Introduction
Severe acute pancreatitis (SAP) is a systemic
disease primarily characterized by pancreatic self-necrosis. SAP
involves a complex array of mediators that are capable of
initiating and exacerbating systemic inflammatory response syndrome
and, in severe cases, multiple organ dysfunction syndrome (MODS).
Despite improvements in treatment, SAP remains associated with a
mortality rate of between 15 and 30% (1,2).
Experimental studies have shown that the intestine is one of the
target organs vulnerable to injury and the development of MODS
(3,4). The intestinal mucosal barrier plays
an important role in maintaining intestinal function by preventing
bacteria and toxins in the enteric cavity from passing into the
bloodstream. The infection complications associated with SAP may be
a result of bacterial translocation from the gastrointestinal tract
due to increased intestinal permeability (5,6).
Berberine is the major constituent of the Coptidis
rhizome, which has been widely used as a traditional drug for the
treatment of gastrointestinal disorders in China. Studies have
revealed that berberine has pleiotropic biochemical and
pharmacological effects, including anti-inflammatory,
anti-bacterial, anti-apoptotic and anti-tumor actions (7–9). Of
note, berberine has been demonstrated to attenuate intestinal
barrier dysfunction in certain animal models. For example,
berberine has been found to ameliorate intestinal mucosal barrier
damage in trinitrobenzene-sulfonic acid (TNBS)- and dextran sulfate
sodium-induced experimental colitis in mice (10,11).
Furthermore, berberine has been shown to protect against
lipopolysaccharide- or pro-inflammatory cytokine-induced intestinal
barrier injury in mice, through the regulation of tight junctions
(TJs) and the myosin light chain kinase (MLCK) pathway (12–14).
The effects of berberine on the intestinal mucosa in
SAP-induced intestinal injury are yet to be fully elucidated. In
the present study, it was hypothesized that berberine was likely to
attenuate damage to the intestinal epithelial barrier in a rat
model of SAP. This study aimed to investigate whether berberine,
which protects the intestinal mucosal barrier, is capable of
reducing intestinal permeability and bacterial translocation in
rats with SAP.
Materials and methods
Animals and experimental design
Healthy, adult Sprague Dawley rats weighing between
250 and 280 g were purchased from Dashuo Laboratories (Chengdu,
China). All animals were individually housed in plastic cages
containing wood shavings, and maintained in a
temperature-controlled environment with a 12-h light/dark cycle and
free access to food and water. All animals were allowed to
acclimate to these conditions for 1 week prior to experimental
treatment. All experimental animal procedures were approved by the
Ethics Committee for Animal Experiments of the General Hospital of
Chengdu Military Command (Chengdu, China), and all animal
experiments were performed according to the National Animal Welfare
Law of China.
Thirty-six Sprague Dawley rats were randomly divided
into sham-operated, SAP and SAP plus berberine groups (n=12/group),
which were referred to as the Sham, SAP and SAP+ber groups,
respectively. Berberine (Sigma-Aldrich, St. Louis, MO, USA; 50
mg/kg dissolved in 1 ml normal saline/200 g body weight) or normal
saline (1 ml/200 g body weight) were administered intragastrically
once a day for 5 days, prior to the induction of pancreatitis.
After 12 h of fasting, rats were anesthetized with
an intraperitoneal injection of 50 mg/kg phenobarbital and a
midline laparotomy was performed. In the Sham group, upon opening
the abdominal cavity, the pancreas and duodenum were only moved
prior to the closure of the abdominal wall, using a double layer of
sutures, and the return of the rats to their cages. In the SAP and
SAP+ber groups, subsequent to opening the abdominal cavity,
pancreatitis was induced using retrograde injection of 3% Na
taurocholate (Sigma-Aldrich; 1 ml/kg body weight) into the
pancreatic duct over a period of 2 min. Following the surgery, rats
in all three groups were injected subcutaneously with 5 ml 0.9%
NaCl solution twice a day to supplement the blood volume.
Twenty-four hours after the surgery, rats were re-anesthetized and
laparotomies were performed. Samples of blood and tissue were
obtained immediately.
Histological examination and pathological
scoring
Ileal and pancreatic specimens were fixed in a 4%
paraformaldehyde solution immediately following isolation. Tissues
were then fixed, dehydrated, paraffin-embedded and sectioned,
according to standard methods. The 4-μm-thick sections were stained
with hematoxylin and eosin (H&E), and the pathological changes
in the small intestine and pancreas were examined using an optical
microscope (Olympus, Tokyo, Japan). A pathologist who was blinded
to the grouping scored the ileal specimens according to the method
described by Chiu et al (15,16).
