Introduction
Glutamate, the major excitatory amino acid
neurotransmitter in the central nervous system, is involved in the
pathological process of excitotoxicity in which neuronal cells are
damaged and killed by excess glutamate present in the brain fluid
following various acute and chronic neurodegenerative disorders,
including stroke, traumatic brain injury, amyotrophic lateral
sclerosis, brain tumors, and human immunodeficiency virus (HIV)
dementia. Accordingly, strategies to interfere with glutamate
activity, including the inhibition of glutamate synthesis, blocking
its release from presynaptic terminals, antagonizing its
interactions with postsynaptic receptors and accelerating its
reuptake from the synaptic cleft, have predominated recent research
in the area of neuroprotection. However, all of these strategies
have proved to be ineffective (1,2).
The failures of the aforementioned strategies
prompted the proposal of an alternative approach to the
neutralization of the deleterious effects of glutamate, which is to
cause an increased pumping of brain cerebrospinal fluid (CSF)
glutamate into the blood in order to accelerate glutamate efflux
from the brain into the blood across the blood-brain-barrier.
Several examples are available, which show that the decrease of
amino acid levels in the blood reduces their concentration in the
CSF in parallel. The administration of asparaginase to the blood
decreases asparagine levels in plasma and in the CSF (3). To achieve a similar objective for
glutamate, it was hypothesized that it may be feasible to boost the
brain-to-blood efflux of glutamate by decreasing its levels in the
blood.
In previous studies (4,5), we
have demonstrated that such a decrease occurs in vitro and
in vivo, upon activation of the blood resident enzymes
glutamate-pyruvate transaminase (GPT) and glutamate-oxaloacetate
transaminase (GOT), which transform glutamate into α-ketoglutarate
in the presence of the respective glutamate cosubstrates, pyruvate
(Pyr) and oxaloacetate (Oxa). The activation of GPT by Pyr and GOT
by Oxa causes a decrease in the blood levels of glutamate. This
decrease was observed to accelerate the efflux of glutamate from
the brain to the blood by increasing the driving force for the
brain-to-blood glutamate efflux, and thereby causing a decrease in
glutamate levels in the CSF. This strategy was assessed in animal
models of closed head injury, stroke, brain glioma and amyotrophic
lateral sclerosis, in which it demonstrated highly significant
neuroprotective effects (4).
As the ability of Pyr/Oxa to decrease blood
glutamate is limited, with the maximum reduction being 50%, the
present study investigated the effects of certain GPT- and
GOT-related compounds, in regard to their blood glutamate
scavenging ability, in order to achieve optimal conditions for
further decreasing the blood levels of glutamate.
Materials and methods
Chemicals
All chemicals were purchased from Sigma-Aldrich
(Shanghai, China). The procedures of the animal experiments
performed in this study were approved by the Animal Care Committee
of Ningxia Medical University (Ningxia, China).
In vitro blood glutamate scavenging
Blood was collected retroorbitally from specific
pathogen-free Sprague Dawley (SD) rats weighing 200–250 g, which
were obtained from the Animal Center of Ningxia Medical University
(Ningxia, China). The animals were incubated at 37°C in the
presence of injected Pyr, Oxa or other chemicals at different
concentrations. Blood aliquots were then taken at different
time-points in order to measure the glutamate levels. In order to
study the depletion of glutamate from the blood cell pool, the
blood was centrifuged at 1,300 × g for 7 min at 4°C and the cell
pellet was resuspended in Ringer-HEPES buffer containing 2.75 mM
glucose, 5 mM HEPES, 0.15 M NaCl, 2 mM CaCl2, 0.2 mM
MgCl2·6H2O, 5 mM KCl and 6 mM
NaHCO3 (pH 7.4). It was then washed three times by
centrifugation and resuspension. Glutamate depletion was achieved
by supplementing the blood cell pool, maintained at 37°C, with 1 mM
Pyr/Oxa or other chemicals added every 15 min, and the glutamate
value was measured at different time-points.
