Introduction
Magnetic nanoparticles (MNPs) are currently under
investigation for their high potential for in vitro and
in vivo diagnostic applications, such as in cellular therapy
(cell labeling, targeting and as a tool for cell-biology research
to separate and purify cell populations), tissue repair, drug
delivery, magnetic resonance imaging (MRI), hyperthermia and
magnetofection (1).
Superparamagnetic particles are suitable for all these
applications, since they do not retain magnetism after removal of
the magnetic field.
In the last few decades, cancer has become one of
the major human diseases that ultimately result in death. Accurate,
sensitive rapid and facile diagnostic methods for
collection/isolation of cancer cells are of critical importance for
the investigation, prevention and treatment of cancer. A number of
methods (2) are used for isolation
of cells, including high-speed fluorescence-activated cell sorting,
dielectrophoresis, parallel-plate flow chambers, immunoadsorption
columns and immunomagnetic selection. Apart from methods of
chemical separation of cells, mechanical methods, such as
size-based separation and differential sedimentation also exist.
Among these methods, MN-based separation of cells has several
advantages in comparison to other techniques. Superparamagnetic
nanoparticles are particularly suitable for cell enrichment, since
they are of the same size scale as cell particles themselves and
provide a larger overall surface area of interaction for the same
bead volume. As an alternative to micrometer magnetic bead-based
selection (3,4), the small size and increased relative
surface area of nanoparticles provide enhanced extraction capacity.
This allows target cells to be directly isolated from crude samples
and is a relatively simple, inexpensive and fast method; to a
certain degree, it may be considered as a sample enrichment step
prior to subsequent analysis.
The magnetism of MNPs commonly comes from magnetic
metallic elements or compounds, which are nevertheless easy to
aggregate. In addition, since the metallic elements or oxides are
reactive in a biological context, they are easily etched in
practice. Therefore, inorganic or organic protective coatings,
including silica, polyelectrolyte, lipid layers and micelles, have
been developed for these particles (5). Among these, polymer wrapping, that
is, encapsulation of already fabricated magnetic nanoparticles into
polymer micro- or nanoparticles, is widely used. The magnetic cores
ensure a strong magnetic response and the polymeric shell provides
favorable functional groups and features that are suitable for
various applications. These particles are stable in aqueous
suspensions and can be easily redispersed after agglomeration in
the presence of a magnetic field. In addition, the structure,
stability and physical properties of nanoscale-size materials can
exhibit a strong dependence on the particle surface. Numerous
polymers are biocompatible and may be used as MNP coating for
biomedical applications. Polyethylenimine (PEI) is a branched
polymer with a high-density amine group,
(CH2CH2NH)n. The ratio of primary
to secondary to tertiary amines is 1:2:1. In each PEI molecule, one
nitrogen atom is protonated per two carbon atoms. Due to the
different pKa values of the primary, secondary and tertiary amino
groups, PEI has the ability to capture protons at different pH
conditions, which is known as the ‘proton sponge’ mechanism. PEI
was developed to condense DNA via the electrostatic interaction
between its positive and negative charge of the phosphate group of
DNA (6). A PEI-modified MALDI
plate was successfully used to concentrate DNA and protein
digestion products (7). Due to its
unique properties, PEI appears to be one of the most appropriate
molecules for the surface modification of MNPs for biomedical
applications.
Cytopathological analysis of sputum is one of the
most promising and effective methods for early diagnosis of lung
cancer. Currently however, there is one main disadvantage of this
method: its high false-positive rate (up to 20–40%). This is
typically caused by the loss of cells during sample preparation,
the poor quality of the smear, complication of cells and the
non-uniform distribution of lung cancer cells in sputum samples.
Therefore, the present study aimed to develop a new method for
enrichment of lung cancer cells from sputum for cytopathological
analysis with increased positive rate, by adopting
superparamagnetic PEI-coated Fe3O4
nanoparticles as convenient vehicles to isolate and enrich lung
cancer cells. A detailed study of the preparation and
characterization of the PEI-coated MNPs is presented below. Our
study demonstrated a highly specific method for the enrichment of
lung cancer cells using a simple approach based on the strong
protonating capacity of PEI (~20% of nitrogen atoms are protonated
under physiological conditions) (8).
