absorbance at 590 nm was read
by a microplate reader and the inhibition ratio of COX-2 enzymatic
activity was calculated according to the manufacturer’s
instructions.
Statistical analysis
All results are presented as the mean ± standard
deviation. Statistical comparison was conducted using Student’s
t-test following analysis of variance (ANOVA). P<0.05 was
considered to indicate a statistically significant difference.
Results
RAW 264.7 cells were treated with various
concentrations of picrasmalignan A for 24 h and the cell viability
was tested by an MTT assay as described in Materials and methods.
Picrasmalignan A did not demonstrate cytotoxicity in the range of
1–100 μM (data not shown). RAW 264.7 cells were then treated with 1
μg/ml LPS with or without the indicated concentrations of
picrasmalignan A or hydrocortisone. After 24 h, nitrite
concentrations were determined as an indicator of the NO
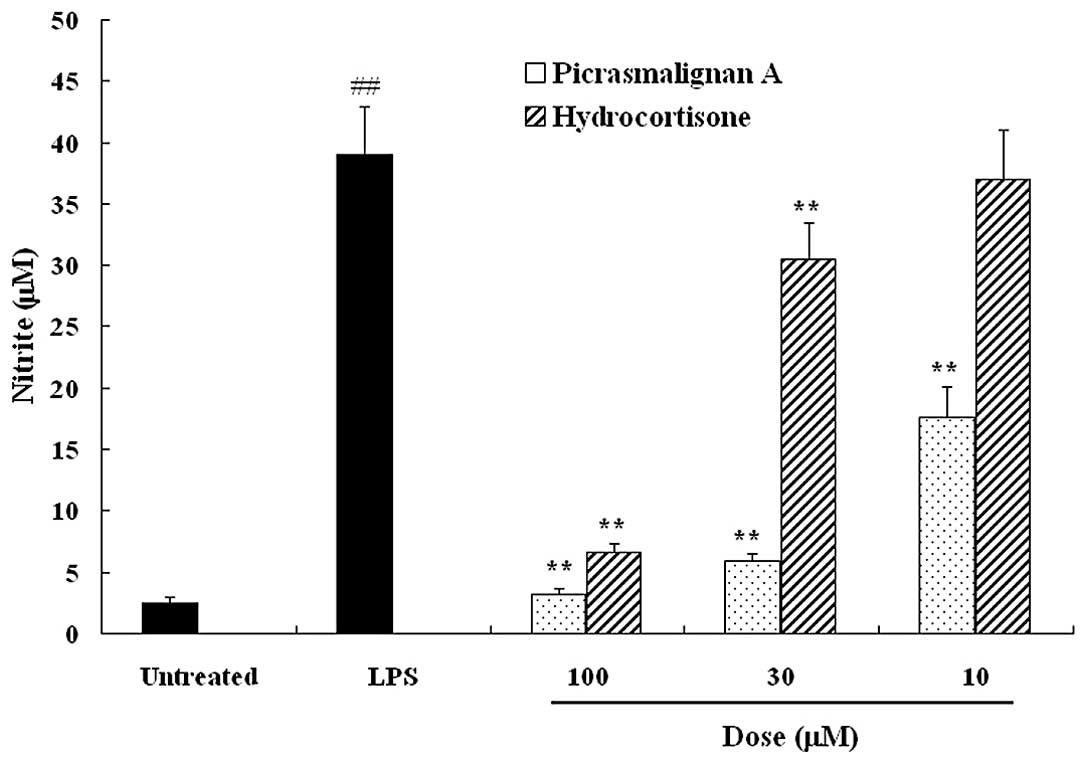
production. As shown in Fig. 2,