Glutamate analysis
Blood samples were deproteinated by adding an equal
volume of ice-cold 1 M perchloric acid (PCA) and then centrifuging
the samples at 16,000 × g for 10 min at 4°C. The pellet was
discarded and the supernatant collected, adjusted to pH 7.2 with 2
M K2CO3, and, when required, stored at −20°C
for later analysis. The glutamate concentration was measured in the
supernatant using the fluorometric method by Graham and Aprison
(6). A 20 μl aliquot of the PCA
supernatant was added to 480 μl reaction buffer containing 15 units
of glutamate dehydrogenase in 0.2 mM nicotinamide adenine
dinucleotide (NAD), 0.3 M glycine and 0.25 M hydrazine hydrate
adjusted to pH 8.6 with 1 N H2SO4. Following
incubation for 30–45 min at room temperature, fluorescence was
measured at 460 nm with excitation at 350 nm. A glutamate standard
curve was established with concentrations ranging between 0 and 6
μM. All determinations were performed at least in duplicate. The
results are expressed as the mean ± standard deviation.
Intravenous injections
In accordance with a previously described method
(4), female SD rats (250–300 g)
were anesthesized by intraperitoneal injection of urethane (0.125
g/0.2 ml per 100 g body weight). Catheterization of the tail vein
(for drug injections) and of the femoral vein (for blood aliquot
withdrawals) were performed using PE10 polyethylene tubing linked
to PE50 polyethylene tubing (Smiths Medical, Barking, UK). All
catheters were secured with 5-0 silk thread (Smiths Medical) and
flushed with heparin (3–5 μl of 182 U/ml). The body temperature was
maintained using a lamp and the rectal temperature was monitored.
Intravenous injections of Pyr/Oxa and lipoamide diluted in
phosphate-buffered saline (PBS) were performed at a rate of 0.05
ml/min for 30 min with a Pharmacia pump P-1 (Pharmacia, Uppsala,
Sweden). During and following the injections, aliquots of 150 μl
blood were removed from the femoral vein every 15 min.
Intracerebroventricular injections
A steel cannula made from a 27G needle was implanted
into the right lateral ventricle of the rat using the following
stereotactic coordinates: 0.8 mm posterior to the bregma; 1.4 mm
lateral from the midline; 4 mm below the skull surface and 3.5 mm
from the dura. A total volume of 11 μl [3H]glutamate
solution in PBS was injected into the lateral ventricle in ~3 min
through the implanted cannula using a Hamilton syringe (25 μl)
connected to a PE20 tube filled with the solution. For
radioactivity determination, 50-μl blood samples, collected from
the femoral vein, were diluted in 500 μl H2O and added
to a 16-ml scintillator (PerkinElmer Lifesciences, Waltham, MA,
USA). The measured counts per minute (cpm) values were corrected
for quenching as determined by comparing the measured cpm of a set
volume of [3H]glutamate added to water or to diluted
blood.
Statistical analysis
Results are presented as the mean ± standard error
of the mean. Data were analyzed by Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Scavenging blood glutamate with Pyr/Oxa
in vitro
The optimal concentration and time-point for
minimizing blood glutamate levels with Pyr/Oxa were evaluated.
Fig. 1 illustrates the changes in
blood glutamate levels upon addition of various concentrations of
the mixture of Pyr/Oxa, at t = 0, 20, 30, 40, and 60 min. The
results show that the blood glutamate levels were reduced by ≤50%
by basal levels of the blood resident transaminases GPT and GOT
with addition of a mixture of 1 mM Pyr/Oxa for 60 min. Pyr/Oxa act
in synergy to decrease glutamate levels but their effects are
limited by the build-up of α-ketoglutarate, which facilitates the
back reaction of glutamate formation.
Impact of cofactors on glutamate
depletion
Blood contains resident enzymes, such as GPT
(substrate, Pyr), GOT (substrate, Oxa) and glutamate dehydrogenase
(substrate, GDH). In the presence of their substrates, glutamate is
transformed into α-ketoglutarate by these transaminases (Fig. 2). The products and cofactors of
these reactions may affect the efficiency of the glutamate
conversion.
In the presence of Pyr, blood glutamate is converted
to α-ketoglutarate by GPT, and Pyr to alanine (Fig. 2). However, this process is
reversible as the enzyme operates equally well in both directions.
As shown in Fig. 3, the addition
of alanine diminished the ability of Pyr to decrease the glutamate
concentration. As the concentration of alanine was increased, the
glutamate conversion into α-ketoglutarate was reduced.
