Introduction
Adrenocorticotrophic hormone (ACTH)-producing
adenoma, accounting for 10–15% of pituitary tumors, result in
Cushing’s disease due to excessive secretion of ACTH and,
consecutively, cortisol (1).
Cushing’s disease possesses a high morbidity and mortality rate if
managed inadequately (1–3). Pituitary surgery, particularly
transphenoidal microsurgery, remains the first choice amongst
therapies. However, complication of hypopituitarism occurs in ~80%
of all cases (4) and the risk of
recurrence reaches 20–25% at 10 years following surgery (5). Radiation therapy, which is often used
in cases of persistence or recurrence, is limited by the risk of
necrosis in the temporal lobe of the brain and high occurrence of
long-term hypopituitarism (6).
Medical therapy with classic adrenal-directed drugs
(steroidogenesis inhibitors) may be highly effective but with
severe side-effects, and these agents cannot inhibit underlying
tumors or restore normal secretory dynamics (7–9).
Pituitary-directed drugs, including somatostatin analogs and
dopamine agonists, demonstrate certain effects on ACTH secretion in
Cushing’s disease; however, further long-term trials are required
to determine the safety and efficacy (10–12).
Other agents, such as peroxisome proliferator-activated receptor γ
(PPAR-γ) agonists for the treatment of Cushing’s disease, are
experimental and not currently available in human clinical trials
(13,14). As noted, the drugs that directly
target the pituitary tumor growth and ACTH secretion are urgently
required and would be an attractive option in the medical
management of ACTH-producing pituitary adenoma.
Ursolic acid (UA) is a triterpenoid compound widely
distributed in food, medicinal herbs and other plants (15). UA has various pharmacological
properties, including anti-oxidant, anti-inflammatory and
anti-hyperlipidemic activities (16,17).
In addition, UA has clinical applications for treating tumor
patients as a promising antitumor agent (18). It has been proven that UA inhibited
tumorigenesis and progression in a broad spectrum of tumors,
including hepatocellular carcinoma, melanoma, and prostate,
colorectal, breast and bladder cancer (19–26).
The reported molecular mechanisms involved in UA-induced apoptosis
include inhibition of nuclear factor κ-light-chain-enhancer of
activated B cells (NF-κB) activity and protein tyrosine kinase
(27,28). However, there is no report in the
literature on the effect of UA on ACTH-producing pituitary adenoma
and its potential molecular mechanisms.
In the present study, the effect of UA on apoptosis
and ACTH secretion in AtT20 cells was investigated. The potential
underlying molecular mechanisms of action, including endogenous,
exogenous signaling pathways and the JNK pathway, were also
explored.
Materials and methods
Cell culture and chemicals
AtT20 cells (mouse corticotroph tumor cell line)
were provided by Shanghai Institute of Materia Medica (Shanghai,
China) (29). AtT20 cells were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS) and 1% penicillin-streptomycin (Invitrogen Life
Technologies, Grand Island, NY, USA) at 37°C in 5% CO2.
UA purchased from Sigma-Aldrich (St. Louis, MO, USA) was dissolved
in dimethyl sulfoxide (DMSO; Sigma-Aldrich) as a 100 mM stock
solution and stored at −20°C. The cells were pretreated with JNK
inhibitor SP600125 (Calbiochem, La Jolla, CA, USA), which was
dissolved in DMSO 1 h prior to UA treatment.
2-(2-Methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
[Cell Counting kit-8 (CCK-8)] assay
Subsequent to being harvested and centrifuged, the
AtT20 cells were resuspended in RPMI-1640 medium with 1% FBS and
seeded in a 96-well plate at a density of 1 × 105/well.
The cells were treated with different concentrations of UA for 24,
48 and 72 h, respectively, while only DMSO was added to the control
wells. A total of 10 μl CCK-8 (Dojindo, Kumamoto, Japan) was added
to each well and the microplate was incubated for 6 h at 37°C with
5% CO2. The optical density (OD) at 450 nm was read with
a 96-well plate reader (Bio-Rad, Reinach, Switzerland). Experiments
were conducted with five replicates.
Annexin V-fluorescein/propidium iodide
(PI) flow cytometric analysis
AtT20 cells were collected, centrifuged and washed
with phosphate-buffered saline following treatment with the
indicated amount of UA for 24 h with or without pretreatment of
SP600125 (JNK inhibitor; Sigma-Aldrich) for 1 h followed by
staining with Annexin V-fluorscein (FLOUS) Staining kit and PI
(Roche Diagnostics, Mannheim, Germany) for 15 min at room
temperature. For each example, 20,000 cells were analyzed on a flow
cytometer (FACStar, BD Biosciences, Franklin Lanes, NJ, USA).
Assessment of caspase-3/7, -8 and -9
activity
The caspase-3/7, -8 and -9 activities were measured
by Caspase-Glo luminescent-based assays (Caspase-Glo kit; Promega,
Madison, WI, USA). The cells were seeded with a total of
105 cells/well in a 96-well plate. Following treatment
with UA (at various concentrations) in combination with SP600125
for 24 h, 100 μl of the Caspase-Glo-3/7, -8, -9 reagents were added
to each well. The mixtures were incubated for 1 h and then
transferred to a fluorescence microtiter plate. Quantification of
luminescence was measured by a luminometer. To normalize the
fluorescence intensity, the optical density values of the CCK-8
assay were measured by a Infinite F500 microplate reader (Tecan,
Männedorf, Switzerland) as the viable cell number.
quantitative polymerase chain reaction
(qPCR) analysis
The total RNA was extracted from cells with
indicated treatments. A volume of 5 μl cDNA (ReverTra Ace qPCR RT
kit, Toyobo, Osaka, Japan) was amplified in a final volume of 0.4
μM of each primer and 25 μl SYBR Green Real-time PCR Master Mix
(Toyobo) in a final volume of 50 μl for 40 cycles (ABI Prism 7500
Sequence Detection system; Applied Biosystems, Carlsbad, CA, USA).