Materials and methods
Materials
Chemical reagents were of analytical grade and were
used without further purification. Iron(III) chloride hexahydrate
(FeCl3·6H2O), iron(II) ferrous chloride
(FeCl2·4H2O), aqueous ammonia and an ethyl
alcohol solution of PEI (molecular weight, 20,000) were used as
purchased from Shanghai Baijin Chemical Group Co., Ltd. (Shanghai,
China). De-ionized water was used in all the experiments.
Nanoparticle synthesis
Magnetic particles were prepared by coprecipitation,
by adding aqueous ammonia solution into a mixed solution of 0.25
mol/l ferrous chloride and 0.5 mol/l ferric chloride (molar ratio
1:2) at 70°C for 1 h until a pH 11.0 was achieved. The entire
process was protected by inactive gas. The precipitate was
alternately washed with distilled water and ethyl alcohol three
times, and separated with centrifugation. It was then dried at 50°C
for 16 h in a vacuum-drying chamber. Following this step, the
magnetite and polyethylenimine were left to mix (mass ratio 1:2) at
room temperature for 24 h at pH 9.0. This procedure was performed
under ultrasonic waves, so that the magnetic nanoparticles could
homogenously disperse in the solution. The PEI-coated magnetic
nanoparticles were thoroughly washed alternately with distilled
water and methyl alcohol at least three times, and were then
separated by centrifugation. The modified nanoparticles were then
dried at 60°C for 24 h. Polyethylenimine molecules bound to
particles by electrostatic interaction and the negative charges on
the surface of the particles were converted to positive
charges.
Characterization of the
nanoparticles
The crystalline phases were studied by X-ray
diffractometry (XRD) operating at a scanning rate of 10°/min from
10° to 85° (D/max2550VB3; Rigaku International Corporation, Tokyo,
Japan). Identification of the synthesized iron-oxide was based on
the position of characteristic peaks in the diffractograms using
the Joint Committee on Powder Diffraction Standards (JCPDS)
database. The XRD patterns were evaluated to determine the lattice
spacing (dhkl values) with the Bragg equation and the
Miller (hkl) indices corresponding to the crystalline phases in the
samples. The particle size and morphology of the naked and modified
particles were examined with a transmission electron microscope
(TEM; FTIR, HYPERION2000, Bruker, Ettlingen, Germany). The Fourier
transform infrared (FTIR) spectrum (500–4,000 cm−1) from
the KBr pellet containing the PEI-coated magnetic nanoparticles was
recorded on a FTIR spectrometer. The samples were also examined by
X-ray photoelectron spectroscopy (XPS; Kratos, Tokyo, Japan). The
magnetic properties were measured with a vibrating sample
magnetometer (VSM; Lakeshore Cryotronics, Westerville, OH, USA) at
room temperature in magnetic fields of up to 20 kOe.
Cell separation and exfoliative
cytopathology
One hundred milligram of PEI-coated
Fe3O4 magnetic nanoparticles were suspended
in 10 ml of 0.01 mol/l PBS (pH 7.2). The concentration of
PEI-coated Fe3O4 magnetic nanoparticles in
the solution was ~3×1013/mol. The solution was then
mixed with 0.5 ml of staphylococcal protein A (SPA) protein
solution. The nanocomposite solution was obtained following uniform
stirring. Sputum (5 ml) was collected by expectorating from five
lung cancer patients in the morning and lysed by 30 ml alkaline
lysate (CytoLyt Solution). All patients provided written informed
consent and the study was approved by the Ethics Committee of
Tongji University, Shanghai, China. The lysed sputum was
mechanically stirred for 15 min and then centrifuged for 5 min. It
was suspended in preservation solution and then 1 ml of the
nanocomposite solution containing the magnetic particles was added.
After stirring, the mixed solution was kept still for 30 min. The
ThinPrep test was used in cytological preparations, with
hematoxylin and eosin staining. The prepared specimens were
observed using a Nikon 90i optical microscope (Nikon Instruments
Inc., Melville, NY, USA) and 600 cells were counted per sample.
Results
Fig. 1 shows the
XRD patterns of the naked and the PEI-coated magnetic
nanoparticles. The characteristic peaks of magnetite were detected
at Miller indices 111, 220, 331, 400, 422, 511 and 440. The
positions of characteristic peaks did not shift but showed limited
broadening, indicating that the nanoparticles have small
crystalline sizes. Using the Debye-Scherrer equation for spherical
particles, this was translated into an average grain size of 10.7
nm.