In regard to the fact that glutamate is converted
into α-ketoglutarate and that the latter is able to drive the GPT-
and GOT-catalyzed back reactions, thereby limiting the conversion
of glutamate, the present study investigated whether
α-ketoglutarate, the common coproduct of GPT and GOT, was able to
serve as a substrate of α-ketoglutarate dehydrogenase, which
converts α-ketoglutarate into succinyl coenzyme A (CoA) in the
presence of CoA and NAD (Fig. 2).
Therefore, the consequences of the addition of CoA and NAD on
glutamate conversion were assessed. In addition, the effects of the
addition of pyridoxal phosphate, which is the cofactor of GPT/GOT,
and of cocarboxylase and lipoamide, which are cofactors of
α-ketoglutarate dehydrogenase (Fig.
2), were examined. As shown in Fig. 4, the extent of glutamate scavenging
in the blood increases with increasing concentration of Pyr/Oxa.
Pyridoxal phosphate exerted a marginal effect, while cocarboxylase
decreased the glutamate conversion. However, lipoamide increased
the conversion from a value of 55% in the control (1 mM Pyr/Oxa) to
83%.
Since, as observed in Fig. 4, lipoamide appeared to contribute
to the scavenging of blood glutamate, the dose-efficiency
correlation was investigated by applying various concentrations of
lipoamide to blood. Fig. 5 shows
that a maximal extent of glutamate conversion was obtained with 100
μM lipoamide.
As blood contains, in addition to GPT and GOT, other
enzymes, such as GDH, the possible contribution of GDH to the
scavenging of glutamate was investigated. GDH is a multimeric
enzyme that uses NAD or nicotinamide adenine dinucleotide phosphate
(NADP) as cofactors to transform glutamate into α-ketoglutarate and
ammonia. It is allosterically activated by leucine and ADP
(Fig. 2). Therefore, the glutamate
scavenging effects of NAD, ADP and leucine (all at 1 mM) were
assessed. Fig. 6 illustrates the
effects of the repeated addition of 1 mM NAD to blood on glutamate
levels in the cell and plasma compartments: While a decrease in
glutamate levels was found to occur in the blood cells, an increase
in glutamate levels occured in the plasma. These results may be
interpreted as NAD possibly activating GDH in the cellular
compartment, with the α-ketoglutarate produced being released from