picrasmalignan A significantly inhibited the LPS-induced production
of NO to an even greater extent than the positive control
hydrocortisone, a commonly used anti-inflammatory drug. RAW 264.7
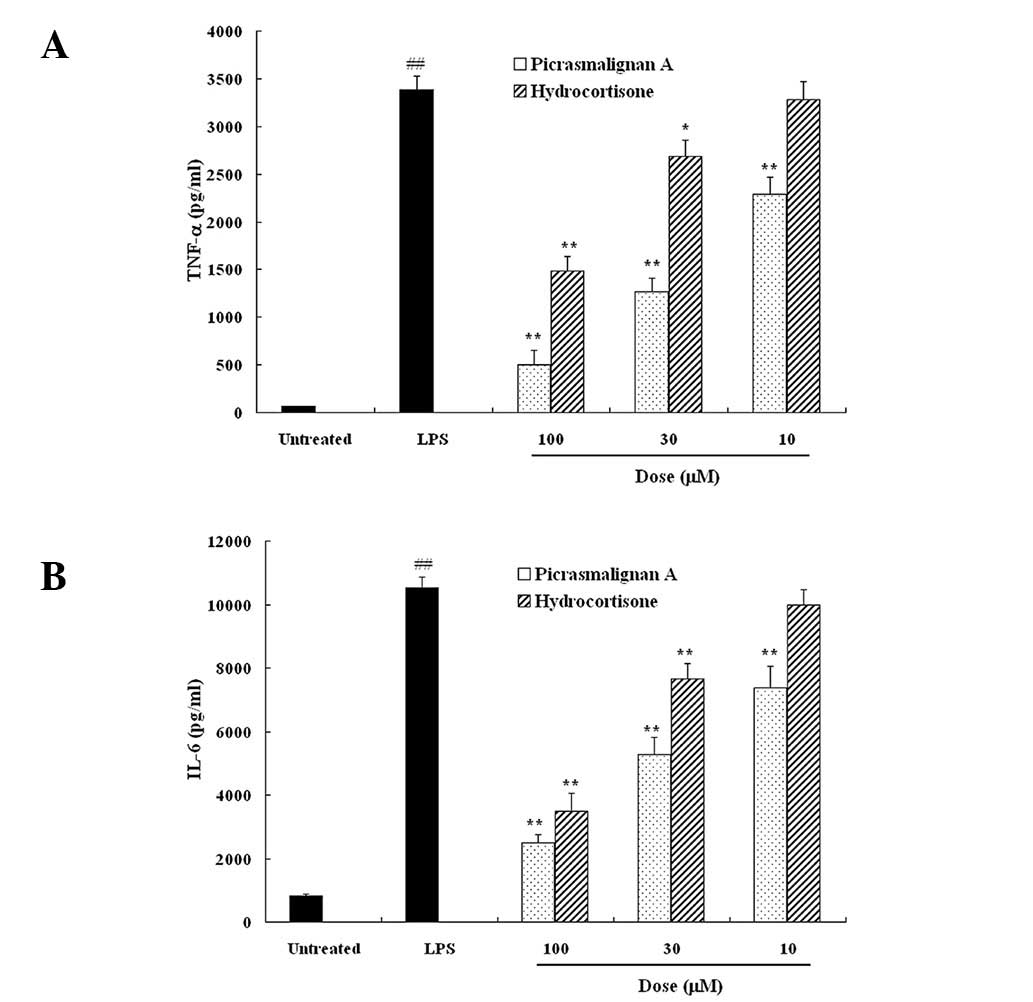
cells were treated with 1 μg/ml LPS with or without the indicated
concentrations of picrasmalignan A or hydrocortisone for 6 h, and
the resulting pro-inflammatory cytokine levels of TNF-α and IL-6 in
the supernatant were determined by an ELISA assay. As shown in
Fig. 3, LPS-induced TNF-α and IL-6
release were significantly suppressed by picrasmalignan A in a
dose-dependent manner. Furthermore, the inhibitive action of
picrasmalignan A was more potent than that of the positive control,
hydrocortisone. These results demonstrated that picrasmalignan A
significantly inhibited the expression of LPS-induced
proinflammatory mediators, such as NO, TNF-α and IL-6, in
macrophages, which may be responsible for its anti-inflammatory
effect.
