Introduction
The elderly population experience various health
issues, particularly non-communicable diseases such as heart
disease, stroke, visual impairment, hearing loss and dementia.
Among these, neurodegenerative diseases account for a significant
percentage. Brain pathology in the form of cerebrovascular and
neurodegenerative disease is known to be a leading cause of
mortality worldwide, with an incidence of ~2/1,000 and a total
mortality rate of 8% (1–3). Examples of neurodegenerative diseases
include Alzheimer’s disease, which is the largest cause of
dementia, Parkinson’s disease, Huntington’s disease and amyotrophic
lateral sclerosis. Currently, there has been little to no success
in discovering cures and breakthroughs for neurodegenerative
diseases. This, along with the ever-growing elderly population, has
become a pressing global health concern.
A common characteristic of these diseases is their
resemblance in symptoms and pathogenesis, leading to their
consideration as a general entity with many subtypes. Another
noteworthy feature is that these diseases mainly affect the elderly
population. Furthermore, pathological findings indicate that
neurodegenerative diseases share similarities in their mode of cell
death, in which apoptosis is implicated (4). Efforts to identify the aetiological
agents for these diseases have been previously undertaken. Examples
of these include protein aggregation, mitochondrial dysfunction and
abnormal metal metabolism. This has led to the proposal of various
different pathogenic mechanisms of disease development, including
oxidative stress (OS), in which reactive oxygen species (ROS) and
reactive nitrative species have been considered to be the main
factors (5).
This has led to a search for antioxidants in an
attempt to find those that can attenuate oxidative damage and
perhaps prevent neuronal cell death. One of the heavily-researched
classes of antioxidants includes flavonoids, which are ubiquitous
in natural dietary sources. Previous studies on orientin, a
less-researched flavonoid, have shown many promising properties
such as radioprotection (6) and
cardioprotection (7). Thereforre,
the potential neuroprotective properties of orientin were
investigated in this study, and the identification of its
mechanisms was considered.
Materials and methods
Determination of the maximum non-toxic
dose (MNTD)
SH-SY5Y cells (1×103) were seeded into
each well of a flat bottom 96-well plate (Corning, Inc., Corning,
NY, USA). The cells were incubated for one day to allow for
attachment and acclimatisation. When 70% confluency was reached,
the cells were treated with different concentrations of orientin
(0, 10, 25, 50, 100, 200, 400, 800 and 1,000 μM) for a period of 24
h. Subsequently, 10 μl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
stock solution was added to each well, followed by 4 h of
incubation at 37°C in a dark environment to allow the formation of
purple formazan dye. After 4 h, the solution was carefully removed.
The formazan that formed was dissolved by adding 100 μl of DMSO and
mixing gently for 10 min. The absorbance reading of each well at
570 nm was then obtained using a microplate reader (Opsys MR; Dynex
Technologies, Chantilly, VA, USA). To determine the MNTD, a graph
of the percentage of cytotoxicity against the concentrations of
orientin was constructed.
Determination of the optimal
concentration of hydrogen peroxide
(H2O2)
The cytotoxicity of H2O2
towards SH-SY5Y cells at concentrations of 0, 50, 100, 150, 200 and
250 μM was determined using the trypan blue exclusion method. In
this method, equal amounts of cell suspension were suspended with
trypan blue dye and 10 μl was transferred into a haemocytometer
(Marienfeld Superior, Lauda-Konigshofen, Germany). The number of
dead cells was then counted under a microscope and their percentage
was calculated. Concurrently, intracellular ROS levels were
measured using a 2′,7′-dichlorodihydrofluorescein-diacetate
(DCFH-DA) assay. All concentrations of H2O2
were freshly prepared by diluting 30% (v/v) stock solution with
DMEM.
Treatment
In order to investigate the neuroprotective effects
of orientin on H2O2-induced apoptosis in
SH-SY5Y cells, cells were assigned to a total of eight treatment
groups and pre-treated (Table I).
Cells treated with 50 μM of D-α-tocopherol succinate acted as the
positive control. After 24 h of pre-treatment, cells were exposed
to the optimal concentration of H2O2 (150 μM
as pre-determined in this study) for another 24 h. Subsequently,
the cells were subjected to cell cycle analysis, and the
measurement of intracellular ROS level and caspase activity.
 | Table ITreatment groups used in the
investigation of the neuroprotective effects of orientin on
H2O2-induced apoptosis in SH-SY5Y cells. |
Table I
Treatment groups used in the
investigation of the neuroprotective effects of orientin on
H2O2-induced apoptosis in SH-SY5Y cells.
| Group | Treatment |
|---|
| 1 | Control (untreated
cells) |
| 2 |
H2O2 only (150
μM) |
| 3 | Orientin at MNTD
(20 μM) |
| 4 | Orientin at ½MNTD
(10 μM) |
| 5 | Vitamin E
(D-α-tocopherol succinate) (50 μM) |
| 6 | Orientin at MNTD
(20 μM) + 150 μM H2O2 |
| 7 | Orientin at ½MNTD
(10 μM) + 150 μM H2O2 |
| 8 | Vitamin E
(D-α-tocopherol succinate) (50 μM) +150 μM
H2O2 |
Analysis of the cell cycle
Cell cycle analysis was performed based on the
principle that cells in different phases of the cell cycle have
varying amounts of genetic material. The cell cycle was divided
into the Sub-G, G1, S and G2/M phases.
For cell cycle analysis, the SH-SY5Y cells
(1×106) were first seeded in separate wells in 6-well
plates (Corning, Inc.). The cells were allowed to reach 70–80%
confluency before the treatment groups were initiated. After 24 h
exposure to H2O2, the cells from each well
were trypsinised, collected and centrifuged at 327 × g for 5 min.
The supernatant was discarded and the pellet was washed with
phosphate-buffered saline (PBS). The cells were then centrifuged
again and fixed with 70% ethanol. The 70% ethanol was diluted from
99.8% (v/v) ethyl alcohol (Chemar® System®,
Shah Alam, Malaysia) with PBS. During fixation of the cells, care
was taken to resuspend the cells gently but thoroughly to avoid
clumps. The cells were then left overnight and kept at 4°C.
After overnight ethanol fixation, the tubes were
centrifuged at 327 × g for 5 min. The supernatant was discarded and
the cells were rinsed twice with PBS. DNA staining reagent (500 μl)
was added to the cells and they were then transferred to glass
tubes for flow cytometry. The programme BD CellQuest™ (BD
Biosciences, Franklin Lakes, NJ, USA) was used to analyze the
results of the cell cycle analysis. Results from the cell cycle
analysis were analysed based on the percentages of cells in
different phases of the cell cycle.