the cells and converted back into glutamate via the GPT or GOT
present in the plasma. In all instances of blood being incubated
with NAD, leucine or ADP either separately or in combination,
increases in plasma glutamate levels occurred (Fig. 7). However, systemic decreases in
the cellular compartment were observed when NAD was present alone
or in combination with leucine and ADP, while the latter did not
significantly affect glutamate levels alone (data not shown). When
blood was incubated with a mixture of NAD, Pyr and Oxa, glutamate
levels decreased to a marginally greater extent than with Pyr or
Oxa alone (Fig. 8).
Scavenging of blood glutamate by Pyr/Oxa
and lipoamide in vivo
Previously, we reported that upon continuous
intravenous administration of Pyr/Oxa, blood, as well as
CSF/interstitial fluid glutamate levels were reduced to ≤50%
(4). Since the present in
vitro study demonstrated that by administration of lipoamide
together with Pyr/Oxa, it was possible to further reduce blood
glutamate levels by 83% compared with 50% for Pyr/Oxa alone, an
in vivo study was performed to examine whether Pyr/Oxa
together with lipoamide was able to further reduce blood glutamate
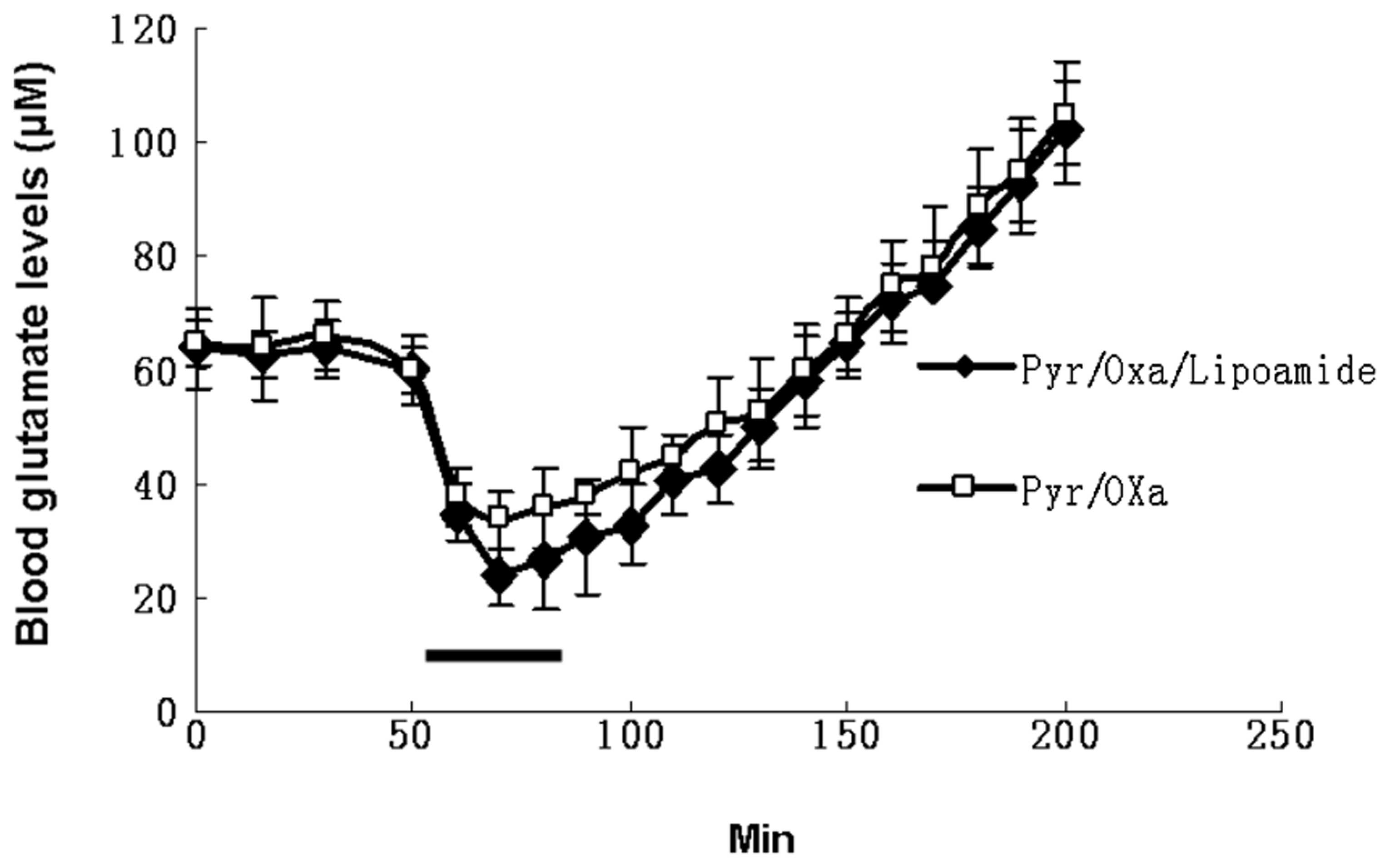
levels compared with the effect of Pyr/Oxa alone. Fig. 9 illustrates the effect of Pyr/Oxa
alone or together with lipoamide administered continuously through
an intravenous catheter at a rate of 50 μmol/min for Pyr/Oxa and
100 μmol/min for lipoamide for a duration of 30 min. A marked
decrease (55% for Pyr/Oxa and 70% for Pyr/Oxa/lipoamide) in blood
glutamate occurred after 15 min. This is consistent with the in
vitro results. However, as soon as the administration of
Pyr/Oxa and lipoamide was ceased, the blood glutamate levels
increased. When the levels of glutamate were monitored for ~200 min
following the completion of the injection of Pyr/Oxa and lipoamide,
glutamate levels clearly exceeded basal blood levels, suggesting
that the increase in glutamate levels is not only due to the back
reactions as a result of the build-up of the enzymatic products,
α-ketoglutarate, alanine and aspartate, but possibly to additional
compensatory processes causing various organs to release their
glutamate content into the plasma to adjust to the novel
conditions.