The primers were as follows: Pro-opio melanocortin (POMC) forward,
5′-AACCTGCTGGCTTGCATCCG-3′ and reverse, 5′-GGGC
TGTTCATCTCCGTTGCCT-3′. β-actin, forward, 5′-TGGAATC
CTGTGGCATCCATGAAAC-3′ and reverse, 5′-TAAAACGCAG
CTCAGTAACAGTCC-3′.
Assessment of ACTH by ELISA
The AtT20 cells were harvested, seeded in 24-well
plates at a density of 2×106/500 μl/well and then added
to different concentrations of UA for 48 h. The cell culture
supernatants were collected and centrifuged. ACTH was assayed using
a mouse ACTH ELISA kit (Uscn Life Science Inc., Wuhan, China)
following the manufacturer’s instructions.
Western blot analysis
Protein samples were extracted in
radio-immunoprecipitation assay lysis buffer containing protease
inhibitors and phosphatase inhibitors (Thermo Fisher Scientific,
Waltham, MA, USA). Mitochondria were extracted using a
mitochondrial isolation kit (Thermo Fisher Scientific). Following
incubation for 10–30 min at 4°C, the supernatant was collected by
centrifugation (10,000 × g, 15 min, 4°C). The cell lysates (20–40
μg) which were determined using the bicinchoninic acid protein
assay kit (Pierce Chemical Co., Rockford, IL, USA) proteins were
separated by 12% SDS-PAGE and electrophoretically transferred onto
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). Membranes were washed twice in Tris-buffered saline and Tween
20 (TBST) and incubated with blocking buffer (5% skimmed milk in
TBST) for 60 min at room temperature. Next, the membranes were
washed three times and incubated overnight at 4°C with primary
antibodies. The membranes were incubated with secondary antibodies
(1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA) for 1
h at room temperature. Following washing three times, the
antibodies bound to the proteins were detected using enhanced
chemiluminescence (Invitrogen Life Technologies). The following
primary antibodies were used: anti-ACTH antibody (Ab) (1:1,000;
Abcam); anti-phospho-p42/44 mitogen-activated protein kinase (MAPK)
(Thr202/Tyr204) Ab, anti-p42/44MAPK Ab, anti-phospho-p38
(Thr180/Tyr182) Ab, anti-p38 Ab, anti-phospho-JNK (Thr183/Tyr185)
Ab, anti-JNK Ab, anti-B cell lymphoma 2 (Bcl-2) Ab,
anti-Bcl-2-associated X (Bax) Ab, anti-β-actin Ab (all 1:1,000;
Cell Signaling Technology, Inc.); anti-phospho-Bcl-2 (Ser70) Ab
(1:1,000; Sigma-Aldrich); anti-cytochrome c Ab (1:1,000;
Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA). First, the
phospho-specific MAPK were detected. Next, the membranes were
stripped and re-probed with anti-JNK, anti-extracellular
signal-regulated protein kinases (ERK) and anti-p38 antibodies. The
densities of phosphorylated MAPK bands were normalized to that of
the total MAPK bands. The expression levels of p-Bcl-2 and total
Bcl-2 were assessed in the same manner. The secondary antibodies
were Goat anti-rabbit IgG.
Statistical analysis
The results were expressed as the mean ± standard
deviation of three separate experiments. Data were analyzed by
one-way analysis of variance followed by Fisher’s least significant
difference test or by Kruskal-Wallits test using SPSS 11 version
software. P<0.05 was used to indicate a statistically
significant difference.
Results
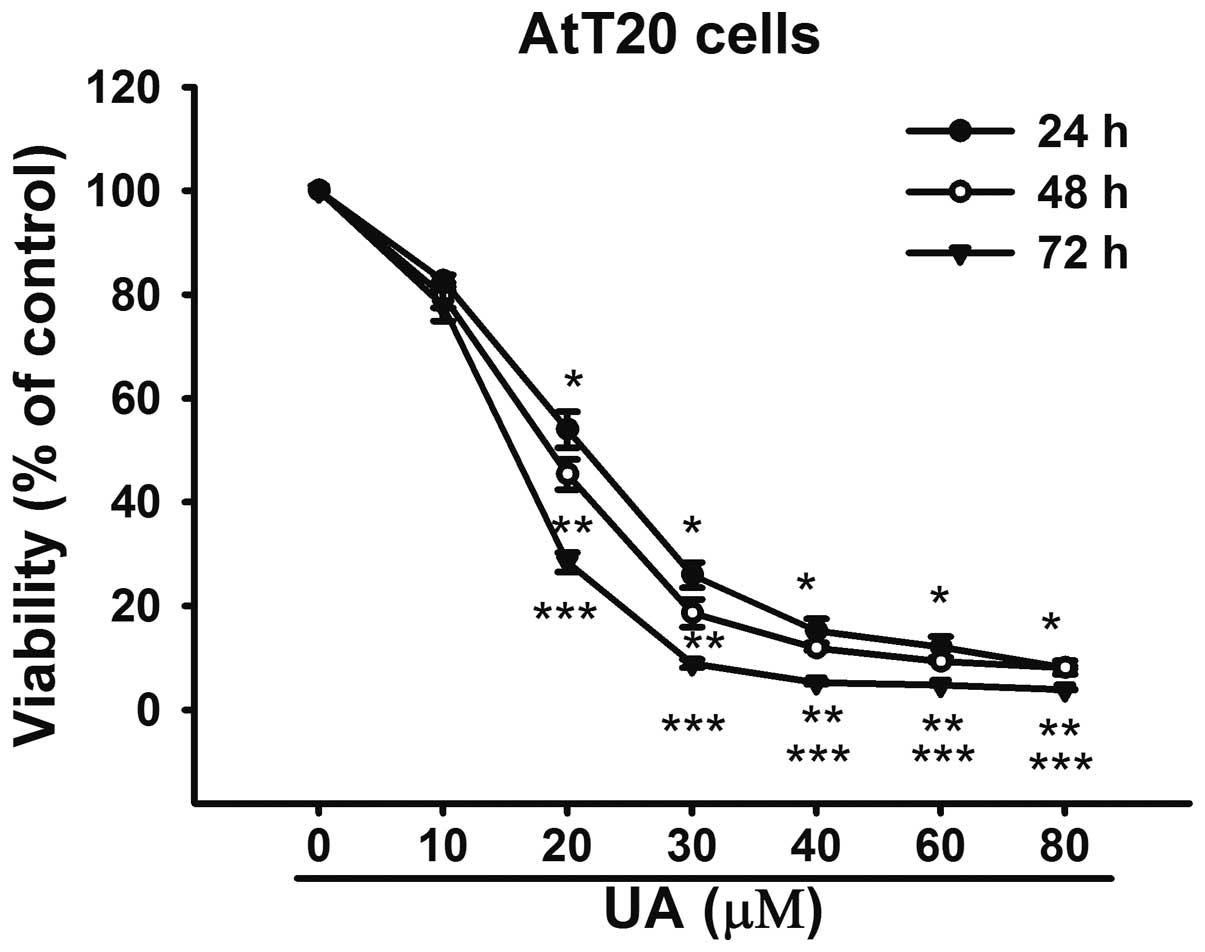
UA reduces viability and induces
apoptosis of AtT20 cells
In order to investigate the effect of UA on
cytotoxicity, the AtT20 cells were exposed to UA (10–100 μM) for
24, 48 and 72 h, respectively. The CCK-8 results revealed that UA
inhibited the viability of AtT20 cells in a dose- and
time-dependent manner (Fig. 1).