The mucosal damage was graded from zero (normal) to five (severely
damaged).
Serum endotoxin and diamine oxidase (DAO)
analysis
Serum DAO activity and endotoxin levels are used as
indices of small intestinal mucosal mass and integrity. Rat blood
samples were collected as described above, measured and then
centrifuged at 1,509 × g for 10 min at 4°C. The supernatant was
transferred to sterile labeled tubes and stored at −80°C until use.
Serum endotoxin levels were assessed using a Chromogenic Limulus
Amebocyte Lysate assay kit (Shanghai Med & Chem Institute,
Shanghai, China). DAO activity was examined using a commercial kit
(Nanjing Jianchen Co. Ltd., Nanjing, China) according to the
manufacturer’s instructions. In brief, DAO catalyzes the oxidation
of the substrate putrescine, and the product is quantitatively
oxidized by peroxidase in proportion to the quantity of hydrogen
peroxide produced, resulting in the production of o-dianisidine,
which has an absorption maximum at 440 nm.
Bacterial culture of mesenteric lymph
nodes (MLNs)
MLNs were harvested under sterile conditions, prior
to being homogenized, incubated at 37°C in an agitated water bath
for 18 h, plated on MacConkey II agar (Oxoid Ltd., Basingstoke, UK)
and incubated aerobically at 37°C for 24 h. A blinded visual
inspection was then performed. Results were recorded as positive
for growth, without quantification, or no growth. The culture was
considered positive if bacterial growth was observed, and the
incidence of bacterial translocation was calculated by determining
the number of rats with a positive bacterial culture divided by the
total number of rats studied.
Quantitative polymerase chain reaction
(qPCR) analysis
The rats were sacrificed by overdose of anesthesia
and segments of the ileum were isolated, flushed and immediately
placed in RNAiso Plus buffer (Takara Biotechnology Co. Ltd.,
Dalian, China). Total RNA was extracted according to the
manufacturer’s instructions. cDNA was synthesized by reverse
transcription using the PrimeScript RT Reagent kit (Takara
Biotechnology Co. Ltd.) with an oligo (dT) primer. qPCR analysis
was performed using SYBR® Premix EX Taq™ II (Takara
Biotechnology Co. Ltd.) in a Bio-Rad IQ5 system (Bio-Rad, Hercules,
CA, USA). Primers for zonula occludens (ZO)-1, occludin and GAPDH
were synthesized by Takara Biotechnology Co. and had the following
sequences: ZO-1, 5′-GCTCCTCCCACCTCG CACGT-3′ (forward) and
5′-GACCTGCTGGAGCATAGG GCTG-3′ (reverse); occludin
5′-TGGAGTTGCGGGAGAGCG ATC-3′ (forward) and
5′-GGGCAGTCGGGTTGACTCCCA-3′ (reverse); GAPDH,
5′-TCCCTCAAGATTGTCAGCAA-3′ (forward) and 5′-AGATCCACAACGGATACATT-3′
(reverse). The reaction conditions were as follows: Initial
denaturation at 95°C for 1 min, template denaturation at 95°C for
20 sec, annealing at 60°C for 30 sec, extension at 72°C for 1 min
(for a total of 40 cycles) and a final extension at 72°C for 10
min. The cycle threshold (CT) was determined using automatic
baseline calculations. A CT value of >30 was considered
unacceptable. The relative gene expression was calculated using the
2−ΔΔCT method. GAPDH was used as an internal
control.
Western blot analysis
Ilea were immediately placed in cold
radio-immunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) subsequent to flushing. Each sample
was then sonicated on ice three times for 30 sec using a Sonic
Dismembrator (Thermo Fisher Scientific, Waltham, MA USA) and
centrifuged at 14,000 × g for 15 min. Supernatants were then
collected and the protein concentrations were determined according
to the Bradford method using bicinchoninic acid assay reagent
(Beyotime Institute of Biotechnology). Samples containing 20 μg
protein were loaded onto SDS-PAGE gels, electrophoresed using a
Bio-Rad mini gel system (Bio-Rad) and then transferred to a
polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA).