Impact of blood glutamate scavenging on
the brain-to-blood glutamate efflux
To monitor the fate of excess glutamate in the CSF,
a bolus injection of 10 μCi [3H]Glu (230 pmol
glutamate/10 μl) was administered to the lateral ventricles of rats
and the appearance of radioactivity in the blood was monitored in
relation to time. Fig. 10 shows
the changes in radioactivity levels in the blood upon
intracerebroventricular injection of 10 μCi
[3H]glutamate followed by blood glutamate scavenging by
Pyr/Oxa and lipoamide. As soon as a constant level of radioactivity
was reached in the blood, blood glutamate levels were transiently
decreased by the intravenous administration of Pyr/Oxa and
lipoamide. The changes in blood radioactivity levels originating
from the brain mirrored those of blood glutamate. While the latter
decreased by ~50% during the administration of Pyr/Oxa with
lipoamide and then increased, the blood radioactivity increased by
~40% and then decreased.
Discussion
The neuroprotective properties of pyruvate have been
thoroughly studied (7–12); however, they have not been used in
the clinic. Our previous studies revealed that it is possible to
regulate blood glutamate levels by activation of the blood resident
enzymes GPT and GOT with their respective substrates Pyr and Oxa
(4,5). This blood glutamate decrease was
observed to accelerate the efflux of glutamate from the brain to
the blood, thereby causing a decrease in glutamate levels in the
CSF by increasing the driving force of the brain-to-blood glutamate
efflux. Due to the ability of Pyr/Oxa to decrease blood and CSF
glutamate levels, these natural GPT and GOT cosubstrates are
potentially suitable for therapeutic applications in the context of
the large number of neurodegenerative conditions, in which excess
CSF glutamate has been indicated to exert a crucial excitotoxicity.
In this study, the optimal conditions for Pyr/Oxa to reduce blood
glutamate levels were investigated, and numerous cofactors of these
reactions for glutamate degeneration were assessed in an attempt to
further reduce glutamate levels in blood concomitant with the
decrease in glutamate levels in CSF.
The results show that the blood glutamate levels
were reduced by ≤50% with 1 mM Pyr/Oxa administered over 60 min.
The GTP-catalyzed conversion reaction of glutamate and pyruvate to
α-ketoglutarate and alanine is reversible. With the accumulation of
α-ketoglutarate and alanine, the reaction is inhibited. Consistent
with this, the results of the present study demonstrate that the
addition of alanine inhibits the ability of pyruvate to reduce
glutamate levels. α-ketoglutarate is the most common product of GPT
and GOT. The reduction or removal of α-ketoglutarate shifts the
reaction towards the conversion of glutamate. α-ketoglutarate
dehydrogenase is another resident enzyme in the blood, which
converts α-ketoglutarate into succinyl CoA in the presence of CoA
and NAD. It was shown that CoA and NAD have no marked effects on
the glutamate conversion by Pyr/Oxa, and cocarboxylase, a cofactor
of α-ketoglutarate dehydrogenase, decreases glutamate conversion.
However, lipoamide, the other cofactor of α-ketoglutarate
dehydrogenase, exerts a significant effect on blood glutamate
levels and decreases them by >80% compared with 50% for Pyr/Oxa
alone. In the in vivo experiments, blood glutamate levels
were reduced to 30% of the basal level and the efflux of glutamate
from the brain to the blood was enhanced upon intravenous
administration of Pyr/Oxa and lipoamide.
Lipoic acid is an essential cofactor for numerous
enzyme complexes. One of the most visible roles of lipoic acid is
as a cofactor in aerobic metabolism, specifically in the pyruvate
dehydrogenase complex. Lipoamide is the functional form of lipoic
acid in which the carboxyl group is attached to protein by an amide
linkage to a lysine amino group. Lipoate was initially termed the
pyruvate oxidation factor (POF) by Irwin C. Gunsalus (13). The structure was determined by
Gunsalus et al, and the synthetic compound designated as
α-lipoic acid proved to be the correct molecule (13). Lipoic acid has a number of uses. It
has been consumed as a dietary supplement since the early 1990s and
has been successfully used for the treatment of chronic liver
disease (viral hepatitis, autoimmune hepatitis) (14). A chronic toxicity/carcinogenicity
study in rats revealed that racemic lipoic acid was
non-carcinogenic and there was no evidence of target organ toxicity
(15). In addition, due to the
ability of lipoic acid to modify gene expression by stabilizing the
transcription factor nuclear factor-κB, Berkson (16) used it for the treatment of various
cancers for which no effective treatments existed. Lipoic acid was
also suggested to be an effective antioxidant when its ability of
preventing the symptoms of vitamin C and vitamin E deficiency was
revealed. Lipoic acid has been shown to have an important
neuroprotective role in numerous brain injury conditions (17–25).
