Introduction
Traditional medicines have been used as primary
healthcare in numerous countries and up to 80% of the world
population relies on traditional remedies that are based on plant
extracts (1). Plant-derived
products, termed phytochemicals, have been extensively investigated
and are being used as drugs or dietary supplements due to their
availability, the ability for multiple applications, high
efficacies and safety (2). The
clinical importance of herbal drugs has been on the increase due to
the low number of side-effects compared with numerous synthetic
antioxidants. Stress management is extremely significant in
day-to-day life. The manner in which the body adapts to stress may
be supported by food supplements, dietary elements, herbs and
minerals, which enhance the immune system. Such compounds are often
termed ‘adaptogens’ (3). It is
well known that various plants or plant-derived products are
composed of multiple nutrients and antioxidants, including
vitamins, flavonoids and polyphenol compounds, and these compounds
exhibit direct effects on various diseases, such as cancer and
pulmonary diseases (4).
Sea buckthorn (SBT) [Hippophae rhamnoides L.
(Elaeagnaceae)] is a thorny nitrogen-fixing arborary shrub,
found in numerous countries, including those in Europe and Asia and
is a rich source of nutrients (5).
SBT has been used as a traditional medicine for thousands of years
and is widely used as a therapeutic agent in various human
diseases, such as flu, cardiovascular diseases, mucosal injuries,
skin disorders, hepatotoxicity and hypoglycemia (6,7).
However, the mechanism underlying the involvement of SBT in these
diseases has not yet been well characterized. Studies have shown
that SBT is a source of nutrients, including vitamins (A, C, E and
K, riboflavin and folic acid), carotenoids (α and β carotene and
lycopene), organic acids (malic acid and organic acid), tocopherol,
lipids, amino acids, tannins and various minerals (7). SBT is also composed of multiple
functional ingredients, including sterols (ergosterol,
stigmasterol, lanosterol and amyrins) and flavonoids (isorhamnetin,
quercetin, myricetin, kaempferol and their glucoside compounds)
(7). The predominant chemical
constituents isolated and characterized from SBT are antioxidants.
Previous studies have investigated the involvement of flavonoids
isolated from SBT in protection against varioustoxins (8–11).
Those flavonoids may also be responsible for its beneficial
involvement in paraquat (PQ)-exposed A549 lung cells. Similarly,
one of the most important protective mechanisms of flavonoids is
their ability to decrease reactive oxygen species (ROS) formation
through the activation of Nrf2-dependent genes (8). Notably, the SBT extract has been
associated with the induction of detoxifying and antioxidant
enzymes as superoxide dismutase (SOD), catalase and glutathione
(GSH) (10). Pharmacological and
clinical studies have also demonstrated that several flavonoids,
which are the active constituents of SBT extract, produce
significant antitumor and anti-apoptotic effects (10,12).
Plant extracts containing numerous classes of chemical entities
with synergistic properties may exhibit greater therapeutic
benefits and applicability in lung protection compared with single
chemical entities. However, the efficacy of SBT in protection from
PQ-poisoning has not yet been elucidated.
PQ (1,1′-dimethyl-44′-bipyridinium chloride) is
widely used as a herbicide and has been of great concern to public
healthcare due to high morbidity and fatality rates resulting from
accidental ingestion or self-poisoning. It is well documented that
the toxicity of PQ is based on the generation of excess quantities
of ROS, which leads to fatal cellular toxicity in various organs,
such as the lung, kidney and liver. PQ toxicity is associated with
redox cyclic oxygen and other free radicals, leading to NADPH
depletion and the lipid peroxidation of cell membranes (13,14).
The lung is the primary target organ of PQ-poisoning and PQ-induced
lung toxicity results in the initial development of pulmonary
edema, infiltration of inflammatory cells and damage to the
alveolar epithelium, which may progress to severe fibrosis
(15). The combinatory procedures,
i.e., haemoperfusion with antioxidants (vitamin) and/or immune
suppressors are used for the treatment of PQ-poisoning; however,
these treatments are not highly effective. The use of natural
antioxidants may therefore be a promising treatment option for
poisoned patients and the effectiveness of such treatments has been
previously demonstrated (13). The
antioxidant activity or free radical scavenging activity is
important in protection against PQ-induced pulmonary toxicity
(7). PQ has been shown to result
in A549 cell death, which leads to subsequent abnormalities
(16).
In the present study, it was hypothesized that SBT
extract ameliorates cytotoxicity in PQ-exposed A549 cells by
inducing the expression of Nrf2 and antioxidant-related genes. The
results demonstrated that SBT was able to protect cell morphology,
membrane integrity and reduce intracellular ROS levels in
PQ-exposed A549 cells. Furthermore, SBT induced the activation and
expression of Nrf2 and several detoxifying phase II enzymes,
such as NAD(P)H dehydrogenase quinone 1 (NQO1), glutathione
peroxidase 1 (GPX1), glutathione reductase (GSR) and
catalase (CAT) under the PQ-poisoning condition. The results
of this study suggests that SBT is likely a therapeutic candidate
for the treatment of PQ-related toxicity.
Materials and methods
Cells and sample treatment
A549, a human carcinoma cell line (no. CCL-185), was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and cultured in Dulbecco’s modified Eagle’s medium (DME) and
Ham’s F-12 Nutrient mixture culture medium (1:1 mixture; Invitrogen
Life Technologies, Carlsbad, CA, USA) containing 10%
heat-inactivated fetal bovine serum and 1% penicillin/streptomycin.