Assessment of intracellular ROS
level
DCFH-DA dye was used to measure the intracellular
ROS levels of the cells in each treatment group. The principle of
this assay is that, when applied to intact cells, the non-ionic,
non-polar DCFH-DA crosses cell membranes and is hydrolyzed
enzymatically by intracellular esterase to non-fluorescent DCFH. In
the presence of ROS, DCFH is oxidised to highly fluorescent
dichlorofluorescein (DCF). Thus, the intracellular DCF fluorescence
can be used as an index to quantify overall ROS in the cells at an
excitation wavelength of 485 nm and an emission wavelength of 535
nm.
Measurement of caspase activity
The induction of apoptosis via
H2O2 has been shown to increase the activity
of the caspase class of enzymes, which is involved in the various
signalling pathways of apoptosis. In particular, caspase 9 is
involved in the intrinsic pathway, caspase 8 is involved in the
extrinsic pathway and caspase 3 is involved in the executioner
pathway. Therefore, three groups of caspase enzymes were monitored
in this study.
A total of 1×104 cells/ml was seeded into
each well of a sterile white flat-bottom 96-well plate (Nunc A/S,
Roskilde, Denmark). Once the cells were 70–80% confluent, they were
pre-treated with their respective treatment groups for 24 h, after
which they were exposed to 150 μM H2O2 for
another 24 h. Subsequently, the activity of caspases 3/7, 8 and 9
were measured using Caspase-Glo® 3/7, 8 and 9 assay kits
(Promega Corporation, Madison, WI, USA), respectively. The
measurement was carried out according to the manufacturer’s
instructions. Luminescence was then read using a luminometer
(Panomics, Santa Clara, CA, USA).
Statistical analysis
All the experiments in this study were performed in
triplicate and repeated once, unless otherwise stated. The data
were presented as the means ± standard deviation (SD) and subjected
to Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference. One-way analysis of variance
(ANOVA), followed by a post-hoc multiple range test (Duncan’s test)
at a significance level of 5%, was conducted on the data obtained
during the cell cycle analysis. Statistical analyses were performed
using SPSS version 18.
Results
Determination of the MNTD
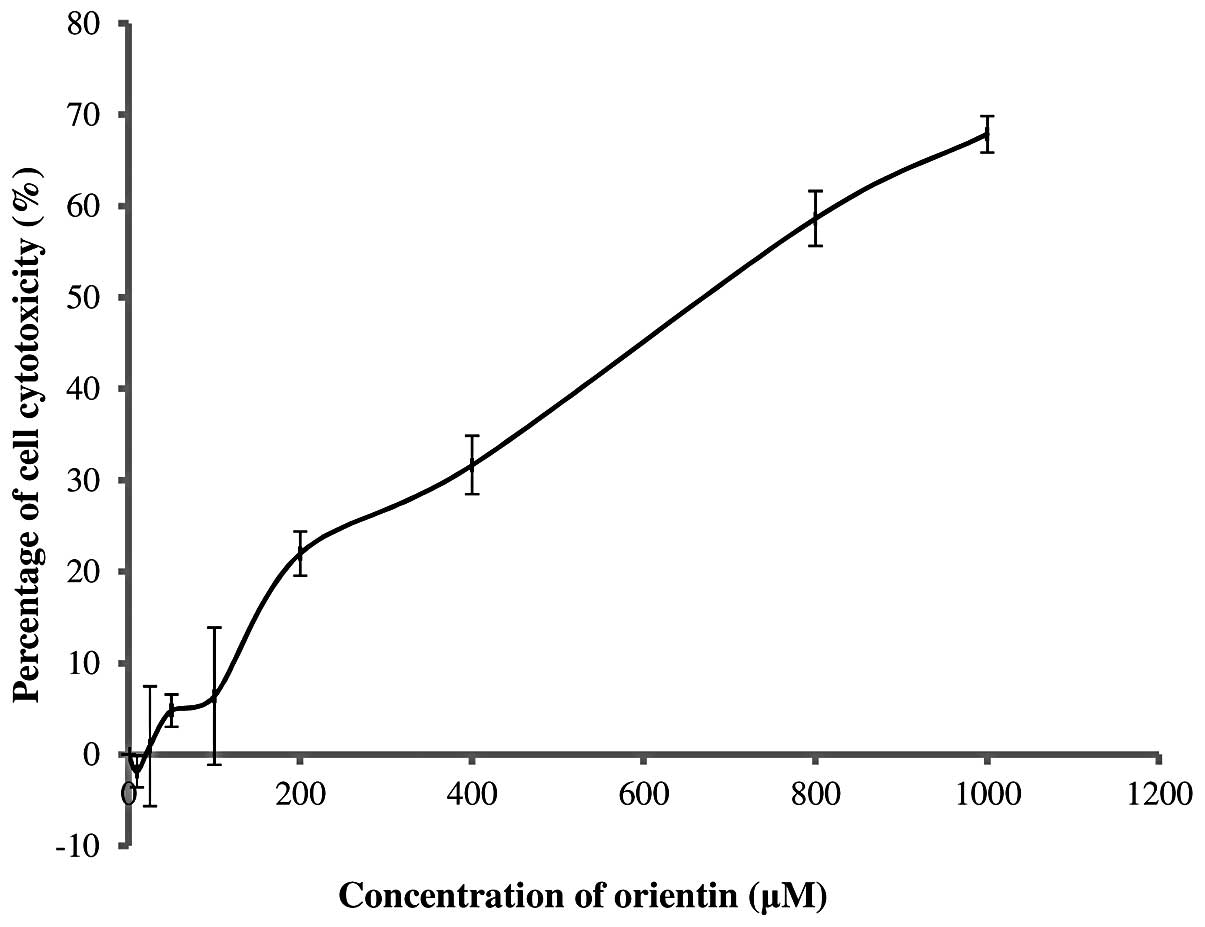
The MNTD of orientin was determined by constructing
graphs of the percentage of cell cytotoxicity against orientin
concentration. The MNTD was regarded as the highest concentration
that allows 0% cytotoxicity towards SH-SY5Y cells, with cell
cytotoxicity measured using an MTT assay.
The concentration of orientin against SH-SY5Y cells
was tested in the range of 0–1,000 μM. Its
cytotoxicity-concentration relationship is presented in Fig. 1. It was clearly observed that
orientin was non-cytotoxic at a concentration of ≤20 μM. This
showed that the MNTD of orientin was 20 μM while its half MNTD
(½MNTD) was as low as 10 μM. The results also revealed that
orientin stimulated the growth of SH-SY5Y cells by ~2% at the
½MNTD. By contrast, increasing the concentration of orientin from
20 to 1,000 μM markedly increased the percentage of cytotoxicity to
as high as 68%.
Determination of the optimal
concentration of H2O2
To determine the optimal concentration of
H2O2, two important parameters were
considered, the ability to induce significant intracellular ROS
levels and cell cytotoxicity. To achieve the objectives of this
study, the optimal concentration selected was required to be the
least cytotoxic concentration of H2O2, while
also being able to induce significant ROS levels in the viable
SH-SY5Y cells within 24 h of incubation. In this study,
intracellular ROS levels were gauged using a DCFH-DA assay while
the cell cytotoxicity was determined using the trypan blue
exclusion method.