The IC50 value for 24 h was 20.02 μM. Considering the
IC50 for 24 h, the concentration of 20 μM was used for
further analysis.
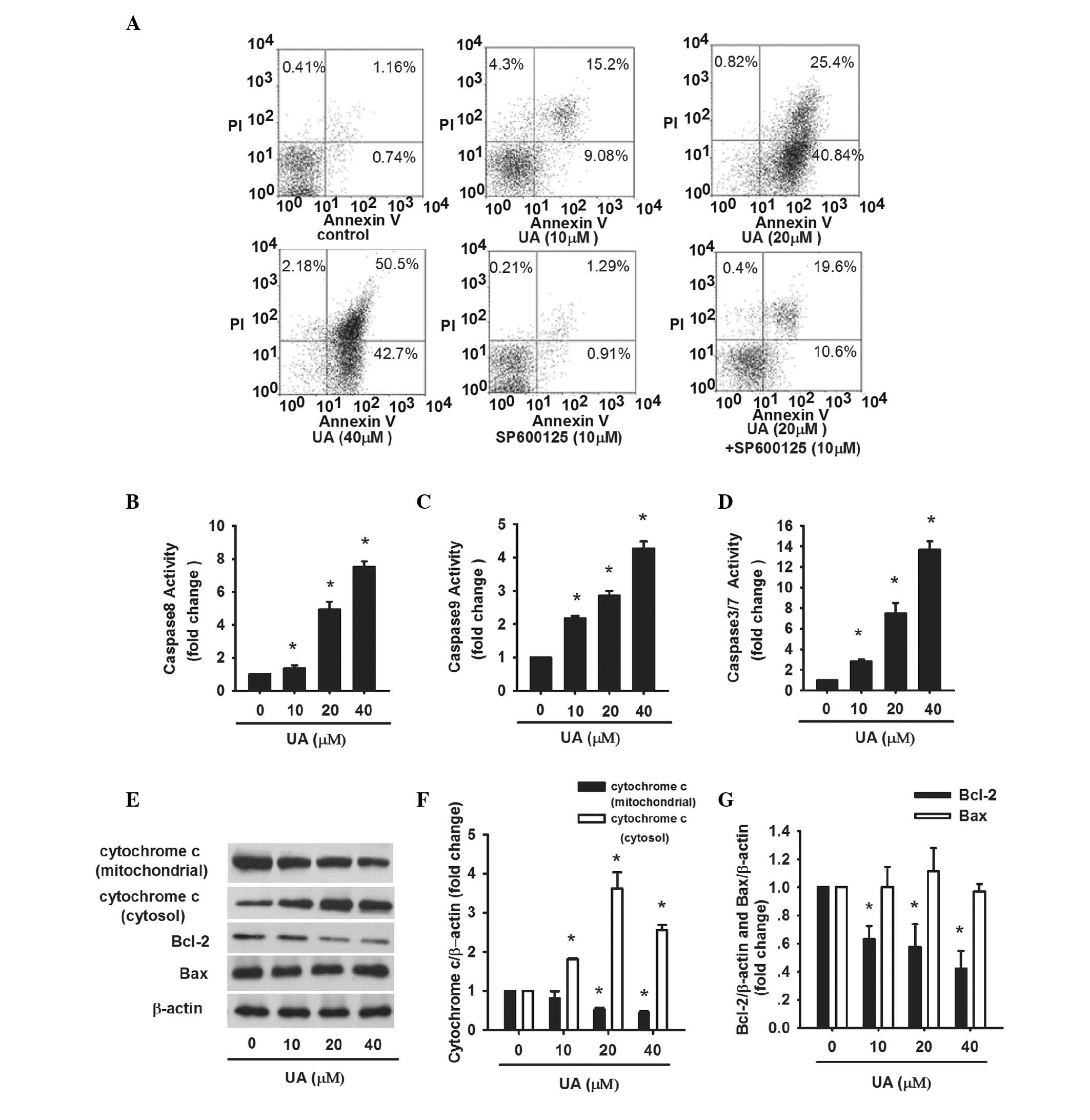
The apoptosis of AtT20 cells induced by UA was
further evaluated. AtT20 cells were treated with UA (10, 20 and 40
μM) for 24 h, respectively. Afterwards, Annexin V-FLOUS/PI staining
was performed and assessed by flow cytometry. The percentage of
apoptotic cells, including early apoptotic cells (Annexin
V-FLOUS+/PI−) and late apoptotic cells
(Annexin V-FLOUS+/PI+) was significantly
increased in a dose-dependent manner (Fig. 3A). In addition, the activity of
caspase-3/7, which is the executioner of apoptosis, was examined.