All the experiments were performed on cells maintained within 15
passages. A549 cells have been used in various PQ-induced lung
toxicity studies (17). The cells
were grown to a confluency of 70–80% prior to use in the
experiments. Cells were treated with different concentrations of
SBT and PQ was then added to the culture medium.
Chemicals and reagents
PQ (1,1′-dimethyl-4,4′-bipyridinium dichloride),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
(MTT), trypan blue stain solution, and Triton X-100 were obtained
from Sigma-Aldrich (St. Louis, MO, USA). DMEM/F-12 was purchased
from Invitrogen Life Technologies. Antibiotics/antimycotics 100
U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin
B were obtained from Gibco-BRL (Carlsbad, CA, USA) and fetal bovine
serum was obtained from HyClone (Thermo Scientific, Rockford, IL,
USA). The total ROS detection kit and lactate dehydrogenase (LDH)
assay kit were obtained from Enzo Life Sciences (Farmingdale, NY,
USA) and Roche (Pleasanton, CA, USA), respectively. The anti-Nrf2
antibody and all secondary antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The primary antibody
against β-actin was obtained from Abcam (Cambridge, MA, USA).
Primers were purchased from Bioneer Corp. (Daejeon, Korea).
Standardized extracts of SBT
A standardized methanol-extract of SBT was purchased
from the International Biological Material Research Center in the
Korea Research Institute of Bioscience and Biotechnology (Daejeon,
Korea). A detailed description of the composition of the SBT
extract was described previously by Kim et al(7). Stock solutions (100 mg/ml) of the
extract were prepared in dimethyl sulfoxide (DMSO) and stored at
−20°C prior to use.
Cell viability assay
MTT was used as an indicator of cell viability as
determined by its mitochondrial-dependent reduction to formazone.
Briefly, 2×105 cells/ml were seeded in a 96-well plate
containing a final volume of 200 μl and incubated for 24 h to allow
the cells to reach a confluency of 80%. The cells were pretreated
with different concentrations of SBT for 6 h prior to 200 μM PQ
treatment and then incubated for different time periods. Following
incubation, 20 μl MTT (5 mg/ml) was added to each well and
intercellular reduction of soluble yellow MTT into insoluble purple
formazan crystals was allowed to proceed. The supernatant was then
removed, and the formazan crystals were dissolved using 100 μl
DMSO. The plate was then incubated for 30 min and the optical
absorbance of the samples was measured at 590 nm using a Victor™ X3
multilabel reader (Perkin Elmer, Waltham, MA, USA). Data on cell
viability are shown as the percentage of control (survival of
control).
LDH release assay
LDH activity assays were performed on A549 cells
using a colorimetric technique. Cells were cultured in 96-well
plates at 1×105 cells/well and grown to a confluence of
70–80%. The cells were then treated with SBT (25, 50, 100 and 200
μg/ml) and PQ (200 μM). Following treatment, the LDH cytotoxicity
assay was performed according to the manufacturer’s instructions.
Briefly, 100 μl cell culture supernatant was transferred from each
well to a 96-well plate and 100 μl freshly prepared reaction
mixture was added to each well. Following 30 min of incubation at
room temperature in the dark, the absorbance was determined at 490
nm using a Victor X3 multilabel reader (Perkin Elmer). The quantity
of LDH was expressed as a percentage compared with the total
quantity of LDH present in cells treated with 2% Triton X-100.
Measurement of total ROS level
Total ROS accumulation in A549 cells was monitored
using a fluorescence-based ROS detection kit (Enzo Life Sciences).
The experiment was performed according to the manufacturer’s
instructions. Briefly, A549 cells (1×105 cells/ml) were
seeded in 24-well plates and incubated for 24 h. Following
incubation, the media were removed from the cell culture plate and
the ROS detection solution was added, covering the whole cell
surface area. The ROS detection solution was incubated for 1 h at
37°C. The cells were then pretreated with SBT for 6 h and oxidative
stress was induced by the addition of PQ (200 μM) for 6 h.
Pyocyanin and N-acetyl-L-cysteine (NAC) served as positive
and negative controls, respectively. Following treatment, the cells
were washed with 1X wash buffer twice to remove all media from the
wells. The fluorescence intensity was then measured using a Zeiss
Axiovert-25 microscope (Carl Zeiss, Thornwood, NY, USA) with a
filter range of Ex/Em: 490/525 nm. The average quantitative value
was determined by measuring five different areas of the same
sample. The intensity of the fluorescence was proportional to the
quantity of total ROS produced by the cells.
Cytoplasmic and nuclear protein
separation
Cytoplasmic and nuclear fractions were separated
using a commercially available nuclear and cytoplasmic extraction
reagent kit (BioVision, Milpitas, CA, USA). A549 cells were seeded
in 6-cm dishes at a density of 4×105 cells/ml and
incubated with SBT for 6 h. Oxidative stress was induced by
incubation with 200 μM PQ for 12 h. Following incubation, the cells
were collected, and 0.2 ml cytosol extraction buffer (CEB)-A
containing DTT and protease inhibitor were added. Ice-cold CEB B
(11 μl) was then added and incubated for 10 min. The supernatant
containing the cytoplasmic proteins was collected. The remaining
pellet was then re-suspended with ice-cold nuclear extraction
buffer mix and vigorously mixed for 15 sec. Nuclear protein
containing supernatant was collected by further centrifugation.