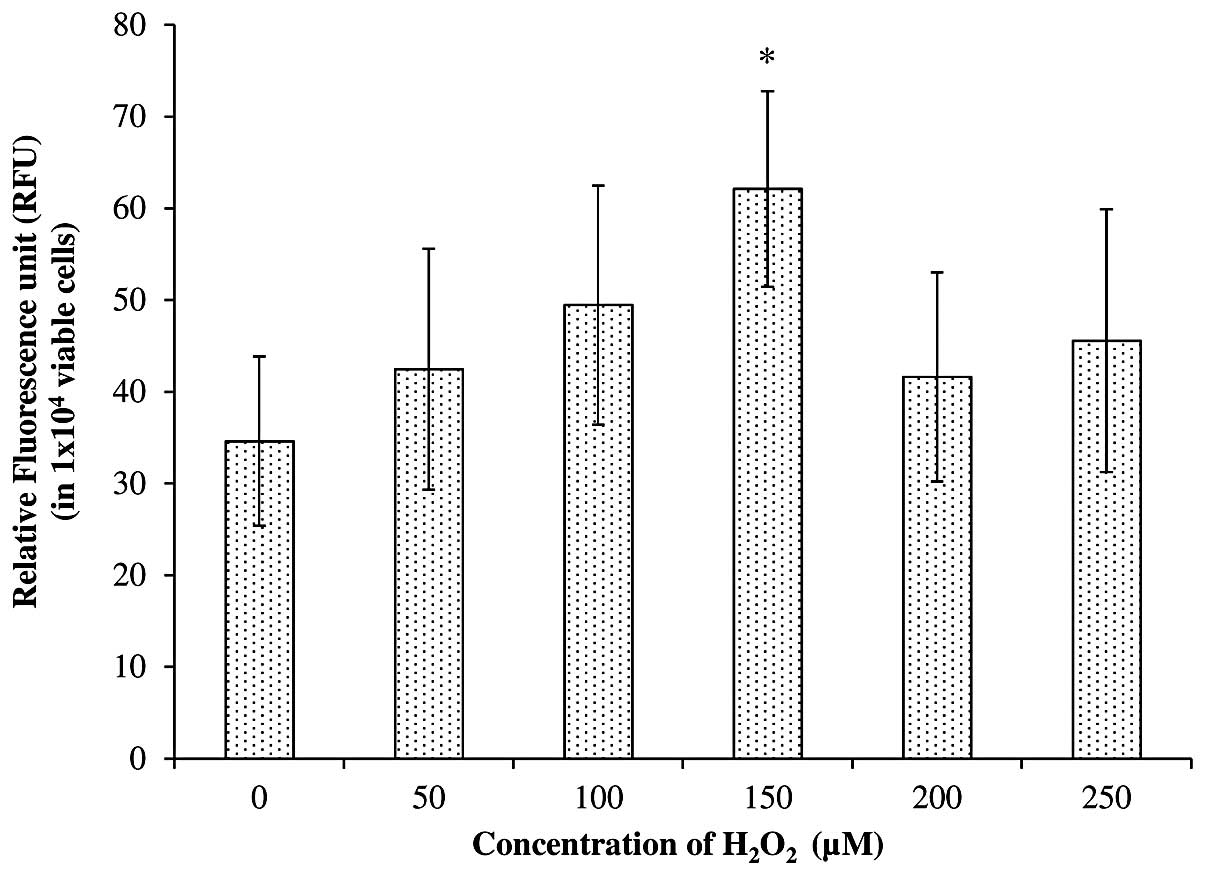
Fig. 2 shows the
intracellular ROS levels (in a total of 1×104 viable
cells) after treatment with freshly prepared
H2O2 at concentrations of 0, 50, 100, 150,
200 and 250 μM. The results demonstrate an increase in
intracellular ROS with an increase in the concentration of
H2O2 from 0 to 150 μM. For instance, a total
increase of 23.5, 42.8 and 79.5% was achieved in cells treated with
50, 100 and 150 μM, respectively, compared with the untreated
SH-SY5Y cells. However, only the cells treated with 150 μM
H2O2 showed a significant increase in
intracellular ROS generation compared with the untreated cells,
based on Student’s t-test at P<0.05. The present study also
revealed that an increase in H2O2
concentration to 200 and 250 μM failed to further increase the
intracellular ROS level. Instead, it reduced the generation of
intracellular ROS to 27–33% compared with a concentration of 150
μM.
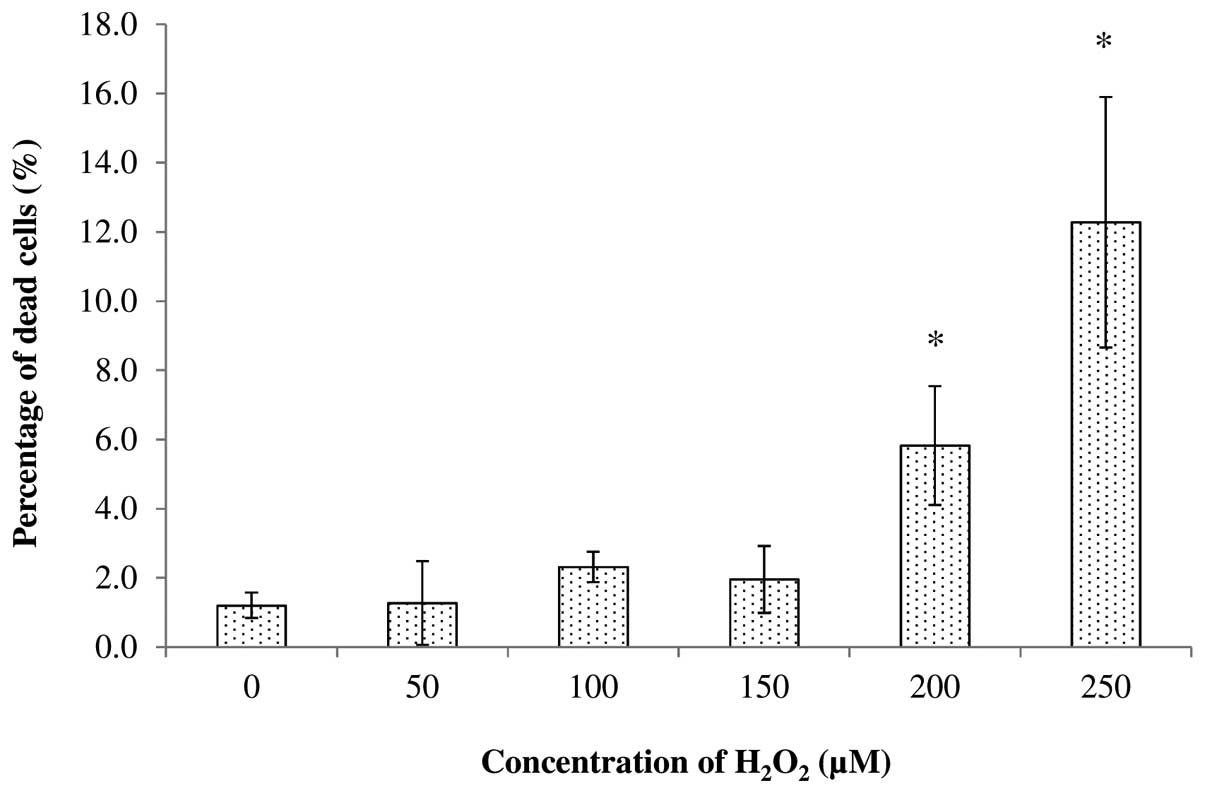
The cytotoxicity of H2O2
towards SH-SY5Y cells was also tested at concentrations of 0, 50,
100, 150, 200 and 250 μM. It was found that treatment with 50, 100
and 150 μM of H2O2 produced <2.32% dead
cells after 24 h of incubation, while treatment with 200 and 250 μM
produced a total of 5.83 and 12.28% dead cells, respectively
(Fig. 3). Statistical analysis
conducted using Student’s t-test at P<0.05 further revealed that
treatment with 200 and 250 μM significantly increased the
percentage of dead cells compared with the untreated cells. This
indicated that the concentrations of 200 and 250 μM were not
suitable for use in subsequent experiments. By considering the
ability to significantly increase the intracellular ROS levels, and
the percentage of dead cells after treatment, the optimal
concentration of H2O2 for SH-SY5Y cells after
24 h of incubation was found to be 150 μM. Thus, this optimal
concentration was used in subsequent experiments.