The size and morphology of the naked and PEI-coated
nanoparticles were investigated by TEM due to their large surface
area and high surface energy (nano effect). Fig. 2 shows the bright-field images and
the corresponding selected area electronic diffraction patterns
(SAED) of naked (Fig. 2A) and
PEI-coated nanoparticles (Fig.
2B). Particles with an approximate spherical shape were
observed. The estimated average diameter of naked nanoparticles was
~10 nm. This result was in accordance with that of X-ray
diffraction analysis. However, the average size for PEI-coated
Fe3O4 nanoparticles in solution was
determined to be ~12.7 nm.
The surface chemical structure of PEI-coated
Fe3O4 MNPs was characterized by FTIR
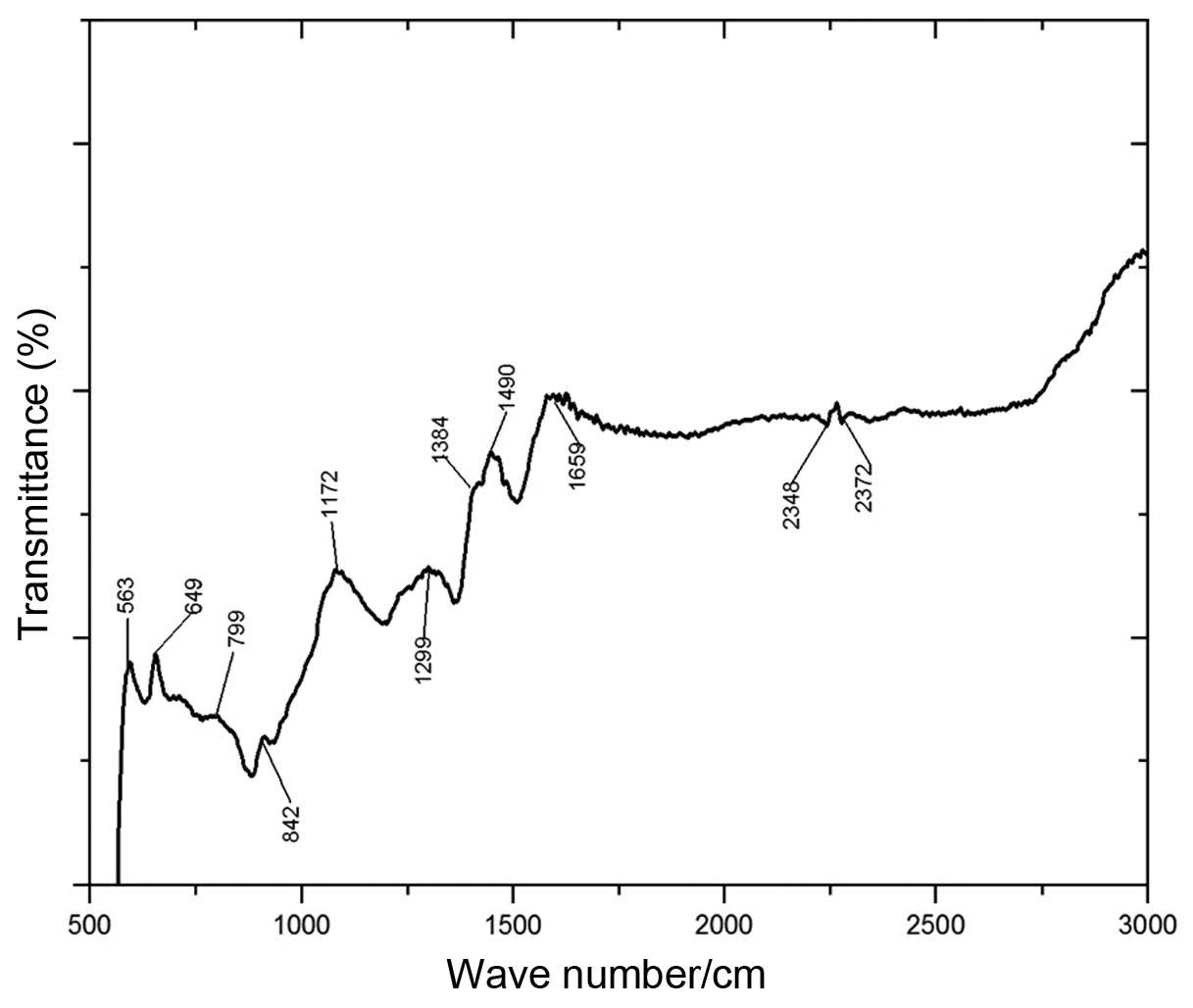
spectroscopy. Fig. 3 shows the
FTIR spectrum for the PEI-coated MNPs from observations in the
infrared spectrometer using the KBr tabletting method. The
absorption peaks at ~563/cm correspond to the characteristic
absorption of Fe-O (9). The
absorption peaks of PEI appear at 649/cm (-NH wagging vibration),
1384–1659/cm (NH2-scissoring vibration and C-H
stretching vibration), 2348 and 2372/cm (C-H2 symmetry
shrinkage). The characteristic peaks corresponding to PEI were
clearly observed at 649, 1560, 2850 and 2372/cm in the FTIR
spectrum. We therefore confirmed that the PEI layer was coated on
the surface of the Fe3O4 magnetic
nanoparticles.
