Introduction
Progranulin (PGRN), also known as proepithelin,
acrogranin or prostate cancer cell-derived growth factor, is a
growth modulating factor that has gained attention in the research
of the cell cycle, wound repair, tumorigenesis, inflammation,
neurodevelopment and more recently, in psychosis and
neurodegeneration (1–3). PGRN is also capable of functioning as
a neurotrophic factor, and its mutations in the PGRN gene are
amongst the most common causes of familial frontotemporal lobar
degeneration with TDP-43-positive inclusions (4,5). The
null and missense mutations in PGRN have also been observed in
patients with clinically diagnosed Alzheimer’s disease (6,7), but
have no major role in the genetic etiology of Parkinson’s disease
(8). Previous animal experiments
demonstrated that PGRN was upregulated following experimental
spinal cord injury and downregulated following traumatic brain
injury (9,10). However, few studies have examined
the involvement of PGRN in cerebral ischemia (11–13).
The expression and functions of PGRN in the central
nervous system are complicated. In the embryonic brain, PGRN is
widely expressed and is involved in the sexual differentiation of
the brain (14). In the adult
brain, PGRN expression is restricted to microglia and specific
neuronal populations, including pyramidal neurons in the neocortex
and hippocampus, and Purkinje cells in the cerebellum (15). PGRN has been indicated to function
in regulating neurite outgrowth and enhancing neuronal survival,
indicating that it has neurotrophic activity (16). Hypoxia increases progranulin
expression in fibroblast cultures and neuroblastoma cell lines
(9,17). Little is known about how hypoxia
affects PGRN expression in neuronal cell lines. In the present
study, the expression pattern of PGRN was examined and the
alterations in PGRN expression were investigated in HT22 mouse
hippocampal cells in hypoxic conditions.
Materials and methods
Chemicals
The sheep polyclonal anti-PGRN antibody was
purchased from R&D Systems (Minneapolis, MN, USA). The rabbit
polyclonal anti-doublecortin (Dcx) antibody was supplied from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The mouse monoclonal
anti-βIII-tubulin antibody was supplied by Abcam (Hong Kong,
China). The rabbit polyclonal anti-β-actin antibody was obtained
from Abmart (Shanghai, China). Fluorescence-labeled secondary
antibodies (goat anti-mouse (Alexa Fluor594), goat anti-rabbit
(Alexa Fluor594) and donkey anti-sheep (Alexa Fluor488). were
supplied from Invitrogen Life Technologies (Carlsbad, CA, USA).
Sodium hydrosulfite (Na2S2O4) was
purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The HT22 cells were a gift from Professor Jun Liu
(The Second Affiliated Hospital, Sun Yat-sen University, Guangzhou,
Guangdong, China). The HT22 cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) with 10% heat-inactivated fetal
bovine serum and antibiotics (100 IU/ml penicillin and 100 μg/ml
streptomycin) at 37°C, with 5% CO2, under standard
conditions. Differentiated HT22 cells were grown in NeuroBasal
medium (Millipore, Billerica, MA, USA) containing 1× N2 supplement
for 24 h prior to use.
Induction of a hypoxia model
A hypoxia model was prepared as previously described
(18). Hypoxia was induced in the
HT22 cells by incubation with
Na2S2O4, which is able to
instantly lower the partial pressure of oxygen (PaO2) of
the solutions (19). Briefly, the
cells were rinsed twice with phosphate-buffered saline (PBS)
solution and treated with various concentrations of
Na2S2O4 (1, 2, 5, 10 or 20 mM) for
a fixed time (6 h) or for different lengths of time (2, 4, 6, 8 or
10 h) at a fixed concentration (5 mM) in DMEM medium. Since
Na2S2O4 solutions are acidic, the
pH was adjusted to 7.4 prior to the experiment by adding extra
NaOH, as described previously (20,21).