Total protein extraction and western blot
analysis
For total cell extracts, A549 cells were grown in
6-well dishes at a density of 4×105 cells/ml for 24 h
and then treated with the different concentrations of SBT (25, 50,
100 and 200 μg/ml) and PQ (200 μM). Following treatment, the cells
were washed twice with cold phosphate-buffered saline and total
cell lysates were prepared using RIPA lysis buffer (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) containing a protease
inhibitor cocktail (Santa Cruz Biotechnology, Inc.). The
supernatant was collected and the protein concentration was
determined using the bicinchoninic acid protein assay kit (Thermo
Scientific, Pittsburgh, PA, USA) with bovine serum albumin as a
standard control. Proteins (30 μg) were separated using a 5%
SDS-PAGE gel at 100 V for 90 min and then transferred onto a
polyvinylidene fluoride membrane (Trans-Blot SD Semi-Dry Cell,
Bio-Rad, Hercules, CA, USA) at 15 V for 1 h. The membranes were
blocked by incubating for 1 h at room temperature with 5% dried
skimmed milk in Tris-buffered saline containing 0.1% Tween-20. The
membranes were then incubated with primary antibodies against Nrf2
(1:500) and β-actin (1:10,000) overnight at 4°C. Horseradish
peroxidase-conjugated anti-rabbit IgG (Santa Cruz Biotechnology,
Inc.) and anti-mouse IgG (Santa Cruz Biotechnology, Inc.) were used
as secondary antibodies for Nrf2 and β-actin, respectively.
Chemiluminescence was performed using an enchanced
chemiluminescence system and images were captured using a ChemiDoc
Imaging system (ChemiDoc™ XRS+ System with Image Lab™ Software,
Bio-Rad).
qPCR analysis
Total RNA was prepared from A549 cells using an RNA
extraction kit (Qiagen, Valencia, CA, USA) according to the
manufacturer’s instructions. Total RNA was quantified using an
ND-1000 spectrophotometer (Thermo Fisher Scientific, Wilmington,
DE, USA) at 260 nm and the purity of the RNA was assessed using the
260/280 nm absorbance ratio. Total RNA (1 μg) was used for the
generation of cDNA with the Maxime RT PreMix kit (Intron
Biotechnology, Korea). qPCR was performed using a CFX96™ Real-Time
PCR detection system (Bio-Rad) with iQTM SYBR-Green Supermix
(Bio-Rad) and gene specific primer sets (for SOD1,
GPX1, GSR, CAT, PRDX1 and LPO),
which were purchased from Intron Biotechnology (PHS-001050,
Accutarget™ human antioxidant Real-Time PCR primer set, Daejeon,
Korea) (17). Gene expression was
measured according to the manufacturer’s instructions (Bio-Rad). In
brief, samples were heated to 95°C for 5 min followed by 40 cycles
of 95°C for 10 sec, 42°C for 10 sec, 72°C for 20 sec for 40 cycles.
Melting curve analysis was performed to ensure the amplification of
a single amplicon. Samples were analyzed via the ΔΔCt method using
GAPDH for normalization and the data are presented as the fold
change relative to the control samples. Data were compiled from
three independent experiments.
Statistical analysis
Values are expressed as the mean ± SD. The
significance of the difference from the respective controls for
each experimental test was analyzed using paired Student’s t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Protection of cell viability
The MTT assay was performed to investigate the
protective effect of SBT in A549 cells exposed to PQ. This cell
viability analysis was assessed in a dose- and time-dependent
manner with PQ and/or SBT treatment. Pretreatment with the SBT