Caspase-3/7 activity was gradually enhanced from 2.8- to 13.7-fold
as the dose of UA increased (Fig.
3D). These results confirm that cell viability loss induced by
UA in AtT20 cells is facilitated by the induction of apoptosis.
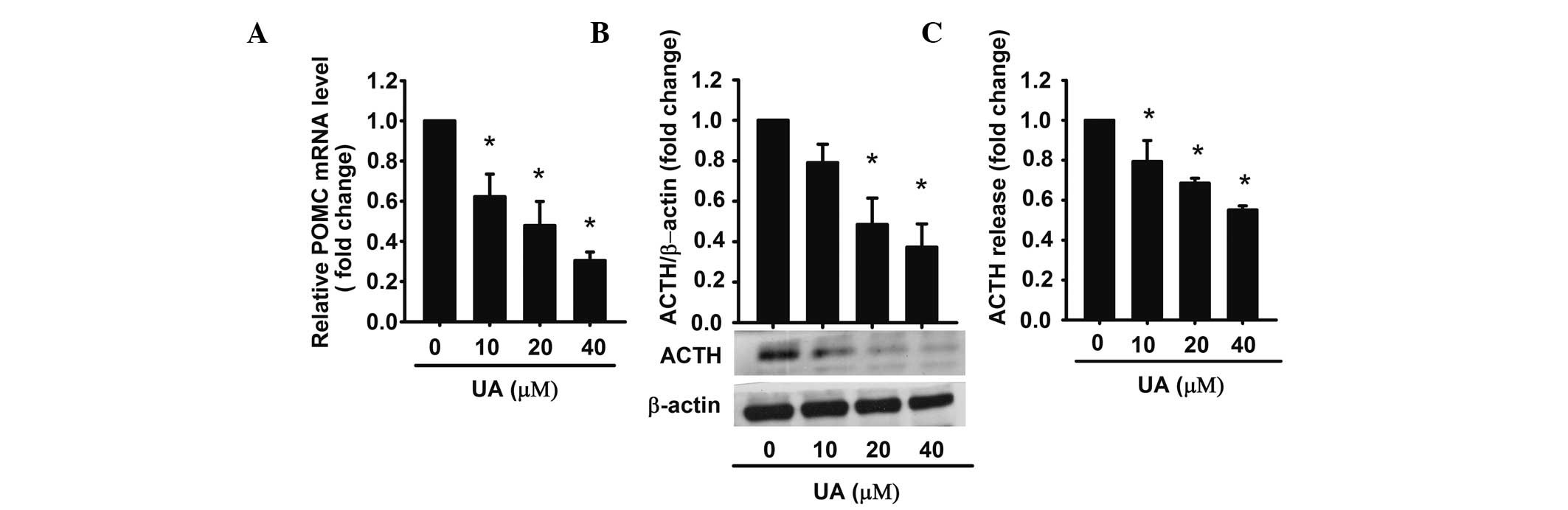
UA decreases ACTH production and
secretion in AtT20 cells
Next, it was investigated whether UA-induced
apoptosis was accompanied with a decreased ACTH secretion. It has
been reported that ACTH is derived from the POMC precursor peptide
(30). In order to examine the
effect on the intracellular levels of ACTH, UA (10, 20 and 40 μM)
was added to AtT20 cells for 48 h, respectively, and the extracted
total RNA was analyzed by qPCR. The results demonstrated that UA
inhibited POMC mRNA (precursor of ACTH) in a dose-dependent manner.
40 μM UA achieved a maximum effect of inhibition (~0.3-fold
compared with the control) (Fig.
2A). Western blot analysis also proved that UA decreased ACTH
synthesis in a dose-dependent manner (Fig. 2B).
For determining the levels of ACTH, the conditioned
medium was harvested and analyzed by ELISA. The data demonstrated
that ACTH was reduced by UA in a dose-dependent manner. Compared
with the control, ACTH levels were 0.62-, 0.48-, 0.3-fold,
respectively (Fig. 2C). These
results indicated that UA decreased the expression of POMC mRNA as
well as ACTH synthesis and secretion in AtT20 cells in a
dose-dependent manner.
Endogenous and exogenous signaling
pathways are involved in UA-induced apoptosis
Apoptosis is mediated by endogenous and exogenous
signal transduction. To address whether the two pathways were
involved in UA-induced apoptosis, caspase-8, -9, cytochrome
c, Bcl-2 and Bax were assayed. AtT20 cells were treated with
UA (10, 20 and 40 μM) for 24 h. Caspase-8 activity increased by
1.35-, 4.93-, 7.5-fold, respectively, as compared with the control
(Fig. 3B). The levels of Bcl-2 and
cytochrome c in mitochondria were reduced while cytochrome
c in cytosol was increased along with increasing dose of UA
(Fig. 3E and F). The protein
levels of Bax were normal while the ratio of Bcl-2/Bax decreased
(Fig. 3E and G). Caspase-9 was
also significantly increased by 4.27-fold subsequent to treatment
with 40 μM UA (Fig. 3C). The
results revealed that endogenous and exogenous signaling pathways
contributed to UA-induced apoptosis.
The JNK pathway is involved in UA-induced
apoptosis
Cell proliferation and apoptosis, and the MAPK
family are closely associated (31). UA has been shown to activate the
MAPK signaling pathway in various cells (23,32).