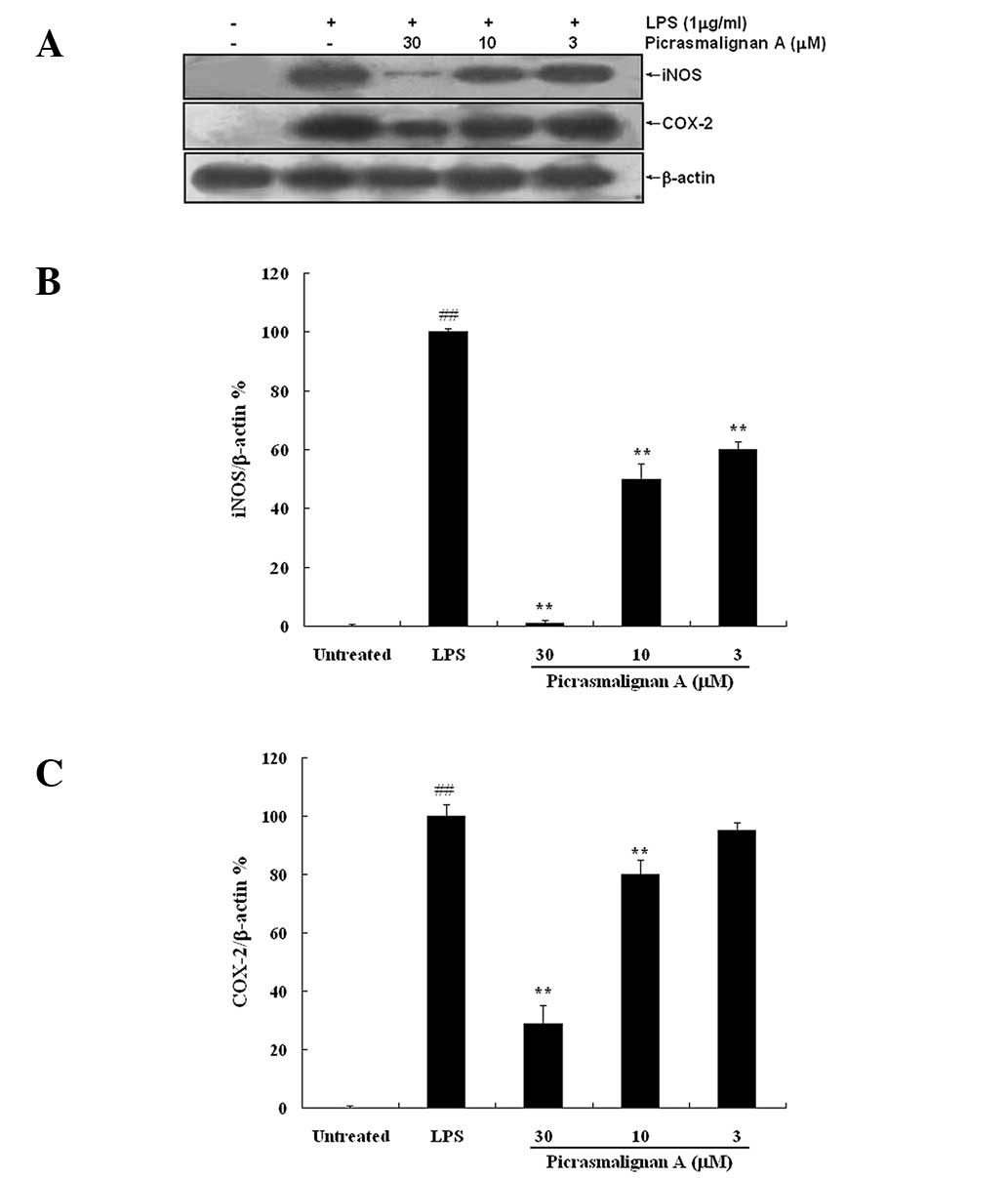
As the excess production of NO is correlated with
the upregulation of inducible nitric oxide synthase (iNOS)
expression, the expression of iNOS and COX-2 was investigated by
western blot analysis. As shown in Fig. 4A, treatment with 30 μM
picrasmalignan A almost completely inhibited the expression of iNOS
and significantly inhibited the LPS-induced overexpression of
COX-2. The density of bands corresponding to the iNOS and COX-2
proteins were normalized on the basis of β-actin and are shown in
Fig. 4B and 4C, respectively.