The membranes were blocked using 5% bovine serum albumin in 50 mM
Tris-HCl (pH 7.5), 140 mM NaCl and 0.1% Tween and incubated at 4°C
overnight with primary antibodies against ZO-1 (1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), occludin (1:1,000; Santa
Cruz Biotechnology, Inc.), MLC (1:1,000; Epitomics Inc.,
Burlingame, CA, USA) or phosphorylated MLC (pMLC; 1:1,000; Abcam
PLC, Cambridge, MA, USA). Membranes were then washed three times
and incubated with horseradish peroxidase-conjugated secondary
antibodies. Membrane imaging was performed using an enhanced
chemiluminesce detection system (Millipore) according to the
manufacturer’s instructions.
Statistical analysis
Kruskal-Wallis and Mann-Whitney U tests were
performed for comparisons among groups. The incidence of
gram-negative bacterial translocation to the MLNs was assessed
using a χ2 test. A value of P<0.05 was considered to
indicate a statistically significant difference. Statistical
analyses were performed using SPSS 18.0 statistical software (SPSS
Inc., Chicago, IL, USA).
Results
Pancreatic pathology
To determine whether SAP rat models were
successfully established, sections of pancreatic tissue were
stained with H&E and examined using light microscopy (Fig. 1). Pancreatic tissues from the Sham
group exhibited a normal macroscopic and histological appearance.
By contrast, the H&E-stained sections from the SAP and SAP+ber
groups revealed atypical pancreatic architectures, with marked
interstitial edema, acinar cell necrosis, leukocyte infiltration
and scattered hemorrhage. These findings suggest that SAP rat
models were successfully established; however, berberine had no
significant effect on the histological changes in the pancreas.
Evaluation of intestinal barrier
function
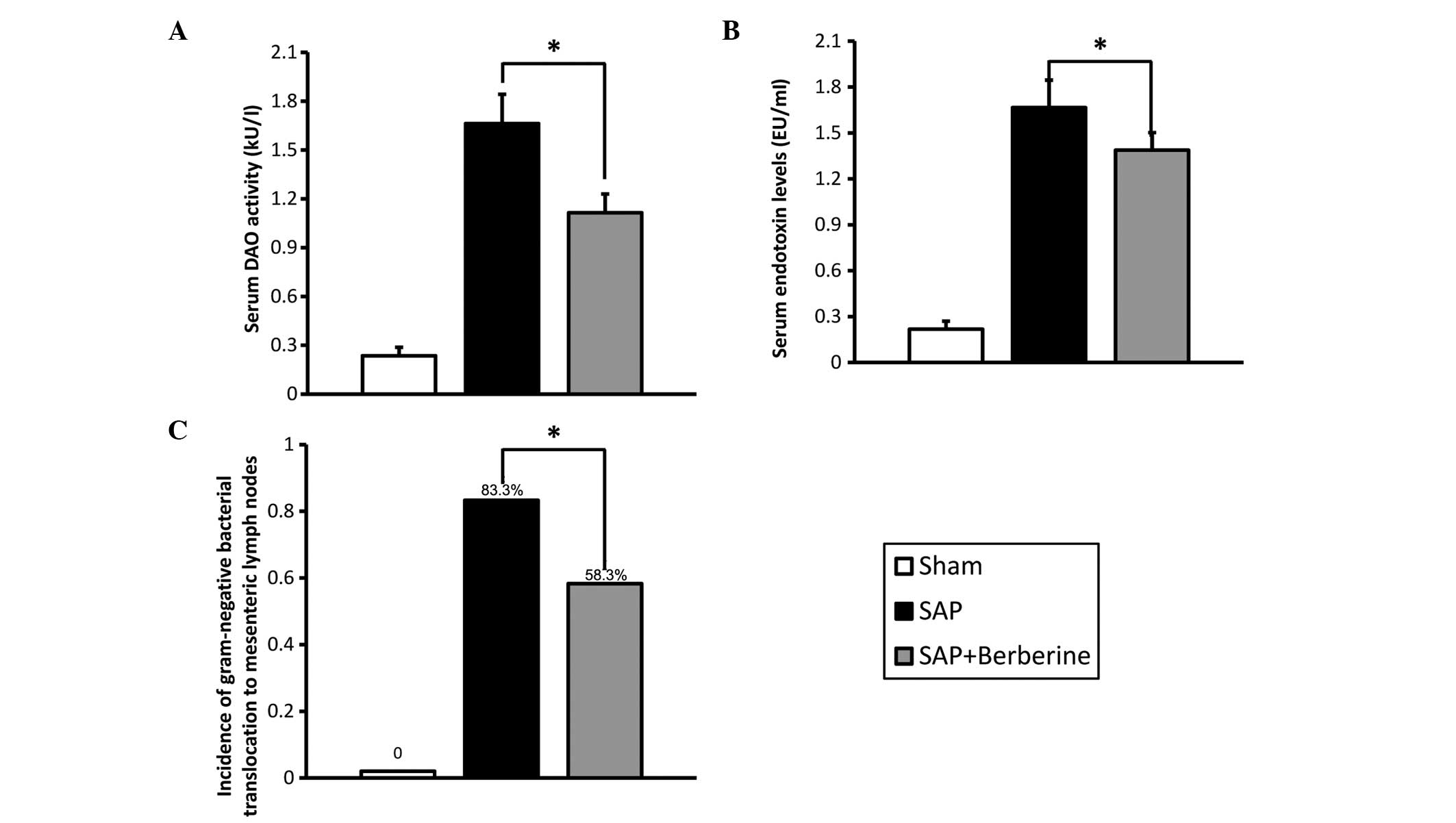
To identify the role of berberine in the maintenance