To further investigate the exact molecular mechanism of UA-induced
apoptosis and identify whether the MAPK signaling pathway was also
activated by UA in AtT20 cells, phosphorylation of JNK, ERK-1/2 and
p38 was detected. The results revealed that 20 μM UA upregulated
the expression of phosphorylation of JNK, which reached a maximum
from 1 to 3 h, but had no effect on the phosphorylation of ERK-1/2
and p38 (Fig. 4A and B). To
further confirm whether JNK activation mediated UA-induced
apoptosis, AtT20 cells were pretreated with 10 μM SP600125 (JNK
inhibitor) for 1 h followed by 20 μM UA for 24 h (Fig. 4C). The percentages of early and
late apoptotic cells were 19.6 and 10.6% respectively, which were
significantly less compared with that of cells in the group treated
with 20 μM UA only (Fig. 3A).
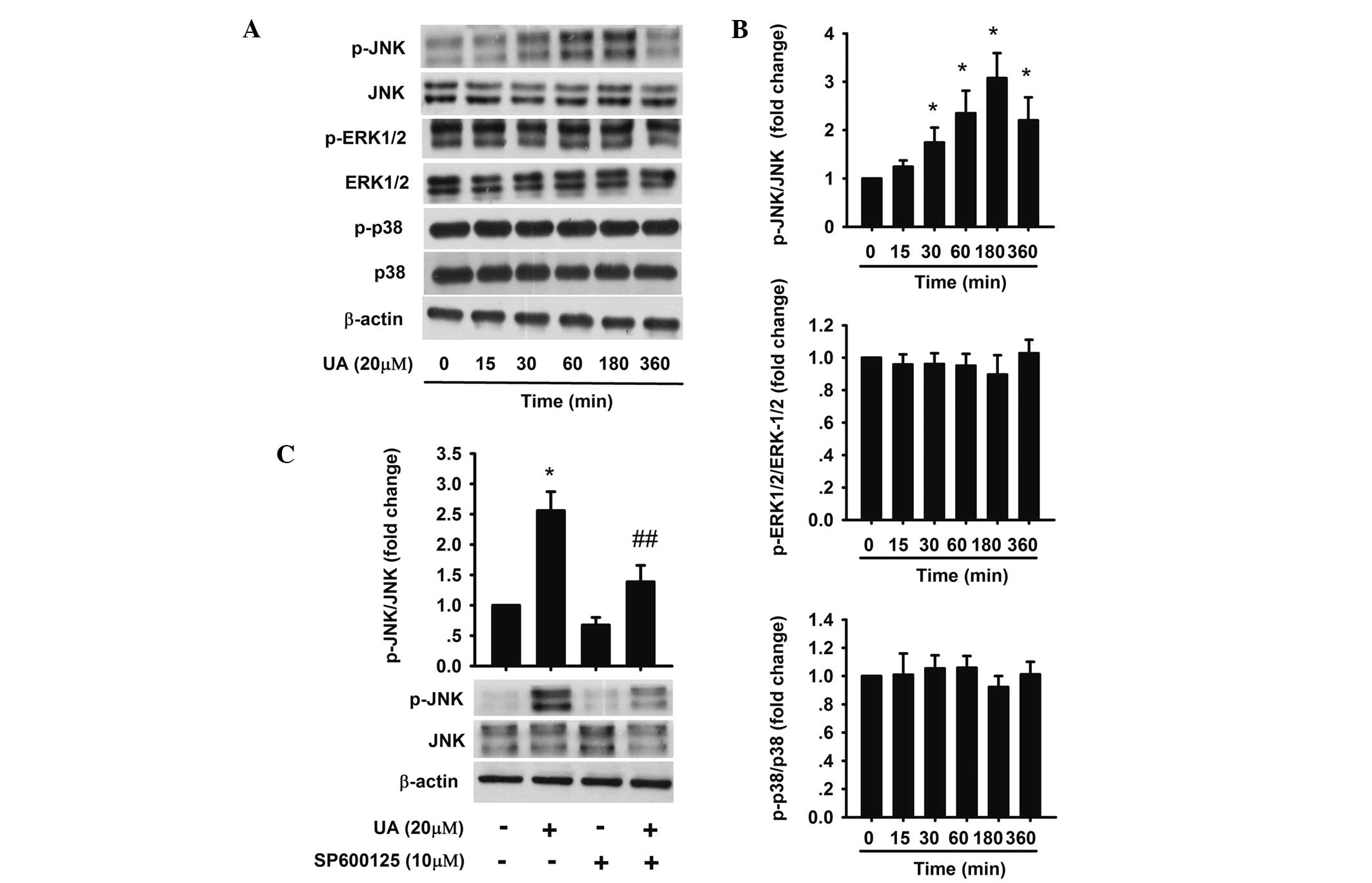 | Figure 4Effect of UA on the activation of
JNK, ERK1/2 and p38 in AtT20 cells. (A,B) AtT20 cells were
incubated with 20 μM UA for 15, 30, 60, 180 and 360 min. Protein
levels of JNK, p-JNK, ERK1/2, p-ERK1/2, p38 and p-p38 were detected
by western blot analysis. (C) AtT20 cells were treated with 10 μM
UA or SP600125 for 1 h prior to 20 μM UA treatment. Next, the
effect of JNK inhibitor SP600125 on JNK and p-JNK expression was
detected by western blot analysis. The data are presented as the
fold-change of the control. Each value was the mean ± standard
deviation of three separate experiments. *P<0.05 vs.