Fig. 4A shows the
XPS spectrum of Fe(2p) for the PEI-coated
Fe3O4 magnetic nanoparticle powder. The
binding energy values for the main peak maxima 2p3/2 and 2p1/2 were
estimated at 711.1 and 724.5 eV, respectively. Fig. 4B shows the typical XPS spectrum of
the N1s region. Notably, one peak was detected for N1s, at 399.8
eV.
The magnetization hysteresis loops of PEI-coated and
naked Fe3O4 nanoparticles were measured by
VSM at room temperature (Fig. 5).
From Fig. 5, it is evident that
the saturation magnetization values for naked and PEI-coated
magnetic nanoparticles were ~58.3 and 53.4 emu/g, respectively. The
variation of magnetization M, on an applied field H, showed no
hysteresis in the two samples; that is, both the remanence and
coercivity of the samples were zero.
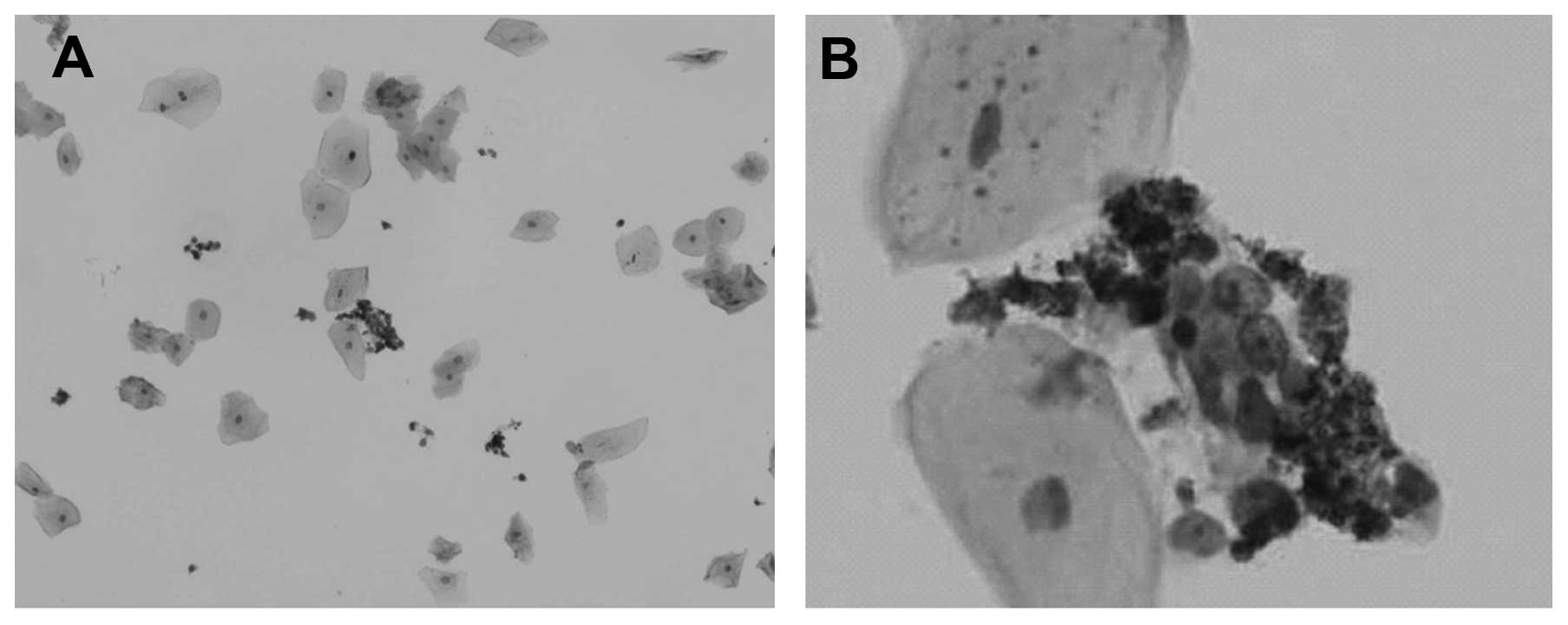
Fig. 6 shows images
from optical microscopy of magnetic nanocomposites adsorbed on the
surface of lung cancer cells. As seen in Fig. 6A, after staining, the
oval/round-shaped cells were dispersed and showed strong light
refraction. The core located at the center of cells and the
nucleolus can be clearly observed. Fig. 6B shows high numbers of magnetic
nanoparticles attached on the surface of the cells, which rendered
the enrichment and the separation of the cells, either by magnetic
or by gravity separation, easy and convenient. Exfoliative
cytopathology analysis showed that the percentage of positive cells
increased from 6.3% (38/600) in the untreated sputum samples to
38.5% (231/600) in the sputum samples treated with PEI-coated
Fe3O4 magnetic nanocomposites.
Discussion
The diffraction peaks of PEI-coated magnetic
nanoparticles agree well with standard Fe3O4
powder diffraction data (JCPDS card 72-2303), and indicate that
there is no crystallographic change after coating, while no
additional peaks are detectable except for those corresponding to
magnetites.
The size of the modified nanoparticles as determined
from TEM images is expected to reflect their actual size in
solution. In addition to dispersal, the naked nanoparticles also