The present study shows that that lipoamide is able to further
decrease blood glutamate levels together with Pyr/Oxa in
vivo and in vitro. The results provide further evidence
for the neuroprotective role of lipoic acid.
The validity of the neuroprotective strategy of
using Pyr/Oxa to reduce blood and CSF glutamate levels has been
tested by Dr V.I. Teichberg in several animal models of
neurodegenerative conditions, including head trauma, stroke, glioma
and exposure to neuronal agents (26). These studies obtained promising
results, which established the proof of concept that blood
glutamate scavenging provides highly effective neuroprotection
against acute and chronic neurodegenerative conditions. Lipoamide
appears to enhance the effects of Pyr/Oxa; however, the validity of
this finding requires further investigation.
The present study further demonstrated that blood
glutamate scavenging enhances glutamate efflux from brain to blood
and is therefore likely to provide highly effective neuroprotection
against acute and chronic neurodegenerative conditions.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 31060140, 81060034,
81060126, 81060112 and 31260243). The Project Sponsored by the
Scientific Research Foundation for the Returned Overseas Chinese
Scholars, State Education Ministry and the Program for New Century
Excellent Talents in University (NCET), State Education Ministry
for Dr Yin Wang
References
|
1
|
Danbolt NC: Glutamate uptake. Prog
Neurobiol. 65:1–105. 2001. View Article : Google Scholar
|
|
2
|
Basuroy S, Leffler CW and Parfenova H:
CORM-A1 prevents blood-brain barrier dysfunction caused by
ionotropic glutamate receptor-mediated endothelial oxidative stress
and apoptosis. Am J Physiol Cell Physiol. 304:C1105–C1115. 2013.
View Article : Google Scholar
|
|
3
|
Hosoya K, Sugawara M, Asaba H and Terasaki
T: Blood-brain barrier produces significant efflux of L-aspartic
acid but not D-aspartic acid: in vivo evidence using the brain
efflux index method. J Neurochem. 73:1206–1211. 1999. View Article : Google Scholar
|
|
4
|
Gottlieb M, Wang Y and Teichberg VI:
Blood-mediated scavenging of cerebrospinal fluid glutamate. J
Neurochem. 87:119–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Gottlieb M and Teichberg VI: An
evaluation of erythrocytes as plasma glutamate scavengers for
enhanced brain-to-blood glutamate efflux. Neurochem Res.
29:755–760. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Graham LT Jr and Aprison MH: Fluorometric
determination of aspartate, glutamate, and gamma-aminobutyrate in
nerve tissue using enzymic methods. Anal Biochem. 15:487–497. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Izumi Y, Benz AM, Zorumski CF and Olney
JW: Effects of lactate and pyruvate on glucose deprivation in rat
hippocampal slices. Neuroreport. 5:617–620. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsumoto K, Yamada K, Kohmura E,
Kinoshita A and Hayakawa T: Role of pyruvate in ischaemia-like
conditions on cultured neurons. Neurol Res. 16:460–464.
1994.PubMed/NCBI
|
|
9
|
Ruiz F, Alvarez G, Pereira R, Hernández M,
Villalba M, Cruz F, Cerdán S, Bogónez E and Satrústegui J:
Protection by pyruvate and malate against glutamate-mediated
neurotoxicity. Neuroreport. 9:1277–1282. 1998.PubMed/NCBI
|
|
10
|
Maus M, Marin P, Israël M, Glowinski J and
Prémont J: Pyruvate and lactate protect striatal neurons against
N-methyl-D-aspartate-induced neurotoxicity. Eur J Neurosci.
11:3215–3224. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mongan PD, Fontana JL, Chen R and Bünger
R: Intravenous pyruvate prolongs survival during hemorrhagic shock
in swine. Am J Physiol. 277:H2253–H2263. 1999.PubMed/NCBI
|
|
12
|
Lee JY, Kim YH and KohJ Y: Protection by
pyruvate against transient forebrain ischemia in rats. J Neurosci.