control; ##P<0.05 vs. UA (20 μM). p-JNK,
phosphorylated c-Jun N-terminal kinase; p-ERK, phosphorylated
extracellular signal-regulated protein kinase; UA, ursolic
acid. |
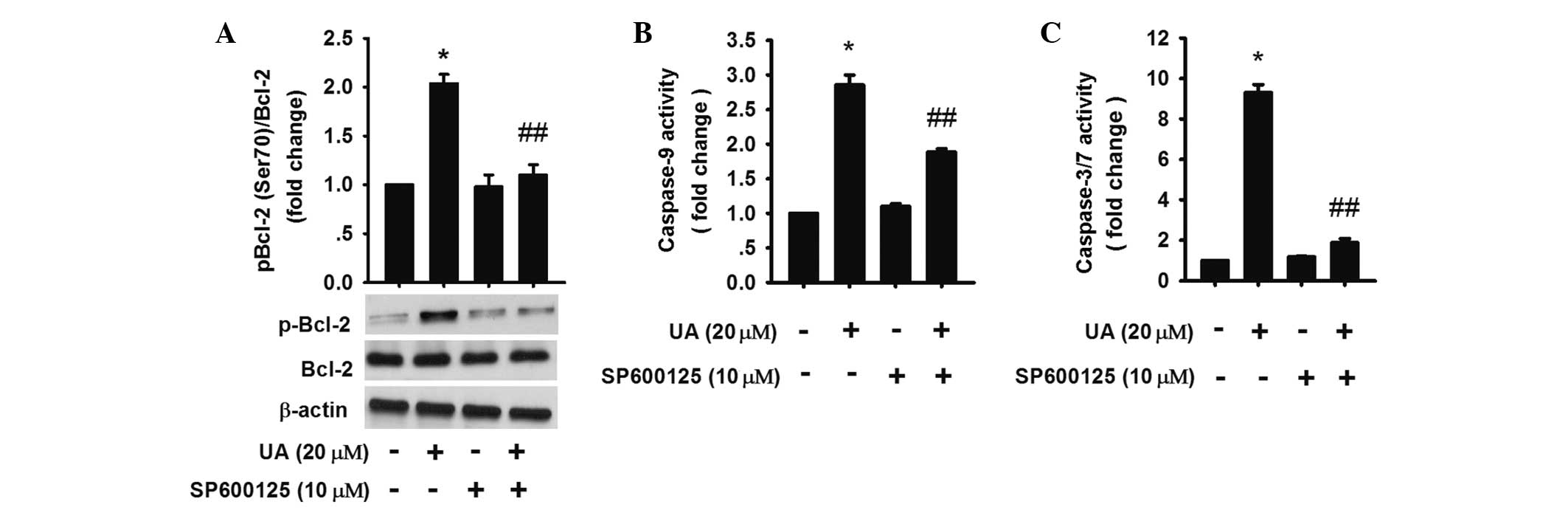
It has been reported that JNK activation inactivated
Bcl-2 by increasing phosphorylation of Bcl-2 (33,34).
The results showed that 20 μM UA treatment for 3 h increased
phosphorylated-Bcl-2 (Ser 70), while inhibition of JNK activation
by SP600125 could downregulate the phosphorylation (Fig. 5A), demonstrating that JNK
activation was involved in the upregulation of UA-induced Bcl-2
phosphorylation and resulting apoptosis.
To evaluate whether the activation of caspases was
affected by Bcl-2, caspase-9 and -3/7 were assessed. The results
revealed that caspase-3/7 and -9 activation were partly blocked in
the UA group in the presence of SP600125 (Fig. 5A–C). Thus, the data indicated that
JNK activation was involved in the endogenous signaling pathway in
UA-induced apoptosis by increasing phosphorylated Bcl-2.
Discussion
Pituitary adenoma accounts for a significant
proportion of primary intracerebral tumors. Although ACTH-producing
pituitary adenoma is benign, it does cause detrimental effects due
to excess hormone secretion resulting in significant mortality and
an impaired quality of life. Removing or shrinking the tumor and
normalization of ACTH excess are the crucial goals of treatment.
Thus far, no reliable medical therapies exist to directly target
the pituitary tumor growth and ACTH secretion. In the present
study, it was demonstrated that UA could function as a potential
novel and potent therapeutic agent targeting directly on
ACTH-producing pituitary adenomas.
The present study revealed that UA was able to
reduce the viability and induce apoptosis of AtT20 cells (mouse
corticotroph tumor cell line) in a dose-dependent manner. The
results were in consistency with other studies in various tumor
types (21,35–39),
which indicated that UA had a pro-apoptotic effect on
ACTH-producing pituitary tumor cells.
Excess ACTH levels and hypercortisolemia may result
in high co-morbidity and mortality. Normalization of hormone excess
is the therapeutic goal of treatment. Numerous compounds, including
dopamine agonists, thiazolidinediones and curcumin have been
reported to inhibit ACTH synthesis and (or) secretion (12,40,41).
However, none of these agents has been proven to be effective in
the management of ACTH-producing pituitary adenoma. In the present
study, it was identified that UA decreased ACTH production and
secretion in AtT20 cells in a dose-dependent manner, demonstrating
the potential of UA to be a novel agent for the management of
ACTH-producing pituitary adenoma.
Apoptosis has an essential role in tumorigenesis.
Two major apoptotic pathways have been identified (42). One is the exogenous pathway, which
involves the binding of a ligand to the death receptor and
subsequent caspase-8 activation (43). The other is the endogenous pathway,
relying on the release of cytochrome c from mitochondria to
the cytosol which recruits the initiator pro-caspase-9, which
yields activated caspase-9 and finally activates caspase-3
(44). The key components of the
mechanism involved in mitochondria-dependent apoptosis are the
Bcl-2 family of proteins, including pro-apoptotic Bax and
anti-apoptotic Bcl-2 proteins (45). Bcl-2 proteins usually form
heterodimers with Bax, resulting in the release of cytochrome
c from mitochondria to the cytosol and triggering the death
program. In the present study, it was revealed that UA increased
caspase-9 and -8 activities, decreased the ratio of Bcl-2/Bax and
promoted cytochrome c release from mitochondria in a
dose-dependent manner. The results indicated that exogenous and
endogenous pathways were involved in UA-induced apoptosis in AtT20
cells.
To elucidate how UA triggered the apoptotic process,
the exact molecular mechanisms were further investigated. It has
been reported that the MAPK pathway had a vital role in UA-induced
apoptosis in various tumor cells (32,37,46).
In the present study, UA was found to increase the phosphorylation
of JNK, but not ERK1/2 or p38, in a time-dependent manner.