extract was conducted instead of co-treatment with PQ due to its
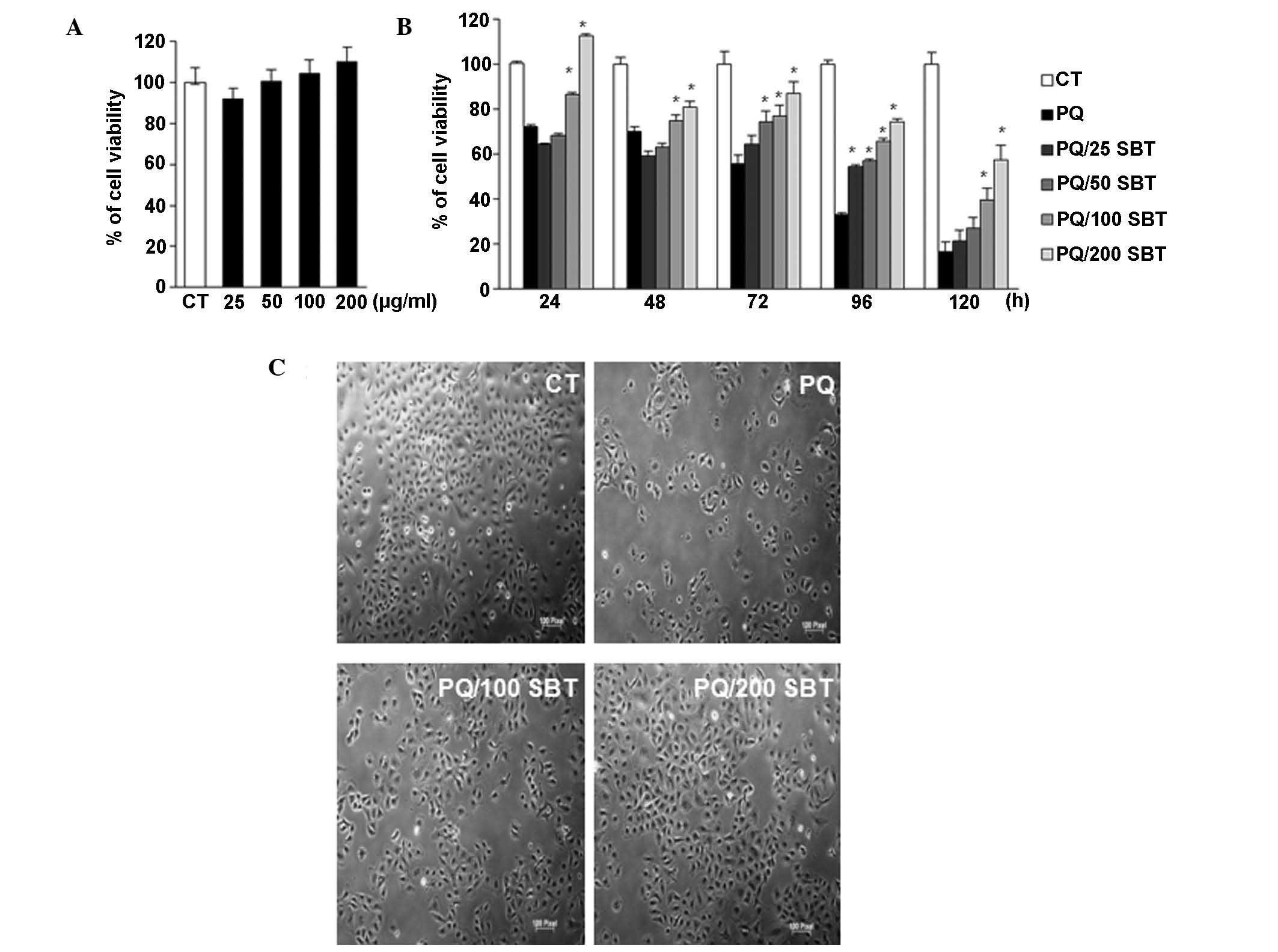
efficacy for cytoprotection(data not shown). As shown in Fig. 1, an SBT extract concentration of up
to 200 μg/ml did not result in any cytotoxic effects (Fig. 1A). However, pretreatment with the
SBT extract produced a significant cytoprotective effect in cells
exposed to PQ (Fig. 1B), whereas
PQ treatment alone led to a marked time-dependent reduction in cell
viability. Notably, higher concentrations of SBT (100 and 200
μg/ml) produced a marked cytoprotective effect at 24 h and SBT
concentrations as low as 25 μg/ml produced a significant
cytoprotective effect after 96 h. Moreover, it was observed that
this protection effect by SBT was more significant at 72, 96 and
120 h. Morphological changes were also observed following PQ
treatment. As shown in Fig. 1C, PQ
treatment resulted in the cells adopting a round shape, detaching
from the surface and aggregating. However, following pretreatment
with 100 and 200 μg/ml SBT, A549 cells showed a healthier
morphology when compared with PQ-treated cells. Data indicated that
SBT demonstrated no cytotoxicity and protected A549 cells against
PQ-induced toxicity.
SBT extract preserves membrane
integrity
To investigate the effect of the SBT extract on
membrane integrity, an LDH enzyme release assay was conducted
(Fig. 2). The quantity of released
LDH was investigated in a dose- and time-dependent manner. PQ
treatment (200 μM) led to a 35% LDH release; however, the quantity
of released LDH was markedly reduced by SBT pretreatment in a
dose-dependent manner (Fig. 2A).
LDH release was low when the cells were pretreated with 100 and 200
μg/ml SBT following 200 μM PQ treatment, which suggested that
pretreatment with SBT extract greatly reduces LDH release induced
by PQ treatment. In addition, the reduction in LDH release was also
shown to be time dependent (Fig.
2B). At 6 h, PQ induced ~40% LDH release; however, LDH release
was only 25% when the PQ-treated cells were pretreated with SBT. In
addition, the SBT extract alone did not result in increased LDH
activity (data not shown), indicating that pretreatment with SBT
extract protected the cell membrane integrity against PQ
treatment.
Pretreatment of SBT extract decreases
total ROS generation
In order to investigate the antioxidant properties
of SBT extract, intracellular ROS generation was measured using a
total ROS detection kit. Total ROS was analyzed based on the
fluorescence intensity from five different regions in three
individual experiments. Pyocyanin treatment was used as the
positive control and NAC, which is a well-known ROS scavenger,
served as the negative control. ROS generation was increased by
>60% following pyocyanin treatment relative to the control
cells. In addition, ROS generation was 55% greater subsequent to PQ
treatment when compared with the control cells. Notably, the total
intracellular ROS level was markedly reduced in SBT-pretreated A549
cells (Fig. 3). Treatment with 100
μg/ml SBT extract significantly reduced PQ-induced ROS levels in
A549 cells. Moreover, PQ-induced ROS generation was almost
completely eliminated with 200 μg/ml SBT extract. In addition,
pretreatment with 200 μg/ml SBT extract reduced ROS generation in
pyocyanin-treated cells by 40%. These results suggest that SBT
extract was able to function as a ROS scavenger following PQ
treatment.
