Introduction
Cholangiocarcinoma is an intractable malignant tumor
originating from the bile duct, and the incidence of this carcinoma
is increasing worldwide. Regardless of recent advances in medical
treatment, surgical resection is the only potentially curative
treatment for the disease. However, less than one-third of patients
recommended for surgical resection as the tumor rapidly progresses
to the perivascular or organ systems and is often only diagnosed at
an advanced stage. Although chemotherapy, including a combination
of molecular targeting-therapies, is the therapy of choice for the
majority of patients, the prognosis remains poor at the present
time (1,2). While most patients do not have a
specific background underlying the occurrence of
cholangiocarcinoma, several risk factors have been demonstrated in
the disease pathogenesis (3).
These factors include the presence of gallstones, chronic
ulcerative colitis, infection by liver flukes, congenital biliary
cysts, primary sclerosing cholangitis (PSC) and nitrosamine
exposure (4,5).
Recently, cholangiocarcinoma has been frequently
highlighted as a high incidence was observed amongst industrial
workers in Japanese printing companies (6). Five cholangiocarcinoma cases were
identified among 33 workers who were employed for >1 year in a
printing factory in Osaka. Further investigation increased the
total case load to 18. In this instance, the onset of the disease
was at a relatively young age, arising between the ages of 25–40
years. Similar cases were also identified in the Tokyo, Fukuoka and
Miyagi provinces in Japan. Etiology of the disease has been
attributed to long-term exposure to chemical substances, such as
dichloromethane and 1,2-dichloropropane (7). However, further investigation is
required to clarify the involvement of these chemicals in the
pathogenesis of cholangiocarcinoma, including tumor initiation,
promotion and/or progression.
In order to understand the mechanism of pathogenesis
of malignant tumors, cellular biological methods, with
morphological, biochemical and molecular approaches utilizing cell
culture, are valuable. With regard to cholangiocarcinoma, studies
have been conducted using several cell lines (8–12).
However, available cholangiocarcinoma cell lines are limited and
results from these lines were predominantly intended for molecular
applications. Thus, these accessible cell lines are not accompanied
by detailed biochemical and morphological data sets and are
insufficient for full understanding of the pathogenic
mechanisms.
Previously, we established a cholangiocarcinoma cell
line from a 78-year-old female patient diagnosed with extrahepatic
cholangiocarcinoma. This line was designated as TK and was
demonstrated to express biochemical markers, such as carbohydrate
antigen (CA) 19-9, CA50 and carcino-embryonic antigen (CEA)
(13). The cell line is able to be
implanted into nude mice to produce xenograft animal models.
However, in addition to these biochemical characterizations,
morphological study is required for a greater understanding of the
nature of the cell line, particularly its utilization as an
experimental cholangiocarcinoma model.
A three-dimensional cell culture method was also
developed with an originally-devised collagen mesh. In the mesh,
cells are able to attach freely to the scaffold and proliferate
without space limitation from the anchors. One application of this
method is in the investigation of cell differentiation. For
example, when fibroblasts were cultured three-dimensionally in the
presence of demineralized bone powder, the cells produced an
extracellular matrix similar to that deposited around chondrocytes
(14,15). This effect was confirmed by kinetic
analysis of gene expression throughout the chondroinduction period
(16). Furthermore, this method of
cell culture is able to be combined with several bioreactors. The
effects of medium perfusion (17),
hydrostatic pressure (18,19) and exposure to low oxygen tension
(20) were analyzed by this
method. In addition, the culture was particularly useful for a
morphological study, simulating the in vivo behavior of a
malignant tumor. The method enabled individual distinction of the
four representative malignant glioma cell lines used for cell
biological experiments, along with their different characteristics.
Behaviors associated with cell differences, migration, attachment
and patterns of proliferationbecame evident only following
three-dimensional cell culture (21).
The use of three-dimensional cell culture is
becoming a more widespread and is being applied to a broader range
of cell lines (22). However,
there are few studies regarding three-dimensional cell culture of
human cholangiocarcinoma cell lines. To add to the biochemical data
obtained in our previous study, in the present study the morphology
of the TK cell line was analyzed in three-dimensional cell culture
to determine whether or not the cell line may be used for a wide
range of experimental studies of cholangiocarcinoma in
vitro.