Effects on cell cycle progression
The apoptosis of neural cells has been reported to
be important in neurodegenerative diseases. Therefore, the effects
of orientin on the apoptosis of SH-SY5Y cells were also determined
using the propidium iodide staining method. The cell cycles of the
treated cells were then analysed and compared with those of the
untreated cells. Data from the cell cycle analysis were then
presented as percentages of cells in each phase of the cell cycle,
i.e., the sub-G, G1, S and G2/M phases (Table II). The sub-G phase represents the
apoptotic cells, while the G2/M phase represents the cells that
underwent mitosis.
 | Table IIPercentage of cells in the various
phases of the cell cycle after treatment with orientin at the MNTD
and ½MNTD. |
Table II
Percentage of cells in the various
phases of the cell cycle after treatment with orientin at the MNTD
and ½MNTD.
| Percentage of cells
in each phase of the cell cycle |
|---|
|
|
|---|
| Treatment | Sub-G | G1 | S | G2/M |
|---|
| Untreated
cells | 0.74±0.09 | 64.56±7.44 | 7.36±0.85 | 17.11±1.97 |
|
H2O2 | 10.50±1.19a | 56.68±6.43 | 8.91±1.01 | 13.67±1.55 |
| Orientin
(MNTD) | 6.70±0.64a,b | 60.15±5.74 | 8.96±0.86 | 15.54±1.48 |
| Orientin
(½MNTD) | 7.27±0.77a | 59.74±5.93 | 8.78±0.87 | 14.89±1.48 |
| D-α-tocopherol
succinate (50 μM) | 9.86±1.08a | 55.83±6.08 | 8.36±0.91 | 16.16±1.76 |
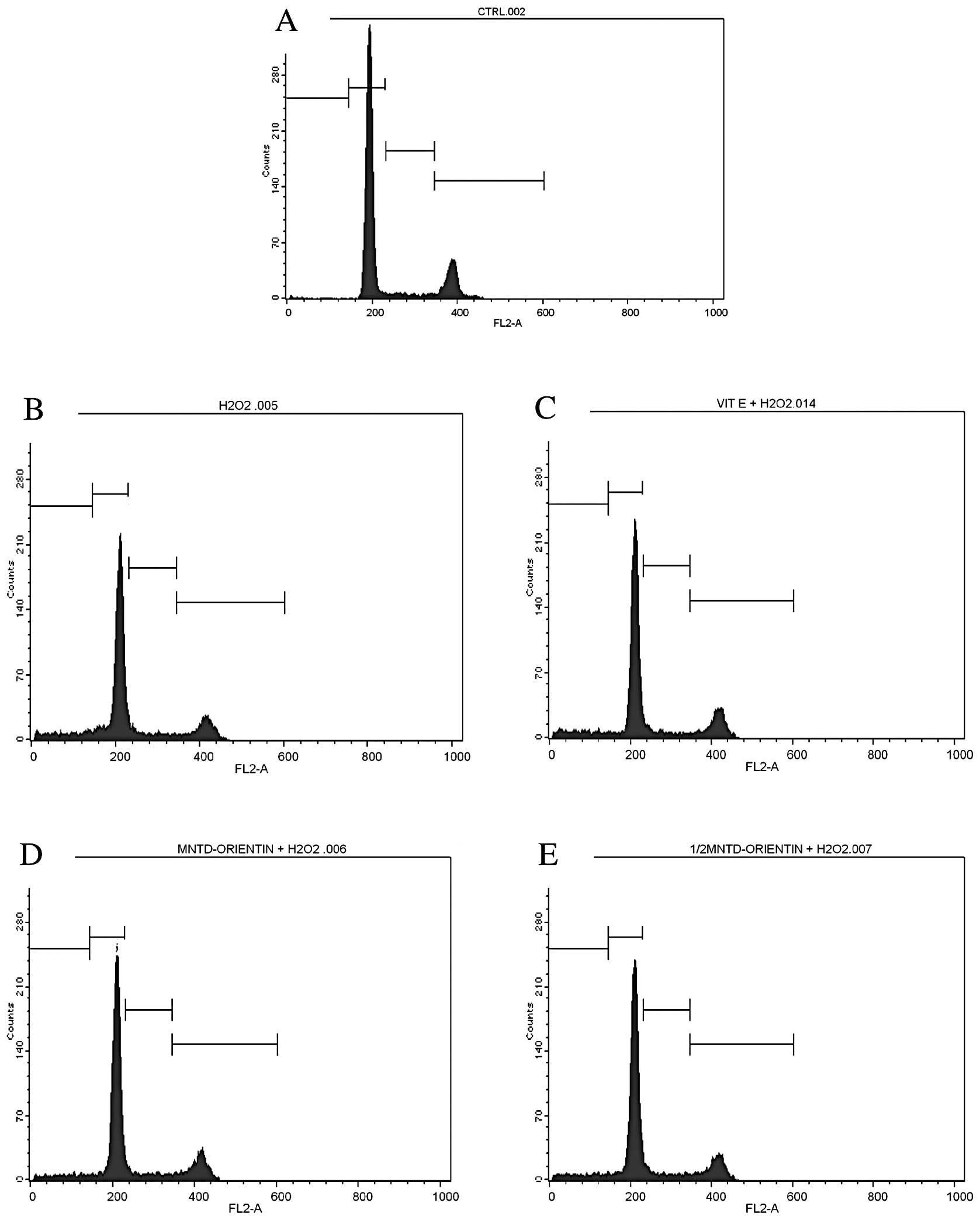
In the untreated SH-SY5Y cells, two peaks were
clearly observed at the G1 and G2/M phases (Fig. 4), which represented 64.56 and
17.11% of the cells, respectively (Table II). A total of 7.36% of the cells
were in the S phase while only 0.74% of the cells were considered
to be apotoptic cells in the sub-G phase. A similar number of peaks
and a similar pattern of distribution were also observed in the
cells treated with 50 μM of D-α-tocopherol succinate, which acted
as the positive control. However, this positive control showed a
significantly higher percentage of apoptotic cells (9.68%) and
cells in the S phase (8.36%) compared with the untreated cells.