of intestinal barrier function, the intestinal membrane
permeability was evaluated by assaying serum DAO activity and
endotoxin concentration (Fig. 2A and
B). In the Sham group, the baseline levels of serum DAO
activity and endotoxin concentration 24 h after the sham surgery
were 0.235±0.053 kU/l and 0.218±0.086 EU/ml, respectively. Serum
DAO activity and endotoxin levels were observed to be higher in the
SAP group than those in the Sham group (P<0.05). Furthermore,
berberine pretreatment was observed to significantly decrease serum
DAO activity (33.0%) and endotoxin levels (16.7%) compared with
levels in the SAP group (P<0.05).
To further assess the effect of berberine on
intestinal barrier function, the incidence of bacterial
translocation was evaluated (Fig.
2C). No bacterial translocation was observed in the MLNs from
the Sham group; however, the incidence of bacterial translocation
to the MLNs in the SAP group 24 h after the retrograde injection of
3% Na-taurocholate was observed to be 83.3%. Berberine treatment
was found to reduce the rate of bacterial translocation to 58.3%,
which was significantly lower than that observed in the SAP group
(P<0.05). Serum DAO activity and endotoxin levels were also
observed to decrease with berberine treatment.
These results indicate that berberine treatment may
ameliorate the intestinal mucosal barrier damage associated with
SAP induced by the retrograde injection of Na-taurocholate.
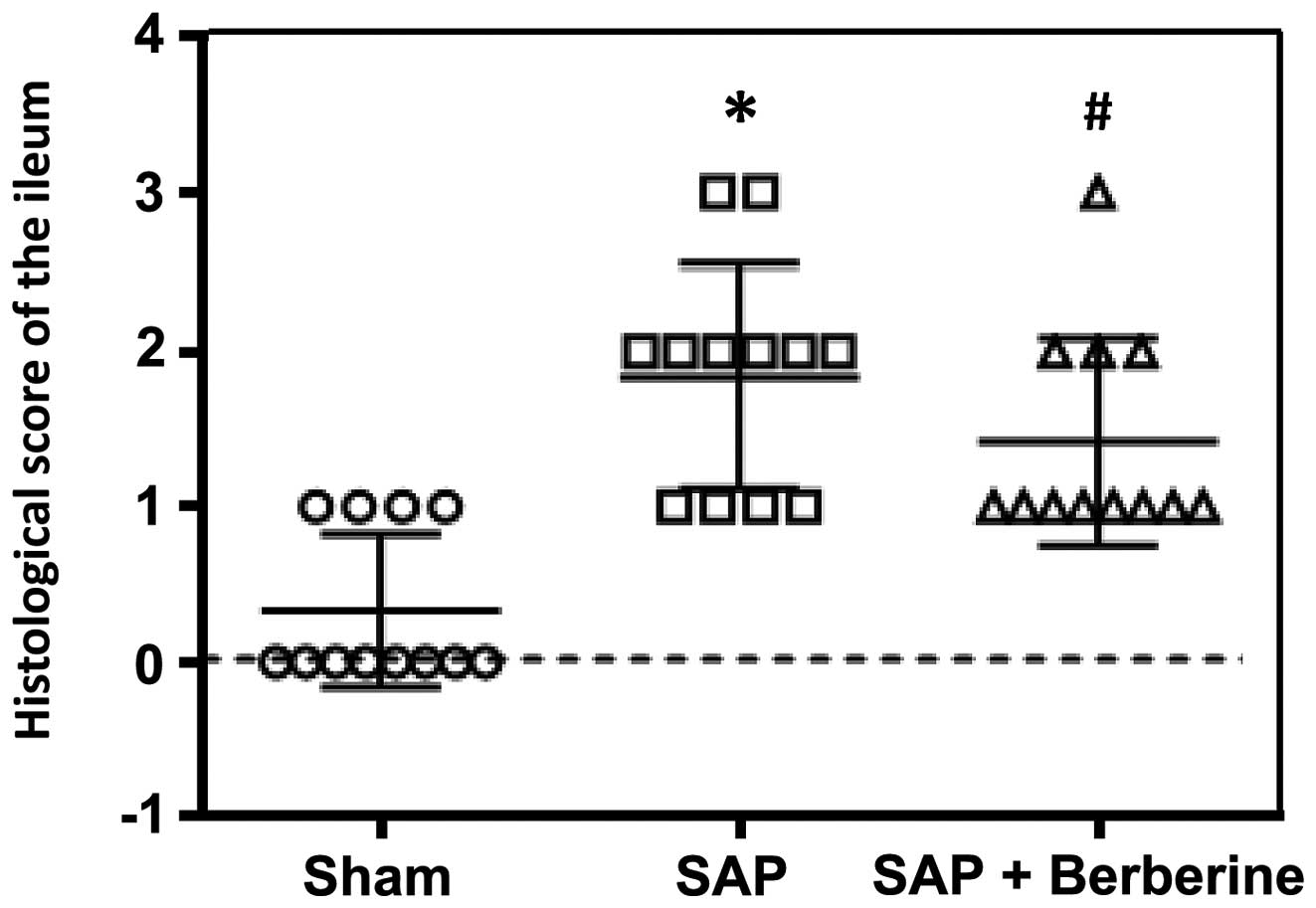
Pathological scoring of the
intestine
Animals were sacrificed 24 h after surgery.
H&E-stained ileal sections were examined using an optical
microscope and scored by a pathologist who was blinded to the
grouping, according to the aforementioned method. The results of
the pathological scoring are shown in Fig. 3. In the Sham group, the epithelial