SBT extract activates the antioxidative
transcription factor, Nrf2
Nrf2 is pivotal in the host defense mechanism and
numerous key phase II enzymes, such as NQO1 and HO1, are controlled
by Nrf2 transcription factor under ROS conditions. Therefore, the
present study aimed to determine whether SBT extract induces the
expression of Nrf2, its downstream target genes and other
antioxidant-related genes in PQ-exposed A549 cells. The SBT extract
was shown to induce Nrf2 expression (Fig. 4A). A549 cells were treated with
different concentrations of the SBT extract for 6 h, total cell
extracts were harvested and western blot analysis was then
performed. Nrf2 expression was markedly induced by SBT extract
treatment (Fig. 4A). Nrf2
mRNA expression was also induced in a dose-dependent manner
following SBT extract treatment (Fig.
4B). The effect of SBT treatment on Nrf2 expression under
PQ-exposed conditions was also observed (Fig. 4C). PQ marginally induced Nrf2
expression at 6 and 12 h and the expression of Nrf2 was
significantly induced subsequent to SBT extract pretreatment
(Fig. 4C). In order to determine
whether the SBT extract affected Nrf2 nuclear translocation,
western blot analysis was also performed using fractionized protein
samples from PQ and SBT extract-treated cells (Fig. 4D). Treatment with 200 μg/ml SBT
extract resulted in the nuclear translocation of Nrf2 in the
absence or presence of PQ treatment; however, PQ treatment alone
did not result in Nrf2 nuclear translocation. These data suggested
that the SBT extract effectively induced the expression of
Nrf2 mRNA and activated Nrf2 followed by nuclear
translocation.
Modulation of antioxidant-related gene
expression by SBT treatment
To investigate the effect of SBT extract on the
expression of antioxidant-related genes and phase II detoxifying
genes, qPCR analysis was performed under various conditions
(Fig. 5). Total RNA was collected
from PQ-exposed cells at 6 and 12 h and qPCR was conducted using
gene-specific primer sets (Table
I). As shown in Fig. 5, PQ
alone marginally induced NQO1, GSR and lipid
peroxidase (LPO) gene expression at 6 and 12 h. However, PQ
treatment did not effectively induce heme oxygenase 1 (HO1),
GPX1, SOD1, CAT and peroxiredoxin 1
(PRDX1) expression. NQO1, GPX1 and GSR
mRNA was significantly induced at 6 and 12 h following SBT
treatment, while HO1, SOD1 and PRDX1
expression was not altered by SBT treatment. CAT mRNA
expression was significantly induced at only 6 h when PQ-induced
A549 cells were exposed to high concentrations (200 μg/ml) of SBT
extract (Fig. 5F). These results
suggested that the SBT extract effectively induced the expression
of NQO1, GPX1, GSR and CAT
antioxidant-related genes, but not HO1, SOD1,
PRDX1 and LPO in PQ-treated A549 cells.
 | Figure 5Differentially regulated expression
of antioxidant-related genes by SBT (25, 50, 100 and 200 μg/ml) in
PQ-exposed A549 cells. (A) NQO1, (B) HO1, (C)
GPX1, (D) GSR, (E) SOD1, (F) CAT, (G)
PRDX1 and (H) LPO. The value was monitored using
SYBR-Green dye and normalized by glyceraldehyde 3-phosphate
dehydrogenase quantitative measurements. The data are expressed as
the mean ± SD of three independent experiments.
*P<0.05, compared with the vehicle-treated control
group. SBT, sea buckthorn; PQ, paraquat; NQO1, NAD(P)H
dehydrogenase quinone 1; HO1, heme oxygenase 1; GPX1,
glutathione peroxidase 1; GSR, glutathione reductase;
SOD1, superoxide dismutase 1; CAT, catalase;
PRDX1, peroxiredoxin 1; LPO, lipid perosidase; CT,
control. |
 | Table IPrimers used for qPCR analysis |
Table I
Primers used for qPCR analysis
| Gene name | Primer
sequence | Amplicon size
(bp) |
|---|
| Nrf2 | Forward:
5′-GCGACGGAAAGAGTATGAC-3′
Reverse: 5′-GTTGGCAGATCCACTGGTTT-3′ | 99 |
| NQO1 | Forward:
5′-CGCAGACCTTGTGATATTCCAG-3′
Reverse: 5′-CGTTTCTTCCATCCTTCCAGG-3′ | 245 |
| HO1 | Forward:
5′-GCAACCCGACAGCATGC-3′
Reverse: 5′-TGCGGTCGAGCTCTTCTG-3′ | 245 |
| GAPDH | Forward:
5′-TCCCATCACCATCTTCCA-3′
Reverse: 5′-CATCACGCCACAGTTTCC-3′ | 380 |
Discussion
Recent studies have demonstrated that SBT extract
contains various functional ingredients, including isoflavonoids,
polyphenols and numerous vitamins, and has clinical benefits such
as antioxidant effects in various human diseases (6,10,17–19).
Therefore, the effect of the SBT extract on ROS-induced
cytotoxicity due to PQ-poisoning was investigated. To obtain
insights into the involvement of SBT in PQ poisoning, several in
vitro experiments were conducted, including an MTT assay, LDH
assay and total ROS measurement, using lung adenocarcinoma A549
cells. A549 cells are a cell line derived from the lung and have
been commonly used to investigate the molecular and cellular
mechanisms of pulmonary-related diseases (20). In addition, A549 cells have been
used extensively to study the mechanisms underlying PQ-induced cell
death (16,20). Furthermore, the optimal conditions
of SBT treatment were determined. In these experiments,
pretreatment with SBT was shown to produce a greater outcome than
PQ co-treatment with regard to cytoprotection (data not shown).