By contrast, the exposure of SH-SY5Y cells to
H2O2 was found to decrease the percentage of
cells in the G1 phase (56.68%), while increasing the percentage of
cells in the sub-G phase (10.50%), compared with the untreated
cells. H2O2 treatment also reduced the
percentage of mitotic cells (those in the G2/M phase) to as low as
13.67%. This G2/M phase value was the lowest among all the
treatments. The results also revealed that the percentage of
apoptotic cells was significantly reduced with the inclusion of
orientin at either the MNTD or ½MNTD. Orientin at the MNTD and
½MNTD was able to decrease the percentage of apoptotic cells from
10.50% in the H2O2 treatment group to 6.70
and 7.72%, respectively. Conversely, the percentage of cells in the
G1 phase was increased by ≥5% after treatment with orientin.
Effects on intracellular ROS levels
As intracellular ROS are involved in in
neurodegenerative diseases, this study also evaluated the effects
of orientin at the MNTD and ½MNTD on intracellular ROS levels in
SH-SY5Y cells. The results of the present study revealed that, by
exposing the cells to 150 μM H2O2,
intracellular ROS levels were significantly increased regardless of
the treatment group, compared with the untreated cells (Fig. 5). However, cells pre-treated with
orientin did not show a significant reduction in intracellular ROS
levels.
Effects on caspase activity
The effectiveness of orientin as a neuroprotective
agent was further evaluated by determining the mechanism of
apoptosis through caspase activation. In this study, SH-SY5Y cells
were pre-treated with orientin for 24 h prior to exposure to 150 μM
of freshly prepared H2O2 for another 24 h.
Upon completion of the treatment period, the activity of caspases
3/7, 8 and 9 was measured using Promega Caspase-Glo®
detection kits.
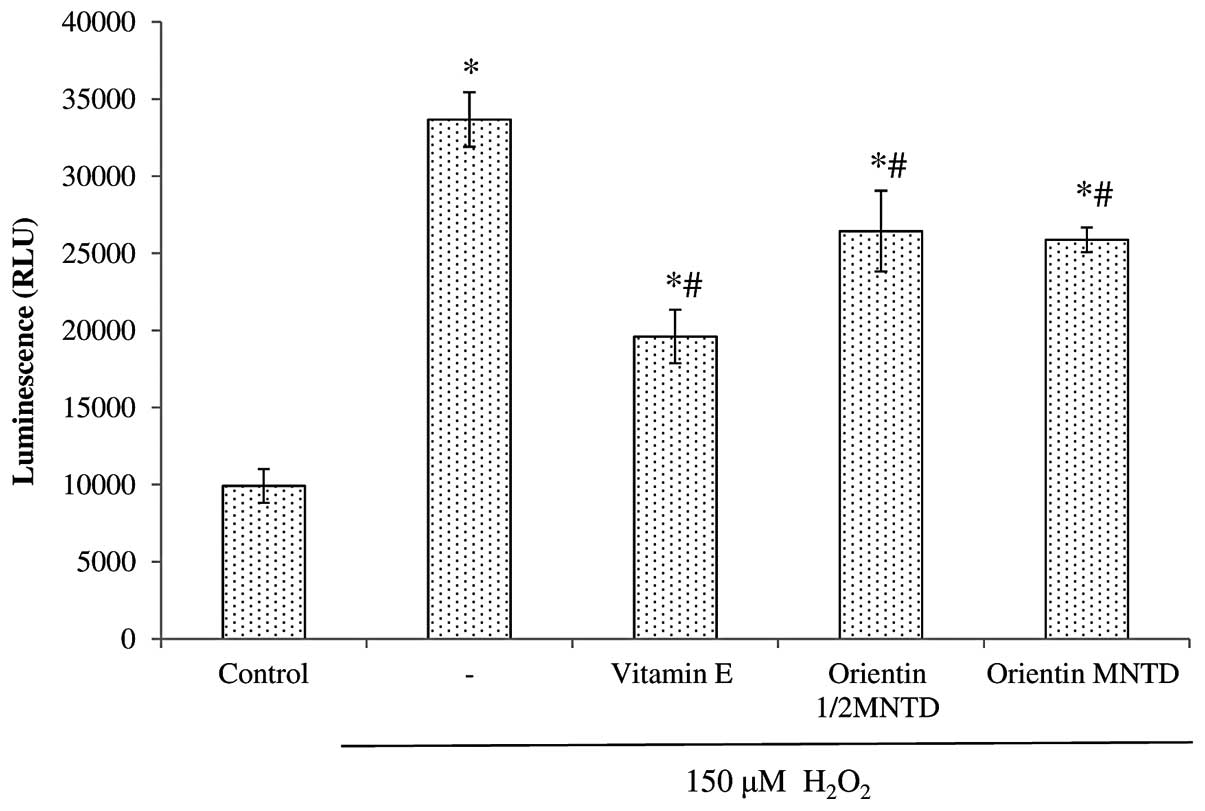
The effectiveness of orientin as a neuroprotective
agent was further evaluated by determining the mechanism of
apoptosis through caspase activation. Fig. 6 shows the comparison of caspase 3/7
activity in the various treatment groups, all of which show
significant increases in caspase 3/7 activity when exposed to
H2O2 (P<0.05 compared with the untreated
cells). Out of the five treatment groups, three [positive control
(D-α-tocopherol succinate), ½MNTD orientin and MNTD orientin] led
to a significant reduction in caspase 3/7 activity compared with
the H2O2 group, in the following ascending
order: positive control, MNTD orientin and ½MNTD orientin.