Materials and methods
TK cell line and three-dimensional cell
culture
TK cells were cultured with RPMI-1640 complete
medium (Gibco Life Technologies Japan, Tokyo, Japan), supplemented
with 15% fetal bovine serum (Lot no. SFB30-1478, Equitech-Bio,
Kershville, TX, USA), 2 mM glutamine and 1 mM sodium pyruvate
(Gibco Life Technologies Japan). The three-dimensional culture
method for the experiments was a modification of the method
described by Mizuno et al (15). The scaffold material used for
three-dimensional culture was a bio-absorbable, degradable gelatin.
In brief, dispersed TK cells (1×104 cells/100 μl
complete RPMI medium) were injected into the scaffolds of
three-dimensional meshes and left to stand for 4–6 hours at 37°C in
a 5% CO2 incubator. Following the attachment of the
cells to the scaffolds, the meshes were transferred onto a 10-cm
dish, immersed in 10 ml culture medium and further cultivated for
3–20 days (21).
Morphological examinations
Phase contrast microscopy
Cell attachment and proliferation in
three-dimensional culture were observed with a phase contrast
microscope (CK2, Olympus Corporation, Tokyo, Japan). The meshes
were directly subjected to microscopy without fixing or staining
during the culture.
Light optical microscopy
Cells in the culture mesh were fixed with 10%
phosphate-buffered formalin and subjected to an automatic paraffin
embedding system (ETP-150CV, Sakura Finetek Japan Co., Ltd., Tokyo,
Japan). Paraffin-embedded specimens were sliced into 6-μl-thick
sections using a microtome and stained with haematoxylin and eosin.
These sections were examined under a light microscope (IX71,
Olympus Corporation). Images were captured using a charge-coupled
device image sensor (VB-7010, Keyence Japan, Osaka, Japan).
Scanning electron microscopy
(SEM)
The cells attached to the mesh were fixed by
treatment with 1.2% glutaraldehyde in 0.1 M phosphate buffer (pH
7.3, 400 mOsm). The specimens were dehydrated with a graded series
of ethanol ranging from 50% through 70, 80, 90 and 100%. Following
further treatment with 100% iso-amylacetate, the samples were dried
by a critical point dryer (Hitachi High-Technologies Corporation,
Tokyo, Japan) and sprayed with Au-Pd. Cells in the mesh were
examined at 15 kV under a JSM-5800LV scanning electron microscope
(JEOL Ltd., Tokyo, Japan).
Transmission electron microscopy
(TEM)
For transmission electron microscopy, the tissues
were fixed with 2% glutaraldehyde in 0.1 M phosphate buffer and
dehydrated by serial dilution of ethanol. Subsequent to treatment
with a substituting agent, the tissues were infiltrated prior to
polymerization in epoxy resin and sectioning with an ultramicrotome
(Leica, Vienna, Austria). Ultra-thin sections were further treated
with uranyl acetate and lead citrate, and observed by the Hitachi
H-7500 transmission electron microscope (Hitachi High-Technologies
Corporation, Tokyo, Japan).
Results
Morphology of cultured cells in the
scaffold
Following injection of dispersed TK cells into the
three-dimensional mesh, the cells attached to the scaffold.
Inoculated cells initially adhered to the material and then started
to grow at the attached spots. Plating efficiency was not
determined, as unattached cells did not remain attached to the mesh
and instead diffused into the culture medium and were removed from
further cultivation. After five days, proliferated cells were
detected in clusters and the TK cells had aggregated and formed
globoid structures (Fig. 1A).
Fig. 1A obtained by phase contrast
microscopy demonstrates the appearance of the cholangiocarcinoma
cluster in three-dimensional culture. The structure grew relative
to the culture duration. On day 14 of culture, light optical
microscopy demonstrated that the TK cells had aggregated and formed
duct-like assemblies (Fig. 1B). At
higher magnification, cells were observed to be filled with
deposits of a secretory substance in the cytoplasm (Fig. 1C). These deposits were periodic
acid schiff-positive as demonstrated in a previous study (13). Fig.
1C also demonstrated the organization of the attachment of the
cell to the scaffold.
SEM shows cell processes on TK cell
surfaces
The cells aggregated and formed globoid shapes in
the scaffold. The overall architecture was clearly demonstrated by
scanning electron microscopy on day 10 of culture (Fig. 2A). The surface of the structure
consisting of TK cells was covered with numerous floral-shaped
microvilli. Closer observation revealed that the pattern was not
homogeneous, despite the cells originating from the same cell line
(Fig. 2B). Higher magnification of
another region of the culture demonstrated that certain cells
possessed relatively sparse plicae whereas others possessed dense
microvilli (Fig. 2C). These two
types of processes were observed on the same globoid structure;
however, were segregated and distributed differently. This was
confirmed by examination at higher magnification (x7,500; Fig. 2D).