cells were observed to be closely arranged in a regular fashion.
Twenty-four hours after the induction of SAP, the ileal mucosa was
injured. Vacuolated epithelial cells, shortened villi and
infiltrating lymphocytes were observed. The mean pathological score
of the ilea in the SAP group increased to 1.833, compared with
0.333 in the Sham group (P<0.05). Compared with the rats in the
SAP group, berberine administration was observed to have a
protective effect against ileal mucosal injury, and the ilea of
rats in the SAP+ber group exhibited relatively normal villi and
mucosal integrity. The pathological scoring also demonstrated that
berberine treatment attenuated the pancreatitis-induced mucosal
injury compared with that observed in the SAP group (1.417 vs.
1.833 for the SAP+ber and SAP groups, respectively; P<0.05).
TJ alteration and MLC
phosphorylation
TJs have a critical role in maintaining the function
of the intestinal barrier (17,18).
The impairment of intestinal barrier function is directly
associated with the aberrant expression of TJ proteins (19). qPCR and western blot analyses were
performed to detect the expression of the TJ proteins ZO-1 and
occludin. Berberine was not observed to influence ZO-1 and occludin
mRNA expression in the SAP-induced rats (Fig. 4A). Furthermore, occludin protein
expression did not differ significantly among the three groups;
however, ZO-1 protein expression was observed to be significantly
higher in the SAP+ber group than that in the SAP group (Fig. 4B and C). Overall, these results
suggest that berberine exerts few effects on TJ proteins in the
ilea of SAP rats.
It is well established that MLCK-mediated
phosphorylation of MLC has a significant role in the physiological
and pathophysiological regulation of intestinal epithelial TJs and
paracellular leak pathways (20,21).