The control cells were maintained in normal DMEM. The cell
viability was measured by optical microscopy (Leica DMI4000B,
Leica, Wetzlar Germany) and a cell counting kit-8 (CCK-8; Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer’s instructions. Briefly, the cells were plated onto a
96-well plate (5×103 cells/well) and were grown for 24 h
at 37°C prior to being subjected to different treatments. Following
the treatments, CCK-8 solution (10 μl) was added to each well of
the plate. Following incubation for 1 h at 37°C in the dark, the
optical density was measured at 450 nm using an absorbance
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
The percentage viability was calculated with viability in the
untreated control cells considered as 100%.
Immunocytochemistry assay
The HT22 cells were seeded on poly-L-lysine and
laminin pre-treated glass coverslips at a density of
1×105/well. Following differentiation and treatment, the
cells were first washed three times with PBS and fixed in 4%
paraformaldehyde solution [4% paraformaldehyde, 0.1 mmol/l
CaCl2 and 0.1 mmol/l MgCl2 (pH 7.4) in PBS]
for 20 min at room temperature. The cells were then washed three
times with PBS, permeabilized in 0.3% Triton X-100 (Beyotime
Institute of Biotechnology)/PBS for 5 min and washed again three
times with PBS. Next, the cells were blocked for 1 h at room
temperature using 10% normal goat serum to reduce non-specific
binding. The cells were then incubated with different primary
antibodies (PGRN, 1:800; βIII-tubulin, 1:1,000; NeuN, 1:1000; and
Dcx, 1:1,000) in PBS at 4°C overnight. Following sufficient
washing, the cells were incubated with the appropriate
fluorescence-conjugated secondary antibodies for 1 h at 37°C.
Finally, the nuclei were stained with Hoechst 33342, and the
coverslips were mounted on slides with FluorSave Reagent (Beyotime
Institute of Biotechnology). The morphologies were captured under a
fluorescence microscope (BX51; Olympus, Tokyo, Japan).
Western blot analysis
Western blotting was used to semi-quantitatively
detect the expression of PGRN following hypoxia treatment as
aforementioned. The expression of β-actin was considered as an
internal control. For the western blot analysis, the HT22 cells
were seeded onto 60-mm dishes at a density of 2×105
cells/dish. The following day, the cells were treated with
Na2S2O4 at different
concentrations and time intervals. Following the treatment, the
cells were rinsed three times with PBS, lysed with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) and incubated on ice for 30 min. The lysates were
then centrifuged at 12,000 × g for 20 min at 4°C and the
supernatant extracts were quantified for the total protein using a
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology) with bovine serum albumin as the standard. Aliquots
from each protein lysate sample were separated in 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
to a poly-vinylidene fluoride membrane (Millipore, Schwalbach,
Germany). The membrane was blocked with blocking buffer
[Tris-buffered saline (TBS) and 5% skimmed milk] for 1 h at room
temperature and incubated overnight in the presence of the primary
antibodies against PGRN (1:8,000) and β-actin (1:10,000), at 4°C.
The membrane was washed three times with TBS containing 0.05% Tween
20 (TBST) and subsequently reacted with the corresponding secondary
antibody for 1 h at room temperature. Following thorough washing
with TBST, the immunoreactive bands were developed using enhanced
chemiluminescence detection reagents (Millipore). The optical
density of the bands on the films was analyzed using imaging
software (ImageQuant Las4000; GE Healthcare, Pittsburgh, PA,
USA).
Statistical analysis
The results are expressed as the mean ± standard
error of the mean from at least three independent experiments. A
statistical evaluation was performed with a one-way analysis of
variance followed by Duncan’s multiple range test, which was used
to compare the control and treated groups. P<0.05 was used to
indicate a statistically significant difference.
Results
Reproduction of the hypoxia model
As shown in Fig. 1,
there was a notable decrease in the cell number and many cells lost
neurites. Following exposure to
Na2S2O4, the majority of the cells
exhibited a round shape and a few were lysed or replaced by cell
debris. Following incubation of the cells with
Na2S2O4, the cell viability was
determined by CCK-8. The results revealed that the cell activity
following hypoxia treatment was much lower than that of the
untreated cells at all the concentrations and time-points, and the
cell viability was decreased in a statistically significant dose-
and time-dependent manner following hypoxia compared with the
control group (Fig. 1C and D). The
results also indicated that the aforementioned methods may be used
for the successful reproduction of a hypoxia model.