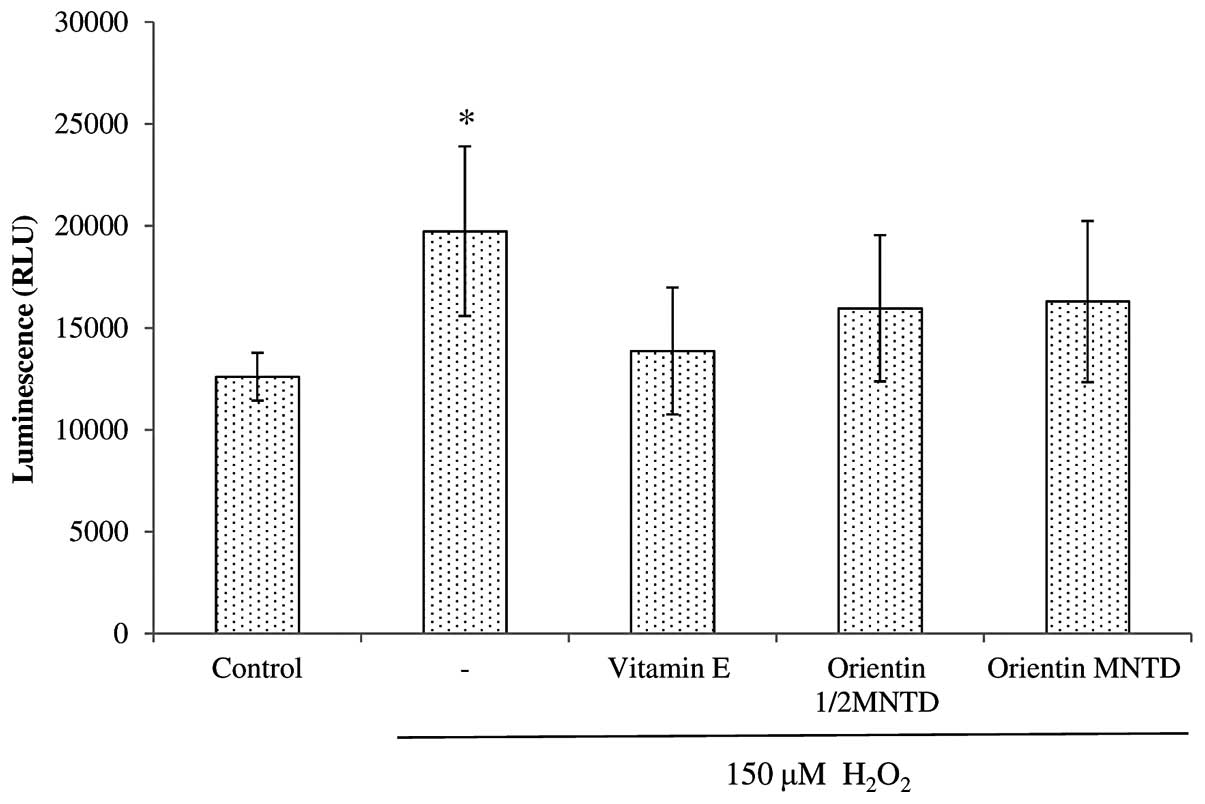
The exposure of SH-SY5Y cells to
H2O2 led to significant increases in caspase
8 activity when compared with the untreated cells (P<0.05)
(Fig. 7). All the treatment groups
showed a capacity to decrease caspase 8 activity, but this capacity
was insignificant compared with that of the
H2O2 treatment group. By contrast, all the
treatment groups significantly increased caspase 9 activity
compared with the untreated cells (Fig. 8). However, only the MNTD of
orientin was able to cause a significant reduction in caspase 9
activity compared with the H2O2 group at
P<0.05.
Discussion
The present study revealed that orientin at
concentrations below the MNTD (<20 μM and 4.0 μg/ml,
respectively) did not exhibit any in vitro cytotoxicity
towards SH-SY5Y cells, which indicated the ability of orientin to
promote cell growth. This may be explained by the antioxidant
properties of orientin as a flavonoid compound (8). Flavonoids are capable of protecting
cells by forming bonds with free radicals that cause cell
abnormalities and cell death, converting them into more stable and
less reactive molecules.
Conversely, flavonoids may protect cells through
interactions with various enzyme systems. An event known as lipid
peroxidation, which results in cell damage, occurs when free
radical species are in the presence of free iron. Flavonoid
compounds may prevent this as they are known to chelate iron,
thereby removing an essential factor for the development of free
radicals (9). Although low
concentrations of flavonoids can protect the cells from free
radicals, high concentrations of flavonoids can generate ROS by
auto-oxidation and redox-cycling, thereby inducing cell apoptosis
as well as DNA damage (10). This
may explain why the percentage of cytotoxicity increased as the
concentration of orientin was increased in this study.
Based on the results of this study, a concentration
of 150 μM of H2O2 produced significant
intracellular ROS levels while maintaining non-significant levels
of cytotoxicity. Up to concentrations of 150 μM, intracellular ROS
levels increased along with the concentration of
H2O2. However, ROS levels were markedly
reduced when H2O2 concentration was increased
to ≥200 μM. This suggests that there is an optimal concentration
for inducing intracellular ROS levels in the SH-SY5Y cell line.
Notably, in studies on prokaryotes such as Escherichia coli,
ROS has been shown to be able to activate the expression of gene
products involved in antioxidant defences such as Mn-SOD (11) and catalase (12). This suggests that ROS are involved
in redox homeostasis by influencing signalling pathways. This is
further supported by studies that show that multicellular organisms
can respond to H2O2 in a variety of ways,
including increased antioxidant gene expression (13–15),
stimulation of cell proliferation (16,17),
differentiation (18,19), migration (20) and apoptosis (21,22).
However, the reason for these cells (SH-SY5Y) reducing ROS levels
following exposure to H2O2 concentrations
≥200 μM is unclear and merits investigation. It is possible that
these cells possess the ability to upregulate the expression of
antioxidative products when in contact with certain concentrations
of ROS, which in this case was H2O2.
Findings of this study also demonstrated an apparent
dose-dependent increase in cytotoxicity, possibly due to the nature
of H2O2 and the antioxidative capacity of the
cells. H2O2 has been shown to be highly
soluble, diffusible and able to cross the cell membrane. Once
across the cell membrane, the antioxidant defenses of the cells
counteract H2O2 and the subsequently-produced
ROS, but reach a threshold and eventually succumb to severe
oxidative damage, leading to cell death (23). This follows the process of
apoptotic cell death reported in fetal rat hepatocytes, where
apoptosis is preceded first by ROS production, and then by the loss
of mitochondrial transmembrane potential, the release of cytochrome
c and the activation of caspase 3 (24).
H2O2 has been used extensively
as an inducer of OS in in vitro models (25,26).