Thus it was speculated that pretreatment rapidly activates the
expression of early host defense genes.
According to the MTT analysis, SBT extract
significantly protected A549 cells against death induced by
exposure to high concentrations of PQ (Fig. 1). A similar cytoprotective effect
has also been observed with other cell lines (17,21,22).
PQ induces intracellular LDH release into the medium, which
indicates a loss in cell membrane integrity. However, when the A549
cells were pretreated with SBT, the LDH level was markedly
decreased. Thus, the SBT extract may block LDH release or suppress
the disruption of membrane integrity by PQ. This cytoprotective
effect of SBT may be due to the presence of other bioactive
compounds, such as quercetin, isorhamnetin, keampferol and
casuarinin in the SBT extract that may suppress the generation of
intracellular ROS induced by PQ. (10,11).
The functional ingredients in the SBT extract have been identified
and characterized. Casuarinin, one of the functional compounds in
the SBT extract, has been shown to exhibit a great antioxidant
effect on hydrogen peroxide-induced oxidative stress in MDCK cells
(23)and an anti-inflammatory
effect on HaCaT cells via the inhibition of NF-κB signaling
(24). However, its function in
the PQ-induced system has not yet been elucidated. Therefore, it is
necessary to investigate the potential role of casuarinin in
PQ-treated conditions. In addition, these compounds may upregulate
proteins associated with the efflux transporter system, including
p-glycoprotein and host defense mechanisms (such as antioxidant and
detoxifying enzymes) (17).
However, more studies are required to determine the mechanisms by
which SBT protects the cell membrane. Total ROS was significantly
increased in PQ-exposed A549 cells (Fig. 3). These results are concurrent with
previous studies that investigated the effect of PQ treatment on
A549 cells (16). However, the ROS
level was gradually reduced when the cells were pretreated with
different concentrations of SBT extract. Certain studies have
demonstrated that SBT extract has dual effects such as ROS
scavenging and antioxidant effects in in vitro and in
vivo models (9,11,21).
Therefore, the decrease in ROS levels is due to the ROS scavenging
effect or an increase in the antioxidant defense activity.
The transcription factor Nrf2 is crucial in the
detoxification mechanisms for scavenging reactive metabolites and
ROS. Under normal physiological conditions, inactive Nrf2 is
localized in the cytoplasm by interactions with its inhibitor Keap1
(25). Under high ROS conditions,
Nrf2 is released from the inhibitory complexes and translocated
into the nucleus, where it regulates the expression of downstream
target genes via antioxidant response elements (ARE) binding sites
on their promoters (26). Previous
studies have demonstrated that the stabilization and translocation
of Nrf2 are critical for cytoprotection against various types of
oxidative stress (27,28). A lack of Nrf2 in MEF cells
led to an increase in ROS production and apoptosis in elevated
chromium (VI) and cadmium systems (29,30).
In the present study, SBT extract was shown to effectively induce
Nrf2 gene expression in a concentration-dependent manner (Fig. 4), which significantly contributed
to protection against PQ-induced cell death. Notably, Nrf2
expression was markedly induced by SBT treatment only (Fig. 4A). In addition, accumulation of
Nrf2 was observed following treatment with SBT extract. Flavonoid
compounds present in the SBT extract may be involved in the
activation of Nrf2, as it has been shown that synthetic flavonoid
compounds are potent inducers of the ARE/Nrf2/Keap1 signaling
pathway (25). Several
plant-derived compounds have previously been identified as inducers
of phase II enzyme genes in other cellular contexts (31,32).
Therefore, based on these results, the effect of SBT on the
expression of antioxidant-related genes, including Nrf2 target
genes, such as NQO1, HO1 and phase II detoxifying
genes, including GPX1, GSR, SOD1, CAT,
PRDX1 and LPO was determined. The SBT extract
significantly induced NQO1 mRNA expression in a
concentration-dependent manner but not HO1 mRNA expression,
although both are Nrf2 target genes. It was hypothesized that the
induction of HO1 requires other transcription factors, such
as NF-κB and AP-1(33). The mechanisms underlying these
differences merits further investigation, however, they are beyond
the scope of the present study.
Glutathione reductase (GSR) is a pivotal enzyme of
the cellular antioxidant defense mechanism, and generates the
sulfhydryl form of GSH from oxidized glutathione disulfide
(34). In this study, GSR
expression was induced by SBT extract treatment in PQ-exposed A549
cells. This result was also supported by previous studies (35,36).
GPX1, which functions in the detoxification of
H2O2, was also observed as it is the most
active antioxidant enzyme in humans and has previously been
associated with the antioxidant defense system (35,36).
In those studies, the SBT extract was shown to significantly induce
GPX1 and CAT mRNA expression, which is consistent
with previous studies (10,11).