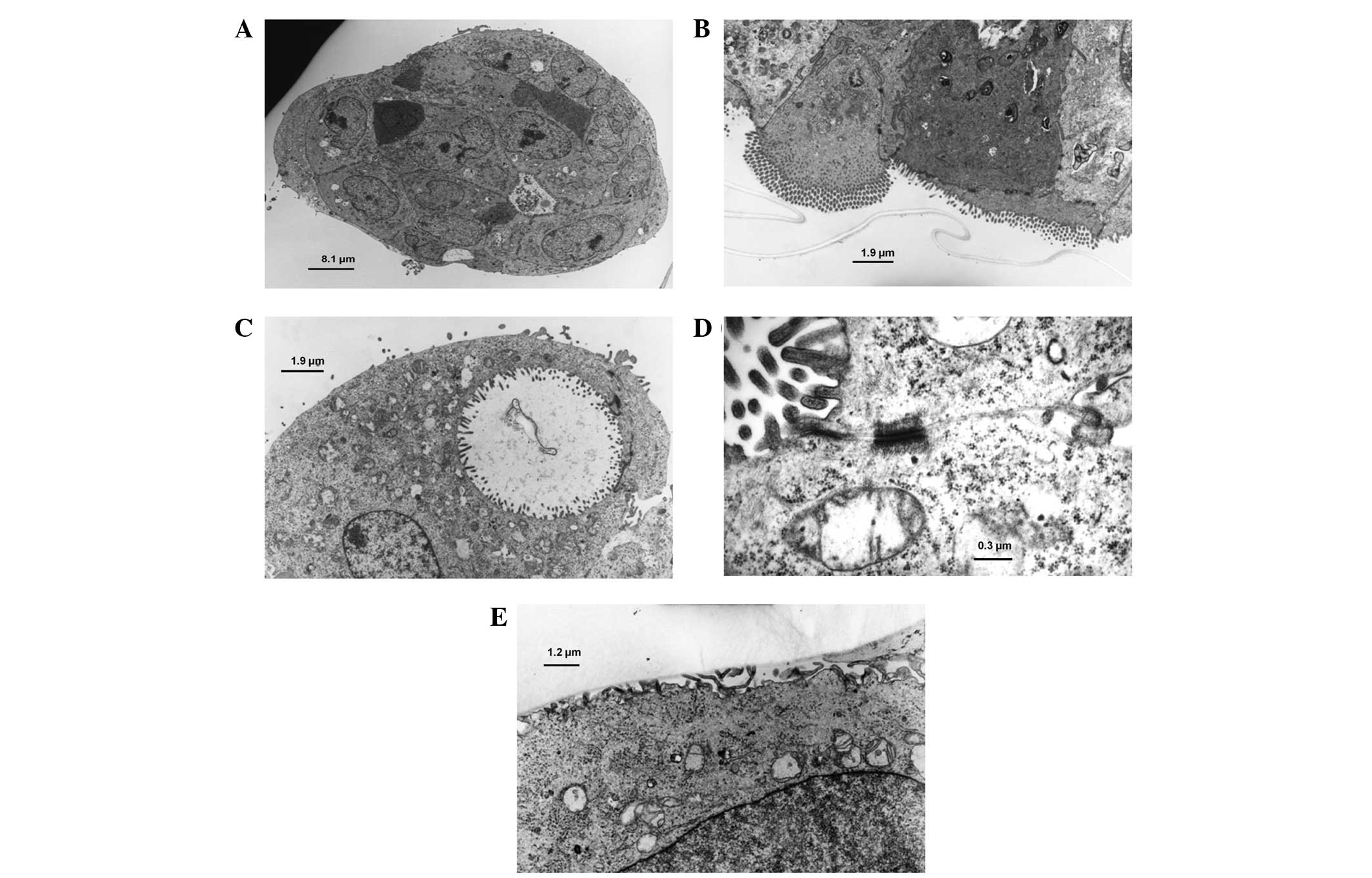
TEM observation of the three-dimensional
culture
A cross-section of the globoid aggregate on day 7 of
the three-dimensional culture was observed by TEM (Fig. 3A), showing the internal arrangement
of the cells to be semi-irregular. Cells of varying
electron-density were observed in the microscopy image. Numerous
microvilli were identified on the surface of the structure and also
protruded into the extracellular space on the inner side of the
aggregate. The cells exhibited irregularly-shaped nuclei and the
endoplasmic reticula and mitochondria were not well-developed. On
the outside of the structure, microvilli were observed only on the
surface layer of the cells and distribution of the microvilli was
demonstrated to be dense when observed under higher magnification
(x5,000; Fig. 3B). When cultured
three-dimensionally, certain cells formed gland-like structures and
the lumen were covered by microvilli (Fig. 3C). These structures consisted of
multiple cells attached to each other by a cell adherent apparatus,
such as a desmosome (Fig. 3D). The
scaffold demonstrated bio-adaptability and cultured cells attached
to the scaffold via cell processes and/or microvilli (Fig. 3E).
