Introduction
Matrine is one of the main active components of the
traditional Chinese medical plant Sophora flavescence
(1). Matrine has been widely
employed in the treatment of chronic viral hepatitis in China
(2) and also exhibits antitumor
effects by inhibiting proliferation and inducing apoptosis or
differentiation in vitro (3–5).
Previously, autophagic cell death, referred to as type II
programmed cell death, has been demonstrated to be induced by
matrine in C6 glioma cells (6).
WB-F344 cells, derived from a single cloned
epithelial cell isolated from rat liver, can be regarded as the
cultured analogue of liver precursor cells. These epithelial cells
share certain phenotypic and functional characteristics with
hepatocytes and bile duct cells. When they were implanted into a
rat liver, they gained morphological and functional properties of
hepatocytes (7,8). WB-F344 cells also have the potential
to differentiate into biliary cells in vitro and develop
cholangiocarcinomas when transplanted with chemically transformed
WB-F344 cells into the liver of syngeneic rats in vivo
(9,10).
The majority of studies of liver oval cells focuses
on hepatocytic differentiation induced by matrine in vitro.
However, its mechanism of liver stem cells under drug treatment
remains to be elucidated. In the present study, apoptosis was
stimulated by matrine in WB-F344 cells and a concomitant increase
in autophagy was detected. Additionally, β-catenin has a role in
the matrine-induced autophagy and apoptosis, while β-catenin is
negatively regulated by autophagy as a feedback mechanism.
Materials and methods
Materials
3-methyladenine (3-MA), rapamycin, dimethylsulfoxide
(DMSO), lithium chloride (LiCl), pifithrin-α (PFT-α) and antibodies
against microtubule-associated protein 1 light chain 3 (LC3) were
purchased from Sigma (St. Louis, MO, USA). Antibodies against p70S6
(Thr389), phosphorylated (p)-Akt (Thr473), p-extracellular
signal-regulated kinases (ERK)1/2 (Thy202/Tyr204) and p-c-Jun
N-terminal kinases (JNK) (T183/Y185) were from Cell Signaling
Technology, Inc. (Beverly, MA, USA), β-catenin, beclin-1, β-actin
and p53 antibody from Santa Cruz Biotechnology, Inc. (Santa Cruz,
CA, USA). Matrine, which was dissolved in Dulbecco’s modified Eagle
medium (DMEM; Invitrogen, Carlsbad, CA, USA) was added to the cells
with a final concentration of 0.2–4 mg/ml matrine that was a gift
from Baiyunshan Mingxing Pharmaceutical Co., Ltd. (Guangzhou,
China) and with a purity >98%. Constitutively, active β-catenin
was a gift from Dr Yanming Li (Peking University School of
Medicine, Beijing, China).
Cell lines and cell culture
WB-F344 cells were purchased by the Institute of
Materia Medica (IMM), Chinese Academy of Medical Sciences and
Peking Union Medical College (all in Beijing, China). The cells
were cultivated in a 25 cm3 flask with DMEM,
supplemented with 15% (v/v) fetal bovine serum, 100 KIU/l
penicillin and 100 mg/l streptomycin (all from Invitrogen). These
were kept at 37°C in a humidified atmosphere containing 5%
CO2. The flasks were subcultured every two days with a
split ratio of 1:2.
Cell viability assay
The cells were trypsinized and cultured in 96-well
flat bottom microtiter plates. Following matrine treatment, 100 μl
MTT (Sigma; 5 mg/ml/well) was added and incubated for 4 h. The
crystals were dissolved in 150 μl DMSO. The absorbance of the
solution was measured spectrophotometrically at 570 nm using a
microtiter plate reader (Becton-Dickinson, Franklin Lanes, NJ,
USA).
Confocal microscopy
The cells were incubated for 1 h with 0.05 mM
monodansylcadaverine (MDC; Sigma). After 1 h incubation at 37°C,
the cells were fixed in 4% paraformaldehyde for 15 min and
immediately analyzed using a scanning confocal microscope (Olympus,
Tokyo, Japan). The percentages of distribution of MDC dots were
counted in five non-overlapping fields and the statistical data
were obtained from three repeated experiments.
Electron microscopy
The trypsinized cells were fixed with ice-cold
glutaraldehyde [(Sigma) 3% in 0.1 M cacodylate buffer (pH 7.4)] for
30 min. The cells were post-fixed in OsO4 (Sigma) and
embedded in Epon (Sigma); 0.1-mm thin sections were stained with
uranyl acetate/lead citrate (Fluka, St. Louis, MO, USA). The
observation was performed on a JEM-1230 electron microscope (Jeol,
Tokyo, Japan).