Those results suggested that the functional SBT extract is a potent
GPX1 antioxidant gene inducer and may activate the pulmonary
defense system (28). Certain
studies have demonstrated that SBT resists ROS stress by activating
SOD1 activity and was shown to protect against butylated
hydroxytoluene treatment by effective induction of SOD1 via Nrf2
activation, respectively (10). In
addition, Tomita and Okuyama (37)
demonstrated that LPO activity was increased in PQ intoxication. In
the present study, SOD1, PRDX1 and LPO mRNA
expression were not activated by pretreatment with SBT extract in
A549 ells. Thus, it is likely that the Nrf2-dependent contribution
of SBT extract to the induction of the expression of these
antioxidant genes may be limited with regard to protecting against
PQ cytoxicity. It was assumed that the non-responsiveness of
SOD1, LPO and PRDX1 expression to the SBT
extract may be due to a lack of key compounds in the SBT extract or
these may be responses that occur in the late stages rather than
the early stages. However, more studies are required to understand
the reason for these results. Thus, SBT may be a therapeutic
candidate to protect against ROS-related diseases and cell
death.
In conclusion, the data from the present study
suggest that the active SBT extract exhibits potent cytoprotective
effects against cell toxicity resulting from exposure to PQ via
scavenging ROS, protecting the membrane integrity and inducing the
expression of antioxidant-related genes, including, Nrf2, which
targets divergent mechanisms. Insights were provided into the
protective mechanisms of SBT under conditions that mimic
PQ-poisoning with the aim of demonstrating its potential
therapeutic uses for the treatment of various ROS stress-related
diseases, such as PQ intoxication. At present, studies are in
progress to identify specific compounds that may be used as
therapeutic candidates against PQ intoxication. In addition,
studies on the molecular mechanisms of SBT with regard to cell
protection are currently underway.
Acknowledgements
The authors would like to thank Mrs. Tamanna Zerin
for her technical support and comments. This project was supported
by a grant from the Rural Development Administration, Republic of
Korea (project no. PJ008246).
References
|
1
|
Schuster BG: Demonstrating the validity of
natural products as anti-infective drugs. J Altern Complement Med.
7(Supp 1): S73–S82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nocentini S, Guggiari M, Rouillard D and
Surgis S: Exacerbating effect of vitamin E supplementation on DNA
damage induced in cultured human normal fibroblasts by UVA
radiation. Photochem Photobiol. 73:370–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar R, Shyam R, Divekar HM, Pahwa ML and
Srivastava KK: Mechanism of increased tolerance to hypothermia
after composite Indian herbal preparation II administration. J
Altern Complement Med. 6:509–517. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duarte J, Andriambeloson E, Diebolt M and
Andriantsitohaina R: Wine polyphenols stimulate superoxide anion
production to promote calcium signaling and endothelial-dependent
vasodilatation. Physiol Res. 53:595–602. 2004.
|
|
5
|
Guliyev VB, Gul M and Yildirim A:
Hippophae rhamnoides L.: chromatographic methods to
determine chemical composition, use in traditional medicine and
pharmacological effects. J Chromatogr B Analyt Technol Biomed Life
Sci. 812:291–307. 2004. View Article : Google Scholar
|
|
6
|
Suryakumar G and Gupta A: Medicinal and
therapeutic potential of sea buckthorn (Hippophae rhamnoides
L.). J Ethnopharmacol. 138:268–278. 2011. View Article : Google Scholar
|
|
7
|
Kim JS, Kwon YS, Sa YJ and Kim MJ:
Isolation and identification of sea buckthorn (Hippophae
rhamnoides) phenolics with antioxidant activity and
alpha-glucosidase inhibitory effect. J Agric Food Chem. 59:138–144.
2011.
|
|
8
|
Saggu S and Kumar R: Effect of
seabuckthorn (Hippophae rhamnoides) leaf aqueous and ethanol
extracts on avoidance learning during stressful endurance
performance of rats: a dose dependent study. Phytother Res.
22:1183–1187. 2008.
|
|
9
|
Padwad Y, Ganju L, Jain M, et al: Effect
of leaf extract of Seabuckthorn on lipopolysaccharide induced
inflammatory response in murine macrophages. Int Immunopharmacol.
6:46–52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maheshwari DT, Yogendra Kumar MS, Verma
SK, Singh VK and Singh SN: Antioxidant and hepatoprotective
activities of phenolic rich fraction of Seabuckthorn (Hippophae
rhamnoides L.) leaves. Food Chem Toxicol. 49:2422–2428. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bao M and Lou Y: Flavonoids from
seabuckthorn protect endothelial cells (EA. hy926) from oxidized
low-density lipoprotein induced injuries via regulation of LOX-1
and eNOS expression. J Cardiovasc Pharmacol. 48:834–841. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurata S, Matsumoto M, Tsuji Y and
Nakajima H: Lipopolysaccharide activates transcription of the heme
oxygenase gene in mouse M1 cells through oxidative activation of
nuclear factor kappa B. Eur J Biochem. 239:566–571. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suntres ZE: Role of antioxidants in
paraquat toxicity. Toxicology. 180:65–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bus JS and Gibson JE: Paraquat: model for
oxidant-initiated toxicity. Environ Health Perspect. 55:37–46.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghio AJ: Disruption of iron homeostasis
and lung disease. Biochim Biophys Acta. 1790:731–739. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mitsopoulos P and Suntres ZE: Cytotoxicity
and gene array analysis of alveolar epithelial A549 cells exposed
to paraquat. Chem Biol Interact. 188:427–436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zerin T, Kim YS, Hong SY and Song HY:
Quercetin reduces oxidative damage induced by paraquat via
modulating expression of antioxidant genes in A549 cells. J Appl
Toxicol. Sep 20–2012.(Epub ahead of print).