Immunocytochemistry assay results
The immunocytochemical experiments demonstrated that
the HT22 cells and hypoxia-treated HT22 cells were capable of
expressing PGRN (Figs. 2 and
3); the present data revealed that
the HT22 cells had high levels of PGRN in the cytoplasm and the
neurites, particularly around the nuclear periphery. Double
labeling results revealed that PGRN was able to co-localize with
neuronal markers, βIII-tubulin and Dcx (Fig. 2).
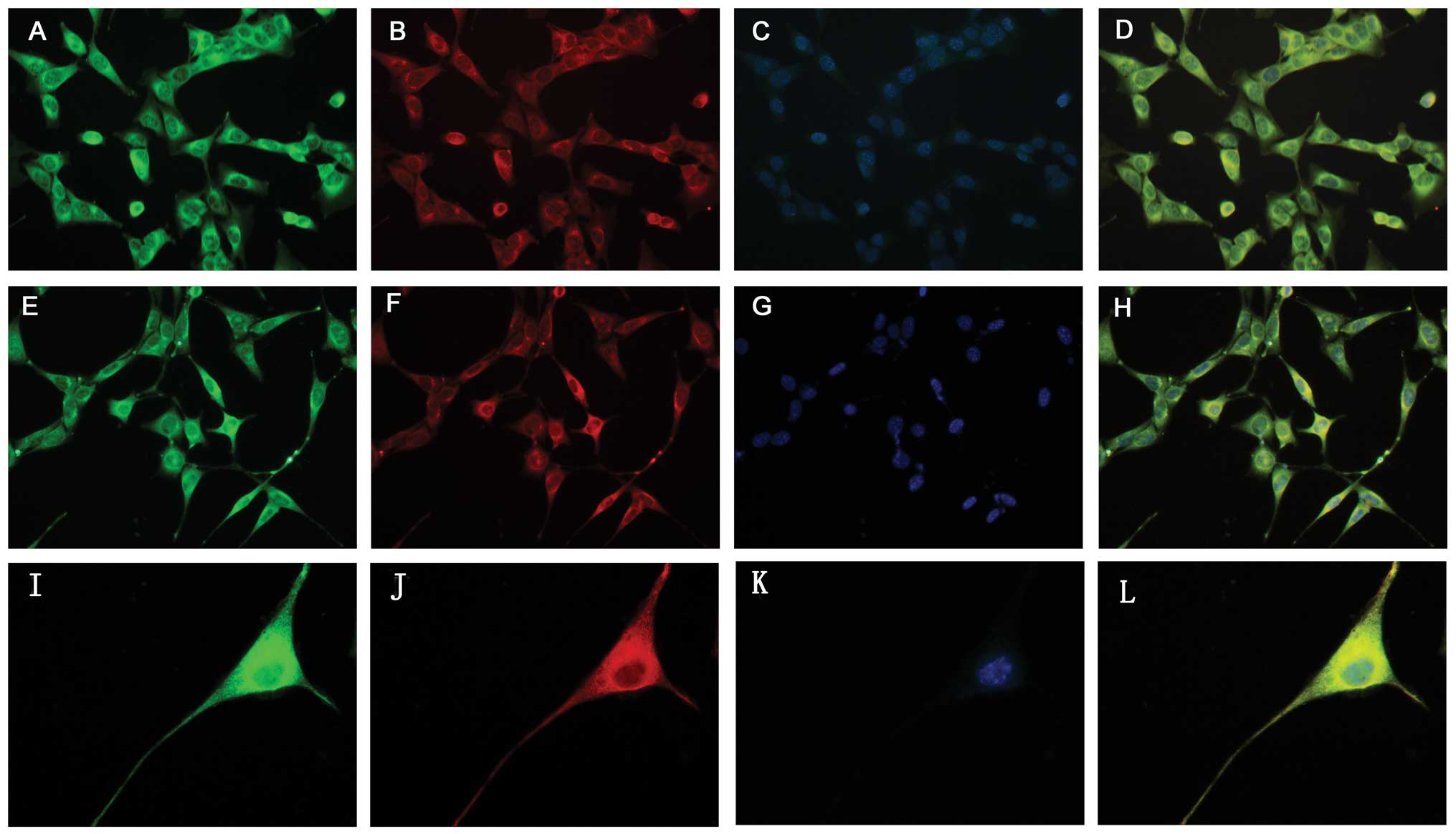 | Figure 2Identification of PGRN expression in
HT22 cells by immunocytochemistry. (A–D) Expression of PGRN in HT22
cells with various neuron markers including βIII-tubulin and Dcx.