In the present study, although berberine was observed to have
little effect on ZO-1 and occludin mRNA and protein expression,
berberine was hypothesized to ameliorate SAP-induced barrier
dysfunction through MLC phosphorylation, which, to the best of our
knowledge, has not been previously investigated. As shown in
Fig. 5, western blot analysis of
MLC and pMLC revealed that berberine treatment had no significant
effect on total MLC expression in SAP-induced rats. However,
significantly higher pMLC levels were observed in rats in the SAP
group compared with those in the Sham group (6.175-fold increase,
P<0.05). This increase was significantly attenuated with
berberine treatment, with pMLC levels in the SAP+ber group reduced
to 0.349-fold those in the SAP group (P<0.05). These data
suggest that the inhibition of SAP-induced MLC phosphorylation may
be one of the mechanisms responsible for the beneficial effect of
berberine on intestinal barrier function during SAP-induced
damage.
Discussion
The present study investigated the effect of
berberine on intestinal epithelial structure and barrier function
in a rat model of SAP. Berberine was found to significantly prevent
the loss of epithelial barrier function induced by pancreatitis in
rat models of SAP, as evidenced by the reduction in permeability
and bacterial translocation upon berberine administration.
Furthermore, berberine was observed to inhibit the SAP-induced
upregulation of pMLC levels, while the TJ proteins ZO-1 and
occludin remained unaffected.
Although the etiology and pathogenesis of
pancreatitis-induced intestinal barrier dysfunction are yet to be
fully elucidated, it is well established that this dysfunction is
characterized by the overproduction of various pro-inflammatory
cytokines within the mucosa and the disruption of epithelial
barrier function. SAP development is associated with the premature
activation of pancreatic enzymes in the acini and an excessive
inflammatory response, stimulating a cascade reaction (22,23).
The subsequent microcirculation disturbance-induced ischemia,
hypoxia and ischemia-reperfusion injury have important roles in the
development of extrapancreatic organ injury in SAP (24,25).
The intestinal mucosal barrier plays a significant role in
maintaining intestinal function and preventing the transfer of
bacteria and toxins from the enteric cavity into the blood
circulation. The infection complications caused by SAP may be a
result of bacterial translocation from the gastrointestinal tract,
and are associated with treatment failure (26,27).
Berberine has been used as a remedy for
gastrointestinal diseases, particularly diarrhea, for centuries.
Recent studies have revealed that berberine has pleiotropic
biochemical and pharmacological effects, including
anti-inflammatory, anti-bacterial, anti-apoptotic and anti-tumor
actions (8,9). In addition, berberine has been
demonstrated to attenuate intestinal barrier dysfunction in certain
animal models. Lee et al (11) reported that berberine may improve
TNBS-induced colitis by suppressing interleukin-8 expression.
Furthermore, berberine has been shown to exhibit a protective
effect against epithelial and endothelial barrier function in
vitro (28,29).
The effect of berberine on the intestinal mucosa in
SAP-induced intestinal injury is yet to be fully elucidated.
Therefore, the present study aimed to assess the effect of
berberine on intestinal membrane permeability and bacterial
translocation by measuring serum DAO activity, endotoxin levels and
the incidence of bacterial translocation to the MLNs, all of which
are techniques that have been widely used to assess intestinal
barrier function. The results of this study demonstrate that
berberine is capable of preventing the intestinal barrier
dysfunction caused by SAP in vivo. However, the mortality
rate, which may be valuable for the evaluation of the effect of
berberine on pancreatitis, was not recorded due to the small sample
size.
Although the molecular mechanism by which berberine
attenuates SAP-induced intestinal barrier dysfunction is yet to be
elucidated, a number of reports have provided novel insights into
potential signaling pathways. Several investigations have shown
that berberine attenuates pro-inflammatory cytokine-induced
intestinal barrier dysfunction by ameliorating the effects on TJs
in vitro and in vivo (14,29,30).
The opening of TJs is primarily dependent on the composition and
organization of TJ proteins, particularly occludin, ZO-1 and
claudins, which are responsible for barrier function. Occludin is a
major TJ component which, upon phosphorylation, becomes
redistributed, resulting in a significant decrease in
transepithelial electrical resistance, indicating the occurrence of
intestinal barrier injury (31).
In a glioma cell line, berberine has been reported to decrease the
activation of protein kinase C-α, which catalyzes occludin
phosphorylation, leading to cytoskeletal rearrangements (32). However, in the present study,
berberine was observed to have little effect on the TJ proteins
ZO-1 and occludin.