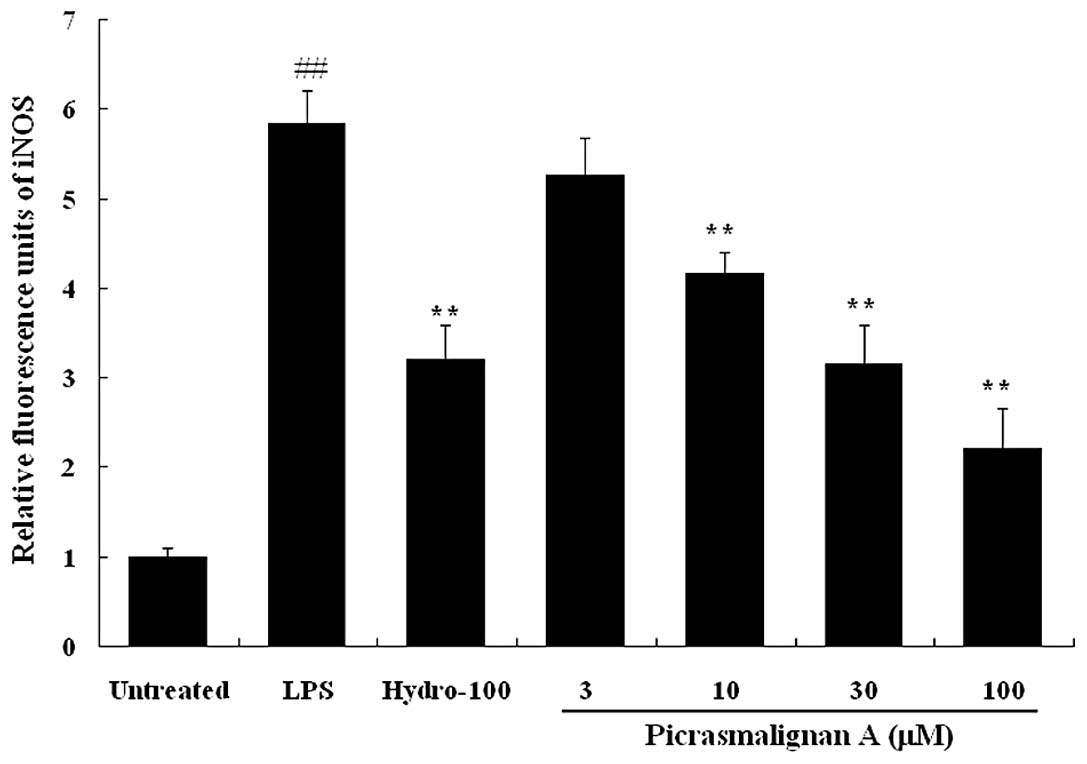
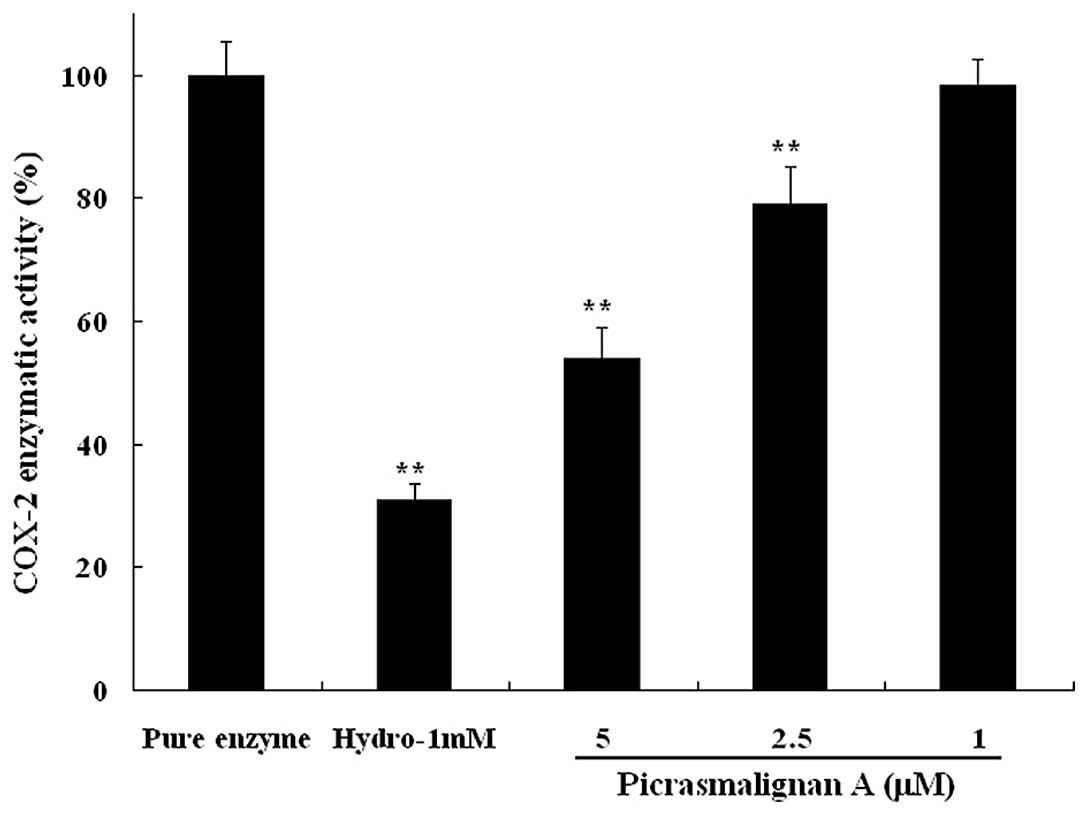
The inhibitory effects of picrasmalignan A on the
activity of the iNOS and COX-2 enzymes were determined. As shown in
Fig. 5, treatment with 1 μg/ml LPS
led to an ~6-fold increase of iNOS enzymatic activity within 2 h.
The indicated concentrations of picrasmalignan A (3, 10, 30 and 100
μM) markedly inhibited the iNOS enzymatic activation in RAW 264.7
cells and demonstrated dose-dependency. Picrasmalignan A (2.5 and 5
mM) also significantly inhibited the activity of the COX-2 enzyme,
similarly to that of the positive control, hydrocortisone (Fig. 6).
Discussion
P. quassioides is a commonly used traditional
Chinese medicine predominantly used to treat fever and
inflammation.. It has previously been demonstrated that β-carboline
alkaloids (the predominant active constituents of this medicinal
plant) exert anti-inflammatory effects through the inhibition of
the iNOS pathway but independent of the COX-2 pathway (16). In the present study, another type
of natural compound neolignan, picrasmalignan A, was further
examined for its possible anti-inflammatory molecular mechanism.
The results demonstrated that picrasmalignan A blocked various
LPS-induced macrophage responses including the increased production
of inflammatory mediators (NO, TNF-α, IL-6), the overexpression of
iNOS and COX-2, and the activity of iNOS and COX-2 enzymes. To the
best of our knowledge, this is the first study to demonstrate the
anti-inflammatory effect of neolignan agents. These results also
suggested that neolignan and β-carboline alkaloids coexist in P.
quassioides and may exert the anti-inflammatory effect through
different pathways.