PGRN (A, green) was expressed in tubulin+ neurons (B,
red, magnification, ×400). (E–H) PGRN (E, green) was expressed in
Dcx+ neurons (F, red, magnification, ×400). (I–L)
Immunocytochemical results showed that PGRN (I, green) expression
exhibited marked cytoplasm and neurite staining for PGRN,
particularly nuclear periphery in HT22 cells (J, red,
tubulin+, magnification, ×1,000). (C, G, K) Cell nuclei
were visualized after DNA staining with Hoechst 33342. (D, H, L)
Co-expression of PGRN in HT22 cells with different neuron markers.
PGRN, progranulin. |
Western blot analysis
The expression of PGRN was determined by western
blot analysis (Fig. 4A and B).
Western blot analysis revealed that a low concentration (1 mM) for
a long time (6 h) and a high concentration (5 mM) for a short time
(2 h) had no effect on the PGRN levels, whereas higher
concentrations (2–20 mM) of
Na2S2O4 were capable of decreasing
the expression of PGRN in a concentration-dependent manner at 6 h
of incubation (P<0.05). PGRN expression was markedly increased
following exposure to 5 mM Na2S2O4
for 4 h (P<0.05), but at the same concentration of
Na2S2O4 the expression of PGRN was
decreased in a time-dependent manner during 6–10 h of incubation
(P<0.05) (Fig. 4C and D).
 | Figure 4Progranulin (PGRN) expression by
western blot analysis. (A) The PGRN expression levels in the HT22
cells following exposure to various concentrations of sodium
hydrosulfate (Na2S2O4; 1, 2, 5, 10
or 20 mM) for a fixed time (6 h). (B) The PGRN expression level
after the HT22 cells were exposed for different lengths of time (2,
4, 6, 8 or 10 h) to a fixed concentration (5 mM) of
Na2S2O4. (C) Western blot analysis
demonstrating that 1 mM Na2S2O4
had no effect on the PGRN levels at 6 h of incubation, whereas
higher concentrations (2, 5, 10 or 20 mM) of
Na2S2O4 decreased the expression
of PGRN in a concentration-dependent manner (P<0.05 compared
with the control). (D) Following 2 h of incubation with 5 mM
Na2S2O4, no effect was shown on
the PGRN levels. PGRN expression was markedly increased (P<0.05
compared with the control) following 4 h of exposure to 5 mM
Na2S2O4; however, 5 mM
Na2S2O4 decreased the expression
of PGRN in a time-dependent manner during 6–10 h of incubation
(P<0.05 compared with the control). *P<0.05 vs.
control. |
Discussion
Stroke is a major cause of mortality from cerebral
ischemia. Cerebral ischemia leads to severe neuronal cell damage
and induces disruption of neuronal function. Hypoxia may be used as
a representative cell model of cerebral ischemic injury by inducing
a number of spatially and temporally regulated intracellular
responses, ranging from reduced channel activity to altered gene
expression (22,23). To evaluate a possible role for PGRN
in neurodegeneration, the changes in PGRN expression were
investigated in the present study following exposure to hypoxic
stimuli in vitro. The study demonstrated that PGRN was
abundantly expressed in HT22 mouse hippocampal cells. In our
present study, the changes of PGRN expression after hypoxia were
first evaluated.