H2O2 induces apoptosis in various cell lines
(25,27) and has been used in numerous other
studies to induce OS in the SH-SY5Y cell line. The optimal
concentration of H2O2 selected for this study
(150 μM) was also the concentration of H2O2
used by Zhang et al (28),
but varies with that used in other studies. This is probably due to
the different periods of exposure to H2O2.
For example, Tarozzi et al (29) used 0.3 mM with an exposure period
of 3 h. Even with 24 h exposure, a different optimal concentration
of H2O2 has been used. Kwon et al
(30) exposed cells to 400 μM
H2O2 while Suematsu et al (31) used 100 μM of
H2O2 for the same exposure period. The
different periods of exposure differ according to the types of
assays used in the particular studies. For example, all the cell
viability tests mentioned in the above studies used a 24-h exposure
period. The studies also observed different parameters, such as in
the study by Tarozzi et al (29) where assays for mitochondrial
functioning were performed on cells exposed to 300 μM of
H2O2 for 3 h; for detecting ROS formation,
the duration of exposure was only 30 min, and for gauging the
extent of DNA fragmentation, the cells were exposed to 300 μM of
H2O2 for 18 h. These different tests gauge
different components of the cells which show changes at varying
times. For example, the mitochondria are extremely sensitive to ROS
levels as their membrane potential may be markedly affected,
impairing function and leading to cell death if the reading is
taken later than 3 h. By contrast, DNA fragmentation requires the
activation of caspase-activated DNase, which is activated during
the caspase cascade of apoptosis, specifically caspase 3 (32). Therefore, these sequences of events
may require extended periods, although much of the data in this
particular cell line are unclear.
The evaluation of orientin in the current study
showed that it confers neuroprotection on an SH-SY5Y neuroblastoma
cell line that has been induced to undergo apoptosis via exposure
to H2O2. The treatment groups were not
compared to vehicle-treated control groups, as previous results
(data not included) have indicated small differences in readings.
Based on the results of the cell cycle analysis, orientin led to a
significant decrease in the percentages of cells in the sub-G
phase, which represent the population of apoptotic cells. This
finding signifies that pre-treatment with orientin may directly or
indirectly reduce the incidence of apoptosis caused by exposure to
H2O2, indicating neuroprotective properties.
These are defined by any therapeutic strategy that is able to slow
or arrest the progression of neuronal loss (33).
However, the mechanism of neuroprotection conferred
by orientin remains unknown. The only indication to this end is
that it belongs to a class of the flavonoid family (6,34).
Flavonoids have been known to exhibit anti-inflammatory,
antiallergic, antiviral and antitumor activities, and these have
been speculated to be heavily dependent on their intrinsic
antioxidative and chelating properties (9), as well as on their role in the
modulation of intracellular signals which promote cellular
survival. For example, severe inhibition of sustained activation of
c-Jun N-terminal kinase was reported in mouse striatal neurons that
had been pre-treated with epicatechin and exposed to low-density
lipoprotein, an apoptosis-inducing agent (35).
The results of this study indicate that orientin at
the MNTD and ½MNTD had no significant effect on the intracellular
ROS levels of viable SH-SY5Y cells that were exposed to
H2O2, indicating that it is unable to mediate
intracellular ROS levels. To confirm the protection against
H2O2-mediated apoptosis by orientin,
triggering of the caspase cascade that results in apoptosis was
also investigated. The present study revealed that orientin was
capable of triggering the caspase cascade. Orientin at the MNTD
exhibited a reduction in the activity of caspase 3/7 and caspase 9,
which are the execution and intrinsic pathway caspases; the ½MNTD
of orientin reduced only the activity of caspase 3/7.
Several other flavonoids, such as apigenin (36,37)
and kaempferol (37), have also
been shown to reduce the activity of caspase 3/7. Therefore, the
neuroprotection conferred by orientin could most likely be
expressed through this mechanism of caspase modulation, or
intracellular signalling leading to decreased caspase expression,
possibly through the expression of cell survival signalling. This
could be due to the expression of the B-cell lymphoma 2 (Bcl-2)
family of proteins, which inhibits the formation of mitochondrial
transition pores. This blocks the release of cytochrome c,
which is essential in the events leading to the caspase cascade
(38). In addition, the
significant reduction in caspase 9 activity in SH-SY5Y cells
pre-treated with orientin indicates that orientin affects the
expression of caspase 9, or is directly involved in its
inactivation. This may also affect the activity of caspase 3/7, as
caspase 9 is involved in its activation.
In conclusion, the present study has demonstrated
that orientin attenuated neuronal cell apoptosis by reducing the
activity of caspases 3/7, 8 and 9. As its underlying mechanism
remains unknown, this could serve as a future point of
investigation. For instance, caspase 3/7 activity could be due to
increased Bcl-2 protein expression, which favours cell survival.
Alternatively, it could be due to other factors such as the direct
inhibition of the enzyme itself. Western blot analysis could be
carried out to determine the expression of pro-survival proteins
and cytochrome c. This may elucidate the promising potential
of orientin as a neuroprotective agent.
Acknowledgements
This study was supported by the International
Medical University under BMS I01-2012(02). The authors would like
to thank Dr Say Yee How for generously providing the SH-SY5Y cells
and Ms. Yee Lian Tiong for technical help with the flow cytometry
work.
Abbreviations:
|
DCFH-DA
|
2′,7′-dichlorodihydrofluorescein-
diacetate
|
|
MNTD
|
maximum non-toxic dose
|
|
½MNTD
|
half maximum non-toxic dose
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
OS
|
oxidative stress
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Kolominsky-Rabas PL, Sarti C, Heuschmann
PU, Graf C, Siemonsen S, Neundoerfer B, Katalinic A, Lang E,
Gassmann KG and von Stockert TR: A prospective community-based
study of stroke in Germany - the Erlangen Stroke Project (ESPro):
incidence and case fatality at 1, 3, and 12 months. Stroke.
29:2501–2506. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Samsa GP, Bian J, Lipscomb J and Matchar
DB: Epidemiology of recurrent cerebral infarction: a medicare
claims-based comparison of first and recurrent strokes on 2-year
survival and cost. Stroke. 30:338–349. 1999.PubMed/NCBI
|
|
3
|
Leppälä JM, Virtamo J, Fogelholm R,
Albanes D and Heinonen OP: Different risk factors for different
stroke subtypes: association of blood pressure, cholesterol, and
antioxidants. Stroke. 30:2535–2540. 1999.PubMed/NCBI
|
|
4
|
Mattson MP: Neuronal life-and-death
signaling, apoptosis, and neurodegenerative disorders. Antioxid
Redox Signal. 8:1997–2006. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Emerit J, Edeas M and Bricaire F:
Neurodegenerative diseases and oxidative stress. Biomed
Pharmacother. 58:39–46. 2004. View Article : Google Scholar
|
|
6
|
Vrinda B and Uma Devi P: Radiation
protection of human lymphocyte chromosomes in vitro by orientin and
vicenin. Mutat Res. 498:39–46. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu N, Sun Y and Zheng X: Orientin-induced
cardioprotection against reperfusion is associated with attenuation
of mitochondrial permeability transition. Planta Med. 77:984–991.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pusztai R, Béládi I, Bakai M, Mucsi I and
Kukán E: Study on the effect of flavonoids and related substances.