21:RC1712001.PubMed/NCBI
|
|
13
|
Reed LJ, DeBusk BG, Gunsalus IC and
Hornberger CS Jr: Crystalline alpha-lipoic acid; a catalytic agent
associated with pyruvate dehydrogenase. Science. 114:93–94. 1951.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berkson BM: Thioctic acid in the treatment
of poisoning with alpha amanitin. Amanita Toxins and Poisonings.
International Amanita Symposium; Heidelberg. Nov. 1–3, 1978;
Faulstich H, Kommerell B and Wieland T: Verlag Gerhard Witzstrock;
Baden-Baden, Koln, New York: pp. 2031980
|
|
15
|
Berkson B: Thioctic acid in treatment of
hepatotoxic mushroom (Phalloides) poisoning. N Engl J Med.
300:3711979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cremer DR, Rabeler R, Roberts A and Lynch
B: Long-term safety of α-lipoic acid (ALA) consumption: A 2-year
study. Regul Toxicol Pharm. 46:193–201. 2006.
|
|
17
|
Mainini G, Rotondi M, Di Nola K, Pezzella
MT, Iervolino SA, Seguino E, D’Eufemia D, Iannicelli I and Torella
M: Oral supplementation with antioxidant agents containing alpha
lipoic acid: effects on postmenopausal bone mass. Clin Exp Obstet
Gynecol. 39:489–493. 2012.PubMed/NCBI
|
|
18
|
Freitas RM: The evaluation of effects of
lipoic acid on the lipid peroxidation, nitrite formation and
antioxidant enzymes in the hippocampus of rats after
pilocarpine-induced seizures. Neurosci Lett. 455:140–144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suchy J, Chan A and Shea TB: Dietary
supplementation with a combination of alpha-lipoic acid,
acetyl-L-carnitine, glycerophosphocoline, docosahexaenoic acid, and
phosphatidylserine reduces oxidative damage to murine brain and
improves cognitive performance. Nutr Res. 29:70–74. 2009.
View Article : Google Scholar
|
|
20
|
Jia Z, Zhu H, Vitto MJ, Misra BR, Li Y and
Misra HP: Alpha-lipoic acid potently inhibits
peroxynitrite-mediated DNA strand breakage and hydroxyl radical
formation: implications for the neuroprotective effects of
alpha-lipoic acid. Mol Cell Biochem. 323:131–138. 2009. View Article : Google Scholar
|
|
21
|
Karunakaran S, Diwakar L, Saeed U, Agarwal
V, Ramakrishnan S, Iyengar S and Ravindranath V: Activation of
apoptosis signal regulating kinase 1 (ASK1) and translocation of
death-associated protein, Daxx, in substantia nigra pars compacta
in a mouse model of Parkinson’s disease: protection by alpha-lipoic
acid. FASEB J. 21:2226–2236. 2007.PubMed/NCBI
|
|
22
|
Zhang L, Xing GQ, Barker JL, Chang Y,
Maric D, Ma W, Li BS and Rubinow DR: Alpha-lipoic acid protects rat
cortical neurons against cell death induced by amyloid and hydrogen
peroxide through the Akt signalling pathway. Neurosci Lett.
312:125–128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim DC, Jun DW, Jang EC, Kim SH, Kim EK,
Lee SP, Lee KN, Lee HL, Lee OY, Yoon BC and Choi HS: Lipoic acid
prevents the changes of intracellular lipid partitioning by free
fatty acid. Gut Liver. 7:221–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Connell BJ, Saleh M, Khan BV and Saleh TM:
Lipoic acid protects against reperfusion injury in the early stages
of cerebral ischemia. Brain Res. 1375:128–136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Emmez H, Yildirim Z, Kale A, Tönge M,
Durdağ E, Börcek AO, Uçankuş LN, Doğulu F, Kiliç N and Baykaner MK:
Anti-apoptotic and neuroprotective effects of α-lipoic acid on
spinal cord ischemia-reperfusion injury in rabbits. Acta Neurochir
(Wien). 152:1591–1601. 2010.
|
|
26
|
Zlotnik A, Gurevich B, Cherniavsky E,
Tkachov S, Matuzani-Ruban A, Leon A, Shapira Y and Teichberg VI:
contribution of the blood glutamate scavenging activity of pyruvate
to its neuroprotective properties in a rat model of closed head
injury. Neurochem Res. 33:1044–1050. 2008. View Article : Google Scholar : PubMed/NCBI
|