|
|
18
|
Geetha S, Singh V, Ram MS, Ilavazhagan G,
Banerjee PK and Sawhney RC: Immunomodulatory effects of
seabuckthorn (Hippophae rhamnoides L.) against chromium (VI)
induced immunosuppression. Mol Cell Biochem. 278:101–109. 2005.
|
|
19
|
Geetha S, Sai Ram M, Singh V, Ilavazhagan
G and Sawhney RC: Anti-oxidant and immunomodulatory properties of
seabuckthorn (Hippophae rhamnoides) - an in vitro study. J
Ethnopharmacol. 79:373–378. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Narayanan S, Ruma D, Gitika B, et al:
Antioxidant activities of seabuckthorn (Hippophae
rhamnoides) during hypoxia induced oxidative stress in glial
cells. Mol Cell Biochem. 278:9–14. 2005.
|
|
21
|
Upadhyay NK, Kumar R, Mandotra SK, et al:
Safety and healing efficacy of Sea buckthorn (Hippophae
rhamnoides L.) seed oil on burn wounds in rats. Food Chem
Toxicol. 47:1146–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ganju L, Padwad Y, Singh R, et al:
Anti-inflammatory activity of seabuckthorn (Hippophae
rhamnoides) leaves. Int Immunopharmacol. 5:1675–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen CH, Liu TZ, Kuo TC, et al: Casuarinin
protects cultured MDCK cells from hydrogen peroxide-induced
oxidative stress and DNA oxidative damage. Planta Med.
70:1022–1026. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kwon DJ, Bae YS, Ju SM, Goh AR, Choi SY
and Park J: Casuarinin suppresses TNF-alpha-induced ICAM-1
expression via blockade of NF-κB activation in HaCaT cells. Biochem
Biophys Res Commun. 409:780–785. 2011.PubMed/NCBI
|
|
25
|
Zerin T, Kim YS, Hong SY and Song HY:
Protective effect of methylprednisolone on paraquat-induced A549
cell cytotoxicity via induction of efflux transporter,
P-glycoprotein expression. Toxicol Lett. 208:101–107. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Surh YJ, Kundu JK and Na HK: Nrf2 as a
master redox switch in turning on the cellular signaling involved
in the induction of cytoprotective genes by some chemopreventive
phytochemicals. Planta Med. 74:1526–1539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jaiswal AK: Nrf2 signaling in coordinated
activation of antioxidant gene expression. Free Radic Biol Med.
36:1199–1207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kode A, Rajendrasozhan S, Caito S, Yang
SR, Megson IL and Rahman I: Resveratrol induces glutathione
synthesis by activation of Nrf2 and protects against cigarette
smoke-mediated oxidative stress in human lung epithelial cells. Am
J Physiol Lung Cell Mol Physiol. 294:L478–L488. 2008. View Article : Google Scholar
|
|
29
|
Cho HY, Jedlicka AE, Reddy SP, et al: Role
of NRF2 in protection against hyperoxic lung injury in mice. Am J
Respir Cell Mol Biol. 26:175–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He X, Lin GX, Chen MG, Zhang JX and Ma Q:
Protection against chromium (VI)-induced oxidative stress and
apoptosis by Nrf2. Recruiting Nrf2 into the nucleus and disrupting
the nuclear Nrf2/Keap1 association. Toxicol Sci. 98:298–309. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen J and Shaikh ZA: Activation of Nrf2
by cadmium and its role in protection against cadmium-induced
apoptosis in rat kidney cells. Toxicol Appl Pharmacol. 241:81–89.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singh M, Murthy V and Ramassamy C:
Standardized extracts of Bacopa monniera protect against
MPP+- and paraquat-induced toxicity by modulating
mitochondrial activities, proteasomal functions, and redox
pathways. Toxicol Sci. 125:219–232. 2012.
|
|
33
|
Liu XP, Goldring CE, Copple IM, et al:
Extract of Ginkgo biloba induces phase 2 genes through
Keap1-Nrf2-ARE signaling pathway. Life Sci. 80:1586–1591. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Camhi SL, Alam J, Wiegand GW, Chin BY and
Choi AM: Transcriptional activation of the HO-1 gene by
lipopolysaccharide is mediated by 5′ distal enhancers: role of
reactive oxygen intermediates and AP-1. Am J Respir Cell Mol Biol.
18:226–234. 1998.
|
|
35
|
Fujii J, Ito JI, Zhang X and Kurahashi T:
Unveiling the roles of the glutathione redox system in vivo by
analyzing genetically modified mice. J Clin Biochem Nutr. 49:70–78.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Singh A, Boldin-Adamsky S, Thimmulappa RK,
et al: RNAi-mediated silencing of nuclear factor
erythroid-2-related factor 2 gene expression in non-small cell lung
cancer inhibits tumor growth and increases efficacy of
chemotherapy. Cancer Res. 68:7975–7984. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rangasamy T, Cho CY, Thimmulappa RK, et
al: Genetic ablation of Nrf2 enhances susceptibility to cigarette
smoke-induced emphysema in mice. J Clin Invest. 114:1248–1259.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tomita M and Okuyama T: Effect of paraquat
on the malondialdehyde level in rat liver microsomes (in vitro).
Arch Toxicol. 68:187–192. 1994. View Article : Google Scholar : PubMed/NCBI
|