MLCK and nuclear factor κ-light-chain-enhancer of
activated B cells (NF-κB) have been reported to have central roles
in the alteration of intestinal epithelial TJs. TJ dysregulation
induced by MLCK activation has been found to cause
apoptosis-mediated barrier loss and experimental colitis (33). Furthermore, additional studies have
shown that MLCK-dependent TJ regulation has an important role in
thermal injury-induced intestinal barrier dysfunction (34,35).
In addition, berberine has been observed to ameliorate intestinal
epithelial TJ damage and to downregulate MLCK pathways in an
endotoxinemia model (14).
Therefore, the present study assessed the MLCK pathway in rat
models of SAP. The results of this study demonstrate that berberine
is capable of suppressing SAP-induced pMLC upregulation, which may
represent the molecular mechanism responsible for the protective
effect of berberine against SAP-induced intestinal epithelial
barrier dysfunction.
In conclusion, the present study showed that
berberine may attenuate the intestinal barrier dysfunction induced
by SAP in vivo. To the best of our knowledge, this is the
first study to demonstrate that berberine is capable of inhibiting
the SAP-induced upregulation of MLC phosphorylation.
References
|
1
|
Morel DR, Frossard JL, Cikirikcioglu B,
Tapponnier M and Pastor CM: Time course of lung injury in rat acute
pancreatitis. Intensive Care Med. 32:1872–1880. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lankisch PG and Lerch MM: Pharmacological
prevention and treatment of acute pancreatitis: where are we now?
Dig Dis. 24:148–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang XP, Zhang J, Song QL and Chen HQ:
Mechanism of acute pancreatitis complicated with injury of
intestinal mucosa barrier. J Zhejiang Univ Sci B. 8:888–895. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gurleyik E, Coskun O, Ustundag N and
Ozturk E: Prostaglandin E1 maintains structural integrity of
intestinal mucosa and prevents bacterial translocation during
experimental obstructive jaundice. J Invest Surg. 19:283–289. 2006.
View Article : Google Scholar
|
|
5
|
Suzuki T: Regulation of intestinal
epithelial permeability by tight junctions. Cell Mol Life Sci.
70:631–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Camilleri M, Madsen K, Spiller R,
Greenwood-Van Meerveld B and Verne GN: Intestinal barrier function
in health and gastrointestinal disease. Neurogastroenterol Motil.
24:503–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boberek JM, Stach J and Good L: Genetic
evidence for inhibition of bacterial division protein FtsZ by
berberine. PLoS One. 5:e137452010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saha P, Bhattacharjee S, Sarkar A, Manna
A, Majumder S and Chatterjee M: Berberine chloride mediates its
anti-leishmanial activity via differential regulation of the
mitogen activated protein kinase pathway in macrophages. PLoS One.
6:e184672011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Liu L, Shi Y, et al: Berberine
induces caspase-independent cell death in colon tumor cells through
activation of apoptosis-inducing factor. PLoS One. 7:e364182012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan F, Wang L, Shi Y, et al: Berberine
promotes recovery of colitis and inhibits inflammatory responses in
colonic macrophages and epithelial cells in DSS-treated mice. Am J
Physiol Gastrointest Liver Physiol. 302:G504–G514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee IA, Hyun YJ and Kim DH: Berberine
ameliorates TNBS-induced colitis by inhibiting lipid peroxidation,
enterobacterial growth and NF-κB activation. Eur J Pharmacol.
648:162–170. 2010.PubMed/NCBI
|
|
12
|
Feng AW, Gao W, Zhou GR, et al: Berberine
ameliorates COX-2 expression in rat small intestinal mucosa
partially through PPARγ pathway during acute endotoxemia. Int
Immunopharmacol. 12:182–188. 2012.PubMed/NCBI
|
|
13
|
Li HM, Wang YY, Wang HD, et al: Berberine
protects against lipopolysaccharide-induced intestinal injury in
mice via alpha 2 adrenoceptor-independent mechanisms. Acta
Pharmacol Sin. 32:1364–1372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu L, Li N, Gong J, Li Q, Zhu W and Li J:
Berberine ameliorates intestinal epithelial tight-junction damage
and down-regulates myosin light chain kinase pathways in a mouse
model of endotoxinemia. J Infect Dis. 203:1602–1612. 2011.