Discussion
In this study, the established human TK
cholangiocarcinoma cell line was cultivated three-dimensionally.
Morphological observations demonstrated characteristics of
cholangiocarcinoma that are not observed by ordinary
two-dimensional culture. The observations of the morphological
characteristics of the cultured TK cells add to the biochemical
characteristics demonstrated in a previous study (13).
Cholangiocarcinoma is one of the most intractable
human diseases (23). The majority
of cases are inoperable and only 30% of patients qualify for
surgical treatment (24). While
total numbers of patients are small compared with those with more
common carcinomas of the colon or lung, the rising incidence
(25) and high mortality rate
(26) require the development of
more effective therapeutic strategies. The median survival time is
<12 months in unresectable cholangiocarcinoma (12) and even in cases of radical
resection, the recurrence rate was reported to be high (27). In the absence of PSC, curative
surgical resection results in a 5-year survival rate of only 2–43%
(28). Therefore, the development
of an additional cholangiocarcinoma cell line with clearly
understood biochemical and morphological characteristics may be
useful for further understanding of the nature of this
malignancy.
The TK cell line originated from ascites of an
extrahepatic cholangiocarcinoma patient. The carcinoma of the
common bile duct had diffusely invaded the liver, gallbladder and
hepatoduodenal ligament. The CT scan revealed swelling of the 12th
lymph node and blood analysis demonstrated elevated levels of
γ-glutamyl transpeptidase, alkaline phosphatase and liver activator
protein (13). The incidence of
cholangiocarcinoma is relatively high in males, possibly due to a
higher incidence of PSC (29);
however, the patient from which the TK cell line was derived was
female. Approximately 90% of patients diagnosed with
cholangiocarcinoma do not have a recognized risk factor for the
malignancy (30,31) and in the present case, parasitic
infection or exposure to toxic risk factors, such as Thorotrast,
were not reported. As this cell line was established from a
Japanese patient, the model may be useful for studying local
specificity, such as the incidence of cases in the Japanese
printing industrial sector.
Under ordinary culture, the TK cells adhered and
grew on the two-dimensional surface of the culture flask or dish as
previously described (13). While
the majority of cells clearly attached to the culture device,
certain cells were observed to accumulate or float suggesting that
the cells were of ascitic origin. Monolayered TK cells were also
demonstrated to frequently form a gland-like structure. However,
these cells did not exhibit steric cell connections, unlike in the
three-dimensional culture in the present study, which demonstrated
the existence of microvilli in the luminal structures. The
morphology was comparable to patterns of three-dimensional culture
of various glioma cell lines (21). Numerous studies have suggested that
three-dimensional cell-to-cell connections are important for
proliferation, adhesion, migration, invasion and cell phenotype,
and that two-dimensional cells on flat and hard plastic dishes or
flasks are not representative of natural cells in living tissues or
organs (22).
A number of methods have been utilized for
three-dimensional cell culture. Representative examples include
reconstituted basement membrane (commercially known as Matrigel)
(32–33) and spheroids (35,36).
Numerous other devices with a collagen scaffold are also available.
One advantage of device used in the present study is its
applicability as a scaffold to various types of extracellular
matrix. This is due to the mesh being directly produced from a
solution of numerous types of matrix. The extracellular matrix is
also known to be important for morphogenesis as it is involved in
the cell-to-cell connection and anchoring of cells (37). In addition, the culture may be used
in combination with bioreactors and the cultured mesh may be
implanted for in vivo experiments. Moreover, by labeling the
cells with radioisotopes or using the release method, the culture
is valuable for cytolytic or cytotoxic assays (38).