I The effect of quercetin on different viruses. Acta Microbiol Acad
Sci Hung. 13:113–118. 1966.PubMed/NCBI
|
|
9
|
Korkina LG and Afanas’ev IB: Antioxidant
and chelating properties of flavonoids. Adv Pharmacol. 38:151–163.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuo M, Sasaki N, Saga K and Kaneko T:
Cytotoxicity of flavonoids toward cultured normal human cells. Biol
Pharm Bull. 28:253–259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hassan HM and Fridovich I: Regulation of
the synthesis of superoxide dismutase in Escherichia coli.
Induction by methyl viologen. J Biol Chem. 252:7667–7672.
1977.PubMed/NCBI
|
|
12
|
Yoshpe-Purer Y, Henis Y and Yashpe J:
Regulation of catalase level in Escherichia coli K12. Can J
Microbiol. 23:84–91. 1977. View
Article : Google Scholar
|
|
13
|
An JH and Blackwell TK: SKN-1 links C.
elegans mesendodermal specification to a conserved oxidative
stress response. Genes Dev. 17:1882–1893. 2003.PubMed/NCBI
|
|
14
|
Inoue H, Hisamoto N, An JH, Oliveira RP,
Nishida E, Blackwell TK and Matsumoto K: The C. elegans p38
MAPK pathway regulates nuclear localization of the transcription
factor SKN-1 in oxidative stress response. Genes Dev. 19:2278–2283.
2005.
|
|
15
|
Sablina AA, Budanov AV, Ilyinskaya GV,
Agapova LS, Kravchenko JE and Churnakov PM: The antioxidant
function of the p53 tumor suppressor. Nat Med. 11:1306–1313. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Foreman J, Demidchik V, Bothwell JH,
Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C,
Jones JD, et al: Reactive oxygen species produced by NADPH oxidase
regulate plant cell growth. Nature. 422:442–446. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Geizst M and Leto TL: The Nox family of
NAD(P)H oxidases: host defense and beyond. J Biol Chem.
279:51715–51718. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Stouffs M, Serrander L, Banfi B,
Bettiol E, Charnay Y, Steger K, Krause KH and Jaconi ME: The NADPH
oxidase NOX4 drives cardiac differentiation: Role in regulating
cardiac transcription factors and MAP kinase activation. Mol Biol
Cell. 17:3978–3988. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sauer H, Rahimi G, Hescheler J and
Wartenberg M: Role of reactive oxygen species and
phosphatidylinositol 3-kinase in cardiomyocyte differentiation of
embryonic stem cells. FEBS Lett. 476:218–223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ushio-Fukai M: Localizing NADPH
oxidase-derived ROS. Sci STKE. 2006:re82006.PubMed/NCBI
|
|
21
|
Cai H: Hydrogen peroxide regulation of
endothelial function: origins, mechanisms, and consequences.
Cardiovasc Res. 68:26–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gechev TS and Hille J: Hydrogen peroxide
as a signal controlling plant programmed cell death. J Cell Biol.
168:17–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whittemore ER, Loo DT, Watt JA and Cotman
CW: A detailed analysis of hydrogen peroxide-induced cell death in
primary neuronal culture. Neuroscience. 67:921–932. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Satoh T, Sakai N, Enokido Y, Uchiyama Y
and Hatanaka H: Free radical-independent protection by nerve growth
factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered
apoptosis. J Biochem. 120:540–546. 1996. View Article : Google Scholar
|
|
25
|
Jacobson MD: Reactive oxygen species and
programmed cell death. Trends Biochem Sci. 21:83–86. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Uberti D, Piccioni L, Colzi A, Bravi D,
Canonico PL and Memo M: Pergolide protects SH-SY5Y cells against
neurodegeneration induced by H2O2. Eur J
Pharmacol. 434:17–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heo SR, Han AM, Kwon YK and Joung I: p62
protects SH-SY5Y neuroblastoma cells against
H2O2-induced injury through the PDK1/Akt
pathway. Neurosci Lett. 450:45–50. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan
C, Cao G and Wang Z: Protective effects of salidroside on hydrogen
peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells.
Eur J Pharmacol. 564:18–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tarozzi A, Morroni F, Hrelia S, Angeloni
C, Marchesi A, Cantelli-Forti G and Hrelia P: Neuroprotective
effects of anthocyanins and their in vivo metabolites in SH-SY5Y
cells. Neurosci Lett. 424:36–40. 2007. View Article : Google Scholar
|
|
30
|
Kwon SH, Kim JA, Hong SI, Jung YH, Kim HC,
Lee SY and Jang CG: Loganin protects against hydrogen
peroxide-induced apoptosis by inhibiting phosphorylation of JNK,
p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem Int. 58:533–541.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suematsu N, Hosoda M and Fujimori K:
Protective effects of quercetin against hydrogen peroxide-induced
apoptosis in human neuronal SH-SY5Y cells. Neurosci Lett.
504:223–227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shoulson I: Neuroprotective clinical
strategies for Parkinson’s disease. Ann Neurol. 32(Suppl):
S143–S145. 1992.
|
|
34
|
Li D, Wang Q, Yuan ZF, Zhang L, Xu L, Cui
Y and Duan K: Pharmacokinetics and tissue distribution study of
orientin in rat by liquid chromatography. J Pharm Biomed Anal.
47:429–434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schroeter H, Spencer JP, Rice-Evans C and
Williams RJ: Flavonoids protect neurons from oxidized
low-density-lipoprotein-induced apoptosis involving c-Jun
N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J.
358:547–557. 2001. View Article : Google Scholar
|
|
36
|
Kang SS, Lee JY, Choi YK, Kim GS and Han
BH: Neuroprotective effects of flavones on hydrogen
peroxide-induced apoptosis in SH-SY5Y neuroblostoma cells. Bioorg
Med Chem Lett. 14:2261–2264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang CN, Chi CW, Lin YL, Chen CF and Shiao
YJ: The neuroprotective effects of phytoestrogens on amyloid beta
protein-induced toxicity are mediated by abrogating the activation
of caspase cascade in rat cortical neurons. J Biol Chem.
276:5287–5295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Woo M, Hakem R, Soengas MS, Duncan GS,
Shahinian A, Kagi D, Hakem A, McCurrach M, Khoo W, Kaufman SA, et
al: Essential contribution of caspase 3/CPP32 to apoptosis and its
associated nuclear changes. Genes Dev. 12:806–819. 1998. View Article : Google Scholar : PubMed/NCBI
|