exhibited aggregated morphology to a certain degree (Fig. 2A) due to their large specific
surface area and the high energy of this surface. By contrast,
after PEI coating, the dispersibility of the cells showed an
obvious improvement (Fig. 2B).
Since polyethylenimine is a linear molecule, the ζ potential of the
MNPs is reduced after functional processing (coating) with PEI,
leading to a steric effect, resulting in disaggregation of the MNPs
(9). Therefore, PEI coating can
reduce the aggregation and improve particle dispersion.
The surface specificity of XPS renders it a useful
analytical technique for the direct characterization of iron oxide
nanoparticles (10,11). The observed position of the
magnetites is consistent with reported iron assignments (12,13).
The appearance of a satellite peak of Fe 2p3/2 in the XPS spectra
is an important feature for discrimination of magnetite from
maghemite (14,15). As seen in Fig. 4, the absence of the satellite peak
further substantiates the formation of magnetite in our
experiments. In addition, the difference in the binding energies of
the main peaks dE, was 13.4 eV, that is, very similar to that
reported for Fe3O4 (13.5 eV) (16). It is notable that the XPS analysis
of N1s detected one peak at 399.8 eV. This has been commonly
interpreted as the formation of nitrogen-coordinated metal
complexes (17,18). Therefore, the peak position of N1s
at 399.8 eV indicates that the nitrogen from the amino groups of
PEI coordinates with the magnetic nanocrystals.
The PEI-coated nanoparticles were uniformly
dispersed in aqueous solution in the absence of a magnetic field. A
suspension of PEI-coated Fe3O4 magnetic
nanoparticles is deep brown. When a magnet was placed under the
glass vial, the particles accumulated on the bottom of the vial
near the magnet within a few minutes. After removal of the external
magnetic field, the aggregates were rapidly redispersed by gentle
stirring. The magnetization hysteresis loops of PEI-coated and
naked Fe3O4 nanoparticles were measured by
VSM at room temperature (Fig. 5).