Pretreatment with JNK inhibitor SP600125 blocked UA-induced
cleavage of caspase-3 and -9. Previous studies have reported that
the JNK pathway participated in UA-induced the apoptotic signaling
pathway via controlling phosphorylation of Bcl-2 (36,37,47).
The phosphorylation of Bcl-2 resulted in the degradation of Bcl-2,
which led to the release of Bax from the Bcl-2/Bax heterodimer and
triggered apoptosis (48). The
present study demonstrated that UA treatment for 24 h decreased the
levels of Bcl-2 and additionally induced the phosphorylation of
Bcl-2 in AtT20 cells. Furthermore, pretreatment with SP600125 was
able to partly block UA-induced Bcl-2 phosphorylation (Ser70) and
degradation. These findings revealed that UA-induced JNK activation
may promote Bcl-2 phosphorylation, degradation and finally induce
apoptosis.
In conclusion, the present study demonstrated that
UA inhibited viability, induced apoptosis and decreased ACTH
production in AtT20 cells. The induction of apoptosis involved
exogenous and endogenous pathways. Increased phosphorylation of
Bcl-2 via JNK activation had a crucial role in UA-induced apoptosis
in AtT20 cells. These findings indicate the potential of UA as a
novel potential therapeutic agent targeting ACTH-producing
pituitary adenoma. Further clinical studies are required to examine
the efficacy and safety of UA.
Acknowledgements
The abstract of this study has been published in
Endocr Rev Vol. 34, (03 Meeting Abstracts): Sun-188, 2013.
References
|
1
|
Boscaro M, Barzon L, Fallo F and Sonino N:
Cushing’s syndrome. Lancet. 357:783–791. 2001.
|
|
2
|
Arnaldi G, Angeli A, Atkinson AB, et al:
Diagnosis and complications of Cushing’s syndrome: a consensus
statement. J Clin Endocrinol Metab. 88:5593–5602. 2003.
|
|
3
|
Witek P, Zielinski G, Szamotulska K, Witek
J and Zgliczynski W: Complications of Cushing’s disease -
prospective evaluation and clinical characteristics. Do they affect
the efficacy of surgical treatment? Endokrynol Pol. 63:277–285.
2012.
|
|
4
|
Swearingen B: Update on pituitary surgery.
J Clin Endocrinol Metab. 97:1073–1081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Atkinson AB, Kennedy A, Wiggam MI, McCance
DR and Sheridan B: Long-term remission rates after pituitary
surgery for Cushing’s disease: the need for long-term surveillance.
Clin Endocrinol (Oxf). 63:549–559. 2005.
|
|
6
|
Heaney AP: Clinical review: pituitary
carcinoma: difficult diagnosis and treatment. J Clin Endocrinol
Metab. 96:3649–3660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beardwell CG, Adamson AR and Shalet SM:
Prolonged remission in florid Cushing’s syndrome following
metyrapone treatment. Clin Endocrinol (Oxf). 14:485–492. 1981.
|
|
8
|
Boscaro M, Sonino N, Rampazzo A and
Mantero F: Response of pituitary-adrenal axis to corticotrophin
releasing hormone in patients with Cushing’s disease before and
after ketoconazole treatment. Clin Endocrinol (Oxf). 27:461–467.
1987.
|
|
9
|
Sonino N, Boscaro M and Fallo F:
Pharmacologic management of Cushing syndrome: new targets for
therapy. Treat Endocrinol. 4:87–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petersenn S, Schopohl J, Barkan A, et al:
Pasireotide (SOM230) demonstrates efficacy and safety in patients
with acromegaly: a randomized, multicenter, phase II trial. J Clin
Endocrinol Metab. 95:2781–2789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bode H, Seiz M, Lammert A, et al: SOM230
(pasireotide) and temozolomide achieve sustained control of tumour
progression and ACTH secretion in pituitary carcinoma with
widespread metastases. Exp Clin Endocrinol Diabetes. 118:760–763.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pivonello R, Ferone D, de Herder WW, et
al: Dopamine receptor expression and function in corticotroph
pituitary tumors. J Clin Endocrinol Metab. 89:2452–2462. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mullan KR, Leslie H, McCance DR, Sheridan
B and Atkinson AB: The PPAR-gamma activator rosiglitazone fails to
lower plasma ACTH levels in patients with Nelson’s syndrome. Clin
Endocrinol (Oxf). 64:519–522. 2006.PubMed/NCBI
|
|
14
|
Pecori Giraldi F, Scaroni C, Arvat E, et
al: Effect of protracted treatment with rosiglitazone, a PPARgamma
agonist, in patients with Cushing’s disease. Clin Endocrinol (Oxf).
64:219–224. 2006.PubMed/NCBI
|
|
15
|
Liu J: Pharmacology of oleanolic acid and
ursolic acid. J Ethnopharmacol. 49:57–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sultana N and Ata A: Oleanolic acid and
related derivatives as medicinally important compounds. J Enzyme
Inhib Med Chem. 23:739–756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sultana N: Clinically useful anticancer,
antitumor, and antiwrinkle agent, ursolic acid and related
derivatives as medicinally important natural product. J Enzyme
Inhib Med Chem. 26:616–642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J: Oleanolic acid and ursolic acid:
research perspectives. J Ethnopharmacol. 100:92–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kassi E, Papoutsi Z, Pratsinis H,
Aligiannis N, Manoussakis M and Moutsatsou P: Ursolic acid, a
naturally occurring triterpenoid, demonstrates anticancer activity
on human prostate cancer cells. J Cancer Res Clin Oncol.
133:493–500. 2007. View Article : Google Scholar
|
|
20
|
Shih WL, Yu FL, Chang CD, Liao MH, Wu HY
and Lin PY: Suppression of AMF/PGI-mediated tumorigenic activities
by ursolic acid in cultured hepatoma cells and in a mouse model.