Morphological assessment of the three-dimensional
culture of TK revealed steric attachments to neighboring cells with
cell-adhering apparatus, such as desmosomes and scaffolds. When
cultured three-dimensionally, the cells constructed globoid
structures and their surface was covered with distinctive
microvilli. These structures were demonstrated in gland-like
structures, the lumen of which was also covered with microvilli.
These observations indicate the morphology of TK cells as being
cholangiocarcinomatous in origin. The results demonstrate that TK
cells may be used as a model of a cholangiocarcinoma cell line in
carious aspects of their morphology in addition to their previously
established biochemical properties. Moreover, this culture method
may be useful for elucidating the pathogenesis of
cholangiocarcinoma and may be beneficial in therapeutic
investigations of a number of factors, such as the sensitivity to
anticancer strategies, using morphological observation. The results
of the present study highlight the requirement for further analysis
of the TK cell line and its potential use in the development of
therapeutics.
Acknowledgements
The authors would like to thank Ms. Keiko Tomaru and
Mayumi Nomura (Department of Molecular Cell Biology, Jikei
University School of Medicine) for their assistance with cell
culture and Ms. Hisako Arai, Emi Kikuchi and Mr. Hideki Saito
(Department of Molecular Cell Biology, Jikei University School of
Medicine) for the morphological images.
References
|
1
|
Faris JE and Zhu AX: Targeted therapy for
biliary tract cancers. J Hepatobiliary Pancreat Sci. 19:326–336.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valle J, Wasan H, Palmer DH, et al:
Cisplatin plus gemcitabine versus gemcitabine for biliary tract
cancer. N Engl J Med. 362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gores GJ: Cholangiocarcinoma: current
concepts and insights. Hepatology. 37:961–969. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan SA, Davidson BR, Goldin RD, et al:
Guidelines for the diagnosis and treatment of cholangiocarcinoma:
an update. Gut. 61:1657–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rustagi T and Dasanu CA: Risk factors for
gallbladder cancer and cholangiocarcinoma: similarities,
differences and updates. J Gastrointest Cancer. 43:137–147. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kubo S, Nakanuma Y, Takemura S, et al:
Case series of 17 patients with cholangiocarcinoma among young
adult workers of a printing company in Japan. J Hepatobiliary
Pancreat Sci. Jan 13–2014.(Epub ahead of print).
|
|
7
|
Kumagai S, Kurumatani N, Arimoto A and
Ichihara G: Cholangiocarcinoma among offset colour proof-printing
workers exposed to 1,2-dichloropropane and/or dichloromethane.
Occup Environ Med. 70:508–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng F, Henson R, Lang M, et al:
Involvement of human micro-RNA in growth and response to
chemotherapy in human cholangiocarcinoma cell lines.
Gastroenterology. 130:2113–2129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mott JL, Kobayashi S, Bronk SF and Gores
GJ: mir-29 regulates Mcl-1 protein expression and apoptosis.
Oncogene. 26:6133–6140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pignochino Y, Sarotto I, Peraldo-Neia C,
et al: Targeting EGFR/HER2 pathways enhances the antiproliferative
effect of gemcitabine in biliary tract and gallbladder carcinomas.
BMC Cancer. 10:6312010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito S, Ghosh M, Morita K, Hirano T, Miwa
M and Todoroki T: The genetic differences between gallbladder and
bile duct cancer cell lines. Oncol Rep. 16:949–956. 2006.PubMed/NCBI
|
|
12
|
Selaru FM, Olaru AV, Kan T, et al:
MicroRNA-21 is overexpressed in human cholangiocarcinoma and
regulates programmed cell death 4 and tissue inhibitor of
metalloproteinase 3. Hepatology. 49:1595–1601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe M, Chigusa M, Takahashi H,
Nakamura J, Tanaka H and Ohno T: High level of CA19-9, CA50, and
CEA-producible human cholangiocarcinoma cell line changes in the
secretion ratios in vitro or in vivo. In Vitro Cell Dev Biol Anim.
36:104–109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mizuno S and Glowacki J: Three-dimensional
composite of demineralized bone powder and collagen for in vitro
analysis of chondroinduction of human dermal fibroblasts.