Acknowledgements
This study was supported by the Project of National
Natural Science Foundation of China (grant no. 81102781) and the
Taishan Scholar Project to Fenghua Fu.
References
|
1
|
Ohmoto T and Koike K: Studies on the
constituents of Picrasma quassioides Bennet I. On the
alkaloidal constituents. Chem Pharm Bull. 30:1204–1209. 1982.
|
|
2
|
Ohmoto T and Koike K: Studies on the
constituents of Picrasma quassioides Bennet II. On the
alkaloidal constituents. Chem Pharm Bull. 31:3198–3204. 1983.
|
|
3
|
Ohmoto T and Koike K: Studies on the
constituents of Picrasma quassioides Bennet III. The
alkaloidal constituents. Chem Pharm Bull. 32:3579–3583. 1984.
|
|
4
|
Jiao WH, Gao H, Zhao F, He F, Zhou GX and
Yao XS: A new neolignan and a new sesterterpenoid from the stems of
Picrasma quassioides Bennet. Chem Biodivers. 8:1163–1169.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiao WH, Gao H, Zhao F, Lin HW, Pan YM,
Zhou GX and Yao XS: Anti-inflammatory alkaloids from the stems of
Picrasma quassioides Bennet. Chem Pharm Bull (Tokyo).
59:359–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiao WH, Gao H, Li CY, Zhao F, Jiang RW,
Wang Y, Zhou GX and Yao XS: Quassidines A–D, bis-beta-carboline
alkaloids from the stems of Picrasma quassioides. J Nat
Prod. 73:167–171. 2010.
|
|
7
|
Jiao WH, Gao H, Li CY, Zhou GX, Kitanaka
S, Ohmura A and Yao XS: Beta-carboline alkaloids from the stems of
Picrasma quassioides. Magn Reson Chem. 48:490–495.
2010.PubMed/NCBI
|
|
8
|
Tilg H, Wilmer A, Vogel W, Herold M,
Nölchen B, Judmaier G and Huber C: Serum levels of cytokines in
chronic liver diseases. Gastroenterology. 103:264–274.
1992.PubMed/NCBI
|
|
9
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Isomäki P and Punnonen J: Pro- and
anti-inflammatory cytokines in rheumatoid arthritis. Ann Med.
29:499–507. 1997.
|
|
11
|
Pokharel YR, Liu QH, Oh JW, Woo ER and
Kang KW: 4-Hydroxykobusin inhibits the induction of nitric oxide
synthase by inhibiting NF-kappaB and AP-1 activation. Biol Pharm
Bull. 30:1097–1101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Emery P: COX-1, COX-2: so what? Scand J
Rheumatol. 28:6–9. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abd-El-Aleem SA, Ferguson MW, Appleton I,
Bhowmick A, McCollum CN and Ireland GW: Expression of
cyclooxygenase isoforms in normal human skin and chronic venous
ulcers. J Pathol. 195:616–623. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruch-Gerharz D, Stahl W, Gerharz CD,
Megahed M, Wingerath T, Sies H and Ruzicka T: Accumulation of the
xanthophyll lutein in skin amyloid deposits of systemic amyloidosis
(al type). J Invest Dermatol. 116:196–197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruch-Gerharz D, Fehsel K, Suschek C,
Michel G, Ruzicka T and Kolba-Bachofen V: A proinflammatory
activity of interleukin 8 in human skin: expression of the
inducible nitric oxide synthase in psoriatic lesions and cultured
keratinocytes. J Exp Med. 184:2007–2012. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao F, Gao Z, Jiao W, Chen L, Chen L and
Yao X: In vitro anti-inflammatory effects of beta-carboline
alkaloids, isolated from Picrasma quassioides, through inhibition
of the iNOS pathway. Planta Med. 78:1906–1911. 2012. View Article : Google Scholar : PubMed/NCBI
|