The present study demonstrated that the cell
survival rate was decreased significantly and that the majority of
the cells lost neurites following treatment with
Na2S2O4 compared with the control.
Different concentrations (1–20 mM) of
Na2S2O4 used for incubation for 6
h and 5 mM Na2S2O4 used for
different lengths of time (2–10 h) significantly affected cell
viability, indicating that Na2S2O4
had remarkable toxicity on the HT22 cells. It has been reported
that PGRN is a neurotropic factor that is able to regulate neurite
outgrowth and enhance neuronal survival (16). Therefore, the loss of neuritis in
HT22 cells may be associated with the low expression of PGRN.
PGRN is expressed in the placenta, epidermis,
microvasculature and brain during murine development (24). In the central nervous system, PGRN
may be expressed in neurons and microglia, but not in astrocytes
and oligodendrocytes. PGRN expression in neurons increases with
cell maturation, whereas expression in microglia depends on the
cells’ state of activation, being specifically upregulated in
microglia in response to excitotoxic injury (25). However, upregulation of PGRN is a
marker of the microglial response that occurs with progression in
motor neuron diseases (26). The
roles of microglial and neuronal progranulin in neurological
disease require addressing. The present study characterized PGRN in
the widely used HT22 hippocampal neuronal cell line, a sub-line
derived from parent HT4 cells that were originally immortalized
from a primary mouse hippocampal neuronal culture (27). An immunochemical characterization
of the HT22 cells revealed that the cells became positively stained
with essential neuron markers, including βIII-tubulin and Dcx. The
data from the present study also indicated that PGRN was mainly
expressed within the cell bodies and in the axons of the HT22
cells. Previous studies have demonstrated that PGRN is expressed in
motor neurons, neuroblastoma cell lines and fibroblasts (9,17,28).
Certain studies demonstrated that extracellular PGRN may protect
cortical neurons from toxic insults induced by glutamate
excitotoxicity and oxidative stress (29). Therefore, the PGRN protein may be
used therapeutically in the field of neurodegenerative
diseases.
Cells of different origins express PGRN in different
ways (28). Previous studies have
demonstrated that hypoxia (1% oxygen) and acidosis may increase
PGRN expression in fibroblasts (17). Hypoxia also induces the
upregulation of progranulin in neuroblastoma cell lines (SK-N-BE,
SK-N-SH and SH-SY5Y), however PGRN is not altered following
hydroperoxide-induced oxidative stress (23). Previous studies have indicated that
PGRN acts as an endogenous neuroprotectant with anti-apoptotic and
anti-inflammatory properties in focal cerebral ischemic mice
(30). PGRN expression is
upregulated following spinal contusion in mice (9), but traumatic brain injury may
increase the risk of frontotemporal dementia through reduced PGRN
(31). To clarify the role of PGRN
in neurodegeneration, the modulation of PGRN expression was
evaluated in the present study following exposure to hypoxia
induced by incubation with
Na2S2O4. The results of the
western blot analysis demonstrated that PGRN expression was
upregulated within 4 h of exposure to 5 mM
Na2S2O4, which indicated that
within a short Na2S2O4 incubation
time, PGRN expression was induced. We hypothesize that PGRN is part
of a neuronal stress response to apoptosis that occurs within a
short time period. The results also demonstrated that PGRN
expression was markedly downregulated in a time-dependent manner
during 6–10 h of incubation, which indicated that with the length
of treated time Na2S2O4 inhibited
PGRN expression. In addition, higher concentrations of
Na2S2O4 (2–20 mM) were able to
significantly downregulate PGRN expression in a
concentration-dependent manner following 6 h of incubation,
indicating that downregulated PGRN expression may be correlated
with a lower cell viability. The changes in PGRN expression induced
by hypoxia may depend on the duration and severity of hypoxia,
therefore the PGRN expression pattern may be associated with
different cell types and their environmental stimuli, including
hypoxia and oxidative stress.