The magnetic data are described by the Langevin equation (19), which indicates that the magnetic
nanoparticles are single-domain, while the samples exhibit
superparamagnetic behavior at room temperature, as expected by the
nanoscale dimension of the particles [the critical size for
superparamagnetic behavior of magnetite is ~20 nm (20)]. The saturation magnetization values
for naked and PEI-coated magnetic nanoparticles are lower than the
reported saturation magnetization of their bulk counterparts
(magnetite, 92–100 emu/g at 300 K). This result is consistent with
that observed for magnetic iron oxide nanoparticles coated with
poly(methacrylicacid) (20). This
phenomenon has been attributed to the presence of nonmagnetic or
‘dead’ surface layers resulting from the chemical reaction between
the stabilizing surfactant and the ferrite particles (21). Such dead surface layers make the
magnetic diameter of the particles smaller than its physical
diameter. Nanoparticles coated with PEI showed an even lower value
of saturation magnetization (Ms, 53.4 emu/g). Davies et al
(22) suggested that particles
containing sufficient concentrations of functional groups allow
spin pinning of the iron oxide surfaces, which gives rise to a
non-collinear spin structure and is known to produce reduced
magnetic moments for the particles (23,24).
The fabricated PEI-coated
Fe3O4 magnetic nanocomposites were used for
separation and enrichment of lung cancer cells in the present
study. The magnetic nanocomposites can be easily aggregated using
an external magnetic field and can be uniformly dispersed when the
magnetic field is removed, owing to their superparamagnetic
properties. Following PEI coating, the Fe3O4
magnetic nanoparticles were functionalized with amino groups on
their surfaces. This feature renders them attractive to cells with
pathological alterations and thus, a
nanoparticle-PEI-pathologically modified cell composite is formed.
This composite can then be easily disaggregated by applying an
external magnetic field.
In our experiments, the PEI-coated
Fe3O4 magnetic nanoparticles effectively
attracted pathologically modified cells and the magnetic
nanocomposites had a measurable effect on the separation and
enrichment of cancer cells. The exfoliative cytopathology results
indicated that using PEI-coated Fe3O4
magnetic nanocomposites can increase the percentage of positive
cells, indicating that these nanocomposites are very effective in
cell separation and enrichment of lung cancer cells.
In summary, we synthesized the superparamagnetic
Fe3O4 magnetic nanoparticles coated with PEI
by a simple precipitation method. The average grain size of
PEI-coated Fe3O4 magnetic nanoparticles is
~12.7 nm while that of naked nanoparticles is ~10 nm. The PEI layer
was successfully coated on the surface of
Fe3O4 magnetic nanoparticles according to XPS
and FTIR analyses. Magnetic measurement showed that the
nanoparticles are superparamagnetic. The synthesized PEI-coated
Fe3O4 magnetic nanocomposites were used for
separation and enrichment of lung cancer cells. Exfoliative
cytopathology analysis showed that the percentage of positive cells
increased from 6.3% (38/600) in untreated sputum samples to 38.5%
(231/600) in sputum samples treated with PEI-coated
Fe3O4 magnetic nanocomposites. This finding
indicates that PEI-coated Fe3O4 magnetic
nanocomposites can be used to efficiently enrich for lung cancer
cells from sputum for cytopathology analysis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 51071109) and the
Natural Science Foundation of Shanghai (grant no. 13ZR1443700). In
addition, the authors would like to thank Professor Gang Chen from
the Shanghai Pulmonary Hospital for his help in the cell separation
experiments and the cytopathology tests.
References
|
1
|
Babic M, Horák D, Trchová M, et al:
Poly(L-lysine)-modified iron oxide nanoparticles for stem cell
labeling. Bioconjug Chem. 19:740–750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong X, Zheng Y, Huang Y, et al: Synthesis
and characterization of multifunctional poly(glycidyl methacrylate)
microspheres and their use in cell separation. Anal Biochem.
405:207–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Imbeault M, Lodge R, Ouellet M and
Tremblay MJ: Efficient magnetic bead-based separation of
HIV-1-infected cells using an improved reporter virus system
reveals that p53 up-regulation occurs exclusively in the
virus-expressing cell population. Virology. 393:160–167. 2009.