View Article : Google Scholar
|
|
15
|
Chiu CJ, McArdle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesion in low-flow states. I A
morphological, hemodynamic, and metabolic reappraisal. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiu CJ, Scott HJ and Gurd FN: Intestinal
mucosal lesion in low-flow states. II The protective effect of
intraluminal glucose as energy substrate. Arch Surg. 101:484–488.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Samak G, Suzuki T, Bhargava A and Rao RK:
c-Jun NH2-terminal kinase-2 mediates osmotic stress-induced tight
junction disruption in the intestinal epithelium. Am J Physiol
Gastrointest Liver Physiol. 299:G572–G584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strauman MC, Harper JM, Harrington SM,
Boll EJ and Nataro JP: Enteroaggregative Escherichia coli
disrupts epithelial cell tight junctions. Infect Immun.
78:4958–4964. 2010.PubMed/NCBI
|
|
19
|
Anderson RC, Cookson AL, McNabb WC, et al:
Lactobacillus plantarum MB452 enhances the function of the
intestinal barrier by increasing the expression levels of genes
involved in tight junction formation. BMC Microbiol. 10:3162010.
View Article : Google Scholar
|
|
20
|
Turner JR: Intestinal mucosal barrier
function in health and disease. Nat Rev Immunol. 9:799–809. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen L, Weber CR, Raleigh DR, Yu D and
Turner JR: Tight junction pore and leak pathways: a dynamic duo.
Annu Rev Physiol. 73:283–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kahl S and Mayer JM: Update on
experimental acute pancreatitis. Minerva Gastroenterol Dietol.
58:355–363. 2012.
|
|
23
|
Lerch MM and Gorelick FS: Models of acute
and chronic pancreatitis. Gastroenterology. 144:1180–1193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mayerle J, Dummer A, Sendler M, et al:
Differential roles of inflammatory cells in pancreatitis. J
Gastroenterol Hepatol. 27(Suppl 2): 47–51. 2012. View Article : Google Scholar
|
|
25
|
Wan MH, Huang W, Latawiec D, et al: Review
of experimental animal models of biliary acute pancreatitis and
recent advances in basic research. HPB (Oxford). 14:73–81. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jha RK, Yong MQ and Chen SH: The
protective effect of resveratrol on the intestinal mucosal barrier
in rats with severe acute pancreatitis. Med Sci Monit.
14:BR14–BR19. 2008.PubMed/NCBI
|
|
27
|
Wang X, Wang B, Wu J and Wang G:
Beneficial effects of growth hormone on bacterial translocation
during the course of acute necrotizing pancreatitis in rats.
Pancreas. 23:148–156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amasheh M, Fromm A, Krug SM, et al:
TNFalpha-induced and berberine-antagonized tight junction barrier
impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell
Sci. 123:4145–4155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li N, Gu L, Qu L, et al: Berberine
attenuates pro-inflammatory cytokine-induced tight junction
disruption in an in vitro model of intestinal epithelial cells. Eur
J Pharm Sci. 40:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gu L, Li N, Li Q, et al: The effect of
berberine in vitro on tight junctions in human Caco-2 intestinal
epithelial cells. Fitoterapia. 80:241–248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marano CW, Garulacan LA, Ginanni N and
Mullin JM: Phorbol ester treatment increases paracellular
permeability across IEC-18 gastrointestinal epithelium in vitro.
Digest Dis Sci. 46:1490–1499. 2001. View Article : Google Scholar
|
|
32
|
Lin TH, Kuo HC, Chou FP and Lu FJ:
Berberine enhances inhibition of glioma tumor cell migration and
invasiveness mediated by arsenic trioxide. BMC Cancer. 8:582008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Su L, Nalle SC, Shen L, et al: TNFR2
activates MLCK-dependent tight junction dysregulation to cause
apoptosis-mediated barrier loss and experimental colitis.
Gastroenterology. 145:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo M, Yuan SY, Frederich BJ, et al: Role
of non-muscle myosin light chain kinase in neutrophil-mediated
intestinal barrier dysfunction during thermal injury. Shock.
38:436–443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zahs A, Bird MD, Ramirez L, Turner JR,
Choudhry MA and Kovacs EJ: Inhibition of long myosin light-chain
kinase activation alleviates intestinal damage after binge ethanol
exposure and burn injury. Am J Physiol Gastrointest Liver Physiol.
303:G705–G712. 2012. View Article : Google Scholar : PubMed/NCBI
|