In conclusion, the results of the present study
demonstrated that PGRN is abundantly expressed in HT22 cells. The
data indicated that hypoxia may induce cell death by exposure to
Na2S2O4 in HT22 cells. The effects
of hypoxia on PGRN expression in the HT22 cells were also described
for the first time. This study presented a link between PGRN and
hypoxic injury. However, the potential mechanisms of PGRN in
ischemic brain injury should be investigated further.
Hypoxic injury may significantly affect the
expression of PGRN, thereby providing novel insights with regard to
the role of PGRN in ischemic brain injury.
Acknowledgements
This study was supported by The National Natural
Science Foundation of China for Youth (grant no. 81000562), the
National Innovation Experiment Program for University Students
(grant no. 101055827) and the Medical Scientific Research
Foundation of Guangdong Province, China (grant no. B2010068).
References
|
1
|
De Muynck L and Van Damme P: Cellular
effects of progranulin in health and disease. J Mol Neurosci.
45:549–560. 2011.PubMed/NCBI
|
|
2
|
He Z and Bateman A: Progranulin
(granulin-epithelin precursor, PC-cell-derived growth factor,
acrogranin) mediates tissue repair and tumorigenesis. J Mol Med
(Berl). 10:600–612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Ayadhi LY and Mostafa GA: Low plasma
progranulin levels in children with autism. J Neuroinflammation.
8:1112011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baker M, Mackenzie IR, Pickering-Brown SM,
Gass J, Rademakers R, Lindholm C, et al: Mutations in progranulin
cause tau-negative frontotemporal dementia linked to chromosome 17.
Nature. 442:916–919. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neumann M, Sampathu DM, Kwong LK, Truax
AC, Micsenyi MC, Chou TT, et al: Ubiquitinated TDP-43 in
frontotemporal lobar degeneration and amyotrophic lateral
sclerosis. Science. 314:130–133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sleegers K, Brouwers N and Van Broeckhoven
C: Role of progranulin as a biomarker for Alzheimer’s disease.
Biomark Med. 4:37–50. 2010.
|
|
7
|
Brouwers N, Sleegers K, Engelborghs S,
Maurer-Stroh S, Gijselinck I, van der Zee J, et al: Genetic
variability in progranulin contributes to risk for clinically
diagnosed Alzheimer disease. Neurology. 71:656–664. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nuytemans K, Pals P, Sleegers K,
Engelborghs S, Corsmit E, Peeters K, et al: Progranulin variability
has no major role in Parkinson disease genetic etiology. Neurology.
71:1147–1151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naphade SB, Kigerl KA, Jakeman LB, Kostyk
SK, Popovich PG and Kuret J: Progranulin expression is upregulated
after spinal contusion in mice. Acta Neuropathol. 119:123–133.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matzilevich DA, Rall JM, Moore AN, Grill
RJ and Dash PK: High-density microarray analysis of hippocampal
gene expression following experimental brain injury. J Neurosci
Res. 67:646–663. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Egashira Y, Suzuki Y, Azuma Y, Takagi T,
Mishiro K, Sugitani S, Tsuruma K, Shimazawa M, Yoshimura S,
Kashimata M, Iwama T and Hara H: The growth factor progranulin
attenuates neuronal injury induced by cerebral ischemia-reperfusion
through the suppression of neutrophil recruitment. J
Neuroinflammation. 10:1052013. View Article : Google Scholar
|
|
12
|
Jackman K, Kahles T, Lane D,
Garcia-Bonilla L, Abe T, Capone C, Hochrainer K, Voss H, Zhou P,
Ding A, Anrather J and Iadecola C: Progranulin deficiency promotes
post-ischemic blood-brain barrier disruption. J Neurosci.
33:19579–19589. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tao J, Ji F, Wang F, Liu B and Zhu Y:
Neuroprotective effects of progranulin in ischemic mice. Brain Res.