Mol Carcinog. 52:800–812. 2013.PubMed/NCBI
|
|
21
|
Shyu MH, Kao TC and Yen GC: Oleanolic acid
and ursolic acid induce apoptosis in HuH7 human hepatocellular
carcinoma cells through a mitochondrial-dependent pathway and
downregulation of XIAP. J Agric Food Chem. 58:6110–6118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prasad S, Yadav VR, Sung B, et al: Ursolic
acid inhibits growth and metastasis of human colorectal cancer in
an orthotopic nude mouse model by targeting multiple cell signaling
pathways: Chemosensitization with capecitabine. Clin Cancer Res.
18:4942–4953. 2012. View Article : Google Scholar
|
|
23
|
Shan JZ, Xuan YY, Zheng S, Dong Q and
Zhang SZ: Ursolic acid inhibits proliferation and induces apoptosis
of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J
Zhejiang Univ Sci B. 10:668–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harmand PO, Duval R, Delage C and Simon A:
Ursolic acid induces apoptosis through mitochondrial intrinsic
pathway and caspase-3 activation in M4Beu melanoma cells. Int J
Cancer. 114:1–11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Angel RE, Smith SM, Glickman RD,
Perkins SN and Hursting SD: Antitumor effects of ursolic acid in a
mouse model of postmenopausal breast cancer. Nutr Cancer.
62:1074–1086. 2010.PubMed/NCBI
|
|
26
|
Zheng QY, Jin FS, Yao C, Zhang T, Zhang GH
and Ai X: Ursolic acid-induced AMP-activated protein kinase (AMPK)
activation contributes to growth inhibition and apoptosis in human
bladder cancer T24 cells. Biochem Biophys Res Commun. 419:741–747.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu W, Ou Y, Li Y, et al: A small-molecule
triptolide suppresses angiogenesis and invasion of human anaplastic
thyroid carcinoma cells via down-regulation of the nuclear
factor-kappa B pathway. Mol Pharmacol. 75:812–819. 2009. View Article : Google Scholar
|
|
28
|
Hollosy F, Meszaros G, Bokonyi G, et al:
Cytostatic, cytotoxic and protein tyrosine kinase inhibitory
activity of ursolic acid in A431 human tumor cells. Anticancer Res.
20:4563–4570. 2000.PubMed/NCBI
|
|
29
|
Gamby C, Waage MC, Allen RG and Baizer L:
Growth-associated protein-43 (GAP-43) facilitates peptide hormone
secretion in mouse anterior pituitary AtT-20 cells. J Biol Chem.
271:10023–10028. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
White A and Gibson S: ACTH precursors:
biological significance and clinical relevance. Clin Endocrinol
(Oxf). 48:251–255. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu XS and Jiang J: Induction of apoptosis
and regulation of the MAPK pathway by ursolic acid in human
leukemia K562 cells. Planta Med. 73:1192–1194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ruvolo PP, Deng X and May WS:
Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia.
15:515–522. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miyoshi N, Uchida K, Osawa T and Nakamura
Y: A link between benzyl isothiocyanate-induced cell cycle arrest
and apoptosis: involvement of mitogen-activated protein kinases in
the Bcl-2 phosphorylation. Cancer Res. 64:2134–2142. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bonaccorsi I, Altieri F, Sciamanna I, et
al: Endogenous reverse transcriptase as a mediator of ursolic
acid’s anti-proliferative and differentiating effects in human
cancer cell lines. Cancer Lett. 263:130–139. 2008.PubMed/NCBI
|
|
36
|
Zhang YX, Kong CZ, Wang HQ, Wang LH, Xu CL
and Sun YH: Phosphorylation of Bcl-2 and activation of caspase-3
via the c-Jun N-terminal kinase pathway in ursolic acid-induced
DU145 cells apoptosis. Biochimie. 91:1173–1179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Kong C, Zeng Y, et al: Ursolic
acid induces PC-3 cell apoptosis via activation of JNK and
inhibition of Akt pathways in vitro. Mol Carcinog. 49:374–385.
2010.PubMed/NCBI
|
|
38
|
Cha HJ, Bae SK, Lee HY, et al:
Anti-invasive activity of ursolic acid correlates with the reduced
expression of matrix metalloproteinase-9 (MMP-9) in HT1080 human
fibrosarcoma cells. Cancer Res. 56:2281–2284. 1996.PubMed/NCBI
|
|
39
|
Yang L, Liu X, Lu Z, et al: Ursolic acid
induces doxorubicin-resistant HepG2 cell death via the release of
apoptosis-inducing factor. Cancer Lett. 298:128–138. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heaney AP, Fernando M, Yong WH and Melmed
S: Functional PPAR-gamma receptor is a novel therapeutic target for
ACTH-secreting pituitary adenomas. Nat Med. 8:1281–1287. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schaaf C, Shan B, Buchfelder M, et al:
Curcumin acts as anti-tumorigenic and hormone-suppressive agent in
murine and human pituitary tumour cells in vitro and in vivo.
Endocr Relat Cancer. 16:1339–1350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Green DR: Apoptotic pathways: paper wraps
stone blunts scissors. Cell. 102:1–4. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nagata S: Apoptosis by death factor. Cell.
88:355–365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar
|
|
45
|
Kuwana T and Newmeyer DD: Bcl-2-family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Achiwa Y, Hasegawa K and Udagawa Y:
Regulation of the phosphatidylinositol 3-kinase-Akt and the
mitogen-activated protein kinase pathways by ursolic acid in human
endometrial cancer cells. Biosci Biotechnol Biochem. 71:31–37.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang YX, Kong CZ, Wang LH, et al: Ursolic
acid overcomes Bcl-2-mediated resistance to apoptosis in prostate
cancer cells involving activation of JNK-induced Bcl-2
phosphorylation and degradation. J Cell Biochem. 109:764–773.
2010.PubMed/NCBI
|
|
48
|
Cheng EH, Wei MC, Weiler S, et al: BCL-2,
BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and
BAK-mediated mitochondrial apoptosis. Mol Cell. 8:705–711. 2001.
View Article : Google Scholar : PubMed/NCBI
|