View Article : Google Scholar
|
|
4
|
Medina F, Segundo C, Salcedo I, et al:
Purification of human lamina propria plasma cells by an
immunomagnetic selection method. J Immunol Methods. 285:129–135.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gupta AK and Gupta M: Synthesis and
surface engineering of iron oxide nanoparticles for biomedical
applications. Biomaterials. 26:3995–4021. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdallah B, Hassan A, Benoist C, et al: A
powerful nonviral vector for in vivo gene transfer into the adult
mammalian brain: polyethylenimine. Hum Gene Ther. 7:1947–1954.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Y, Bruening ML and Watson JT: Use of
polymer-modified MALDI-MS probes to improve analyses of protein
digests and DNA. Anal Chem. 76:3106–3111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choosakoonkriang S, Lobo BA, Koe GS, et
al: Biophysical characterization of PEI/DNA complexes. J Pharm Sci.
92:1710–1722. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Waldron RD: Infrared spectra of ferrites.
Phys Rev. 99:1727–1735. 1955. View Article : Google Scholar
|
|
10
|
Banerjee I, Khollam YB, Balasubramanian C,
et al: Preparation of γ-Fe2O3 nanoparticles
using DC thermal arc-plasma route, their characterization and
magnetic properties. Scr Mater. 54:1235–1240. 2006.
|
|
11
|
Fujii T, De Groot FMF, Sawatzky GA, et al:
In situ XPS analysis of various iron oxide films grown by
NO2-assisted molecular-beam epitaxy. Phys Rev B.
59:3195–3202. 1999. View Article : Google Scholar
|
|
12
|
Qi HP, Chen QW, Wang MS, et al: Study of
self-assembly of octahedral magnetite under an external magnetic
field. J Phys Chem C. 113:17301–17305. 2009. View Article : Google Scholar
|
|
13
|
Asuha S, Zhou XG and Zhao S: Adsorption of
methyl orange and Cr(VI) on mesoporous TiO2 prepared by
hydrothermal method. J Hazard Mater. 181:204–210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamashita T and Hayes P: Analysis of XPS
spectra of Fe2+ and Fe3+ ions in oxide
materials. Appl Surf Sci. 254:2441–2449. 2008. View Article : Google Scholar
|
|
15
|
Graat PCJ and Somers AJ: Simultaneous
determination of composition and thickness of thin iron-oxide films
from XPS Fe 2p spectra. Appl Surf Sci. 100:36–40. 1996. View Article : Google Scholar
|
|
16
|
Cheng JP, Ma R, Chen X, et al: Effect of
ferric ions on the morphology and size of magnetite nanocrystals
synthesized by ultrasonic irradiation. Cryst Res Technol.
46:723–730. 2011. View Article : Google Scholar
|
|
17
|
Finn P and Jolly WL: Nitrogen 1s binding
energies of transition metal nitrosyls. Inorg Chem. 11:893–895.
1972. View Article : Google Scholar
|
|
18
|
Incorvia MJ and Contarin S: X-ray
photoelectron spectroscopic studies of metal/inhibitor systems:
structure and bonding at the iron/amine interface. J Electrochem
Soc. 136:2493–2498. 1989. View Article : Google Scholar
|
|
19
|
Berkowitz AE, Lahut JA and VanBuren CE:
Properties of magnetic fluid particles. IEEE Trans Magn.
16:184–190. 1980. View Article : Google Scholar
|
|
20
|
Gnanaprakash G, Philip J, Jayakumar T, et
al: Effect of digestion time and alkali addition rate on physical
properties of magnetite nanoparticles. J Phys Chem B.
111:7978–7986. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chantrell RW, Popplewell J and Charles SW:
Measurements of particle size distribution parameters in
ferrofluids. IEEE Trans Magn. 14:975–977. 1978. View Article : Google Scholar
|
|
22
|
Davies KJ, Wells S and Charles SW: The
effect of temperature and oleate adsorption on the growth of
maghemite particles. J Magn Magn Mater. 122:24–28. 1993. View Article : Google Scholar
|
|
23
|
Coey JMD: Noncollinear spin arrangement in
ultrafine ferrimagnetic crystallites. Phys Rev Lett. 27:1140–1142.
1971. View Article : Google Scholar
|
|
24
|
Morup S: Mössbauer spectroscopy studies of
suspensions of Fe3O4 microcrystals. J Magn
Magn Mater. 39:45–47. 1983.
|