1436:130–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuwaki T, Asakura R, Suzuki M,
Yamanouchi K and Nishihara M: Age-dependent changes in progranulin
expression in the mouse brain. J Reprod Dev. 57:113–119. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Daniel R, He Z, Carmichael KP, Halper J
and Bateman A: Cellular localization of gene expression for
progranulin. J Histochem Cytochem. 48:999–1009. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van Damme P, Van Hoecke A, Lambrechts D,
Vanacker P, Bogaert E, van Swieten J, et al: Progranulin functions
as a neurotrophic factor to regulate neurite outgrowth and enhance
neuronal survival. J Cell Biol. 181:37–41. 2008.PubMed/NCBI
|
|
17
|
Guerra RR, Kriazhev L, Hernandez-Blazquez
FJ and Bateman A: Progranulin is a stress-response factor in
fibroblasts subjected to hypoxia and acidosis. Growth Factors.
25:280–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang XQ and Eyzaguirre C: Effects of
hypoxia induced by Na2S2O4 on
intracellular calcium and resting potential of mouse glomus cells.
Brain Res. 818:118–126. 1999.PubMed/NCBI
|
|
19
|
Lawson WH Jr, Holland RA and Forster RE:
Effect of temperature on deoxygenation rate of human red cells. J
Appl Physiol. 20:912–918. 1965.PubMed/NCBI
|
|
20
|
Abudara V and Eyzaguirre C: Modulation of
junctional conductance between rat carotid body glomus cells by
hypoxia, cAMP and acidity. Brain Res. 792:114–125. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pang L and Eyzaguirre C: Hypoxia affects
differently the intracellular pH of clustered and isolated glomus
cells of the rat carotid body. Brain Res. 623:349–355. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meloni BP, Meade AJ, Kitikomolsuk D and
Knuckey NW: Characterisation of neuronal cell death in acute and
delayed in vitro ischemia (oxygen-glucose deprivation) models. J
Neurosci Methods. 195:67–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Piscopo P, Rivabene R, Adduci A, Mallozzi
C, Malvezzi-Campeggi L, Crestini A and Confaloni A: Hypoxia induces
up-regulation of progranulin in neuroblastoma cell lines. Neurochem
Int. 57:893–898. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daniel R, Daniels E, He Z and Bateman A:
Progranulin (acrogranin/PC cell-derived growth
factor/granulin-epithelin precursor) is expressed in the placenta,
epidermis, microvasculature, and brain during murine development.
Dev Dyn. 227:593–559. 2003. View Article : Google Scholar
|
|
25
|
Petkau TL, Neal SJ, Orban PC, MacDonald
JL, Hill AM, Lu G, et al: Progranulin expression in the developing
and adult murine brain. J Comp Neurol. 518:3931–3947. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Philips T, De Muynck L, Thu HN, Weynants
B, Vanacker P, Dhondt J, et al: Microglial upregulation of
progranulin as a marker of motor neuron degeneration. J Neuropathol
Exp Neurol. 69:1191–1200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu J, Li L and Suo WZ: HT22 hippocampal
neuronal cell line possesses functional cholinergic properties.
Life Sci. 84:267–271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ryan CL, Baranowski DC, Chitramuthu BP,
Malik S, Li Z, Cao M, et al: Progranulin is expressed within motor
neurons and promotes neuronal cell survival. BMC Neurosci.
10:1302009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu J, Xilouri M, Bruban J, Shioi J, Shao
Z, Papazoglou I, et al: Extracellular progranulin protects cortical
neurons from toxic insults by activating survival signaling.
Neurobiol Aging. 32:2326.e5–2326.e16. 2011.PubMed/NCBI
|
|
30
|
Tao J, Ji F, Wang F, Liu B and Zhu Y:
Neuroprotective effects of progranulin in ischemic mice. Brain Res.
1436:130–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jawaid A, Rademakers R, Kass JS, Kalkonde
Y and Schulz PE: Traumatic brain injury may increase the risk for
frontotemporal dementia through reduced progranulin. Neurodegener
Dis. 6:219–220. 2009. View Article : Google Scholar : PubMed/NCBI
|


