Biomaterials. 17:1819–1825. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizuno S and Glowacki J: Chondroinduction
of human dermal fibroblasts by demineralized bone in
three-dimensional culture. Exp Cell Res. 227:89–97. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yates KE, Mizuno S and Glowacki J: Early
shifts in gene expression during chondroinduction of human dermal
fibroblasts. Exp Cell Res. 265:203–211. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizuno S, Allemann F and Glowacki J:
Effects of medium perfusion on matrix production by bovine
chondrocytes in three-dimensional collagen sponges. J Biomed Mater
Res. 56:368–375. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manome Y, Saeki N, Yoshinaga H, Watanabe M
and Mizuno S: A culture device demonstrates that hydrostatic
pressure increases mRNA of RGS5 in neuroblastoma and CHC1-L in
lymphocytic cells. Cells Tissues Organs. 174:155–161. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizuno S, Tateishi T, Ushida T and
Glowacki J: Hydrostatic fluid pressure enhances matrix synthesis
and accumulation by bovine chondrocytes in three-dimensional
culture. J Cell Physiol. 193:319–327. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizuno S and Glowacki J: Low oxygen
tension enhances chondroinduction by demineralized bone matrix in
human dermal fibroblasts in vitro. Cells Tissues Organs.
180:151–158. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Manome Y, Mizuno S, Akiyama N, et al:
Three-dimensional cell culture of glioma and morphological
comparison of four different human cell lines. Anticancer Res.
30:383–389. 2010.PubMed/NCBI
|
|
22
|
Pampaloni F, Reynaud EG and Stelzer EH:
The third dimension bridges the gap between cell culture and live
tissue. Nat Rev Mol Cell Biol. 8:839–845. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Noel MS and Hezel AF: New and emerging
treatment options for biliary tract cancer. Onco Targets Ther.
6:1545–1552. 2013.PubMed/NCBI
|
|
24
|
de Marsh RW, Alonzo M, Bajaj S, et al:
Comprehensive review of the diagnosis and treatment of biliary
tract cancer 2012. Part I: diagnosis-clinical staging and
pathology. J Surg Oncol. 106:332–338. 2012.PubMed/NCBI
|
|
25
|
Patel T: Cholangiocarcinoma. Nat Clin
Pract Gastroenterol Hepatol. 3:33–42. 2006. View Article : Google Scholar
|
|
26
|
Farley DR, Weaver AL and Nagorney DM:
‘Natural history’ of unresected cholangiocarcinoma: patient outcome
after noncurative intervention. Mayo Clin Proc. 70:425–429.
1995.
|
|
27
|
Jarnagin WR and Shoup M: Surgical
management of cholangiocarcinoma. Semin Liver Dis. 24:189–199.
2004. View Article : Google Scholar
|
|
28
|
Malhi H and Gores GJ: Review article: the
modern diagnosis and therapy of cholangiocarcinoma. Aliment
Pharmacol Ther. 23:1287–1296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Henson DE, Albores-Saavedra J and Corle D:
Carcinoma of the extrahepatic bile ducts. Histologic types, stage
of disease, grade, and survival rates. Cancer. 70:1498–1501. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ben-Menachem T: Risk factors for
cholangiocarcinoma. Eur J Gastroenterol Hepatol. 19:615–617. 2007.
View Article : Google Scholar
|
|
31
|
Lazaridis KN and Gores GJ:
Cholangiocarcinoma. Gastroenterology. 128:1655–1667. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kleinman HK and Jacob K: Invasion assays.
Curr Protoc Cell Biol. Chapter 12(Unit 12.2)2001.
|
|
33
|
Kleinman HK and Martin GR: Matrigel:
basement membrane matrix with biological activity. Semin Cancer
Biol. 15:378–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marques MM, Martins MD and França CM:
Effect of Matrigel on adenoid cystic carcinoma cell line
differentiation. Int J Exp Pathol. 87:405–410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Ridder L, Cornelissen M and de Ridder
D: Autologous spheroid culture: a screening tool for human brain
tumour invasion. Crit Rev Oncol Hematol. 36:107–122.
2000.PubMed/NCBI
|
|
36
|
Kunz-Schughart LA, Kreutz M and Knuechel
R: Multicellular spheroids: a three-dimensional in vitro culture
system to study tumour biology. Int J Exp Pathol. 79:1–23. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kleinman HK, Philp D and Hoffman MP: Role
of the extracellular matrix in morphogenesis. Curr Opin Biotechnol.
14:526–532. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manome Y, Furuhata H, Hashimoto A, et al:
Application of therapeutic insonation to malignant glioma cells and
facilitation by echo-contrast microbubbles of levovist. Anticancer
Res. 29:235–242. 2009.PubMed/NCBI
|