Flow cytometric analysis
The cells were treated with the indicated
concentrations of chemotherapeutic agent, then cells were collected
and incubated with Annexin V-fluorescein isothiocyanate (FITC)
(Beijing Biosea Biotechnology Co., Ltd., Beijing, China) for 30 min
at 4°C and in the dark, then incubated with propidium iodide (PI)
(Beijing Biosea Biotechnology Co., Ltd.) for 5 min. Analysis was
immediately performed by flow cytometry (FACSAria,
Becton-Dickinson, Franklin Lakes, NJ, USA).
The cells were cultured in six-well tissue culture
plates and received different treatments. They were then
trypsinized and fixed in 70% ethanol at 4°C overnight. Then cells
were collected and resuspended in staining buffer (50 μg/ml PI in
phosphate-buffered saline) for 5 min in the dark at room
temperature and then analyzed by flow cytometry.
Small interfering (si)RNA
transfection
The cells were seeded at 40% confluence per well in
six-well plates overnight and transfected with β-catenin siRNA or
control siRNA duplex (Santa Cruz Biotechnology, Inc.) using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA). The successful targeted knockdown was confirmed by western
blot analysis.
Reverse transcription-polymerase chain
reaction (RT-PCR) and quantitative PCR (qPCR)
Total RNA was purified using TRIzol agent
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. The total RNA was reverse-transcribed using cDNA
reverse transcription kits (Invitrogen Life Technologies).
Hot-start PCR was then performed. The PCR results were verified by
varying the number of PCR cycles for each cDNA and set of primers.
The target gene primer pairs were as follows: Bax forward,
5′-CCAAGAAGCTGAGCGAGTGTC-3′ and reverse,
3′-TGAGGACTCCAGCCACAAAGA-5′; Bcl-2 forward,
5′-CCGGGAGATCGTGATGAAGT-3′ and reverse, 3′-ATCCCAGCCTCCGTTATCCT-5′;
p53 forward, 5′-GGCCTCTGTCATCTTCCG-3′ and reverse,
3′-CCGTCACCATCAGAGCAAC-5′; GAPDH forward, 5′-ATCGGACGCCTGGTTACC-3′
and reverse, 3′-GACTGTGCCGTTGAACTTGC-5′. The amplified products
were separated on 1.5–2% agarose gels and visualized under
ultraviolet transillumination.
qPCR was performed using the ABI Prism 7700 Sequence
Detection system (Applied Biosystems, Grand Island, NY, USA). For
each PCR run, the samples were incubated in a 96-well plate at 95°C
for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min. The data were analyzed using the ΔΔCt method according to the
manufacturer’s instructions. The data were normalized to the
housekeeping gene GAPDH. Changes in the gene expression were
illustrated as a fold increase/decrease, as compared with the
control. The experiments were repeated thrice.
Western blot analysis
The cell lysates were separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
nitrocellulose membranes (Millipore Co., Bedford, MA, USA). The
membranes were incubated with the primary antibodies overnight at
4°C, then the secondary antibodies at room temperature for 2 h. The
antibodies used included LC3, p70S6 (Thr389), Akt and p-Akt
(Thr473), ERK1/2 and p-ERK1/2 (Thy202/Tyr204), JNK and p-JNK
(T183/Y185), β-catenin, beclin-1 and p53. β-actin antibody was used
as a control. The protein bands were observed on X-ray film or
using an enhanced chemiluminescence system (Pierce Biotechnology,
Rockford, IL, USA).
Statistical analysis
Statistical analysis was performed using SPSS 17
statistics software (SPSS, Inc., Chicago, IL, USA). The data are
expressed as the mean ± standard deviation with the number of
individual experiments described in the Figure legends. P<0.05
was used to indicate a statistically significant difference.
Results
Matrine inhibits cell cycle and induces
apoptosis of WB-F344 cells
The effect of matrine treatment on WB-F344 cells was
examined with an MTT assay. The cell viability was inhibited by
matrine (0–4 mg/ml) in a dose- and time-dependent manner. The
IC50 for matrine of WB-F344 cells for 24 h was 0.84
mg/ml (Fig. 1A). Based on these
results, 0.8 mg/ml matrine for 24 h was used for further
experiments. The cell cycle and apoptosis were further analyzed by
flow cytometric analysis. An evident decline in the percentage of
the S-phase cells, but a significant elevation tendency of the
G0/G1-phase was observed in rat oval cells following exposure to
matrine (Fig. 1B). Additionally,
the Annexin-V-FITC/PI assay revealed that matrine induced a
significant increase in the number of apoptotic oval cells
(Fig. 1C). These results indicated
that matrine blocked the oval cell cycle progression at the G0/G1
phase and induced apoptosis. Next, the mRNA expression of Bcl-2 and
Bax, the key regulators of cell proliferation and apoptosis, were
examined. As shown in Fig. 1D,
RT-PCR and qPCR assays indicated a decrease in Bcl-2 mRNA
expression, but an increase of Bax mRNA expression in
matrine-treated cells in a dose-dependent manner, leading to an
upregulation of the Bax/Bcl-2 ratio in comparison with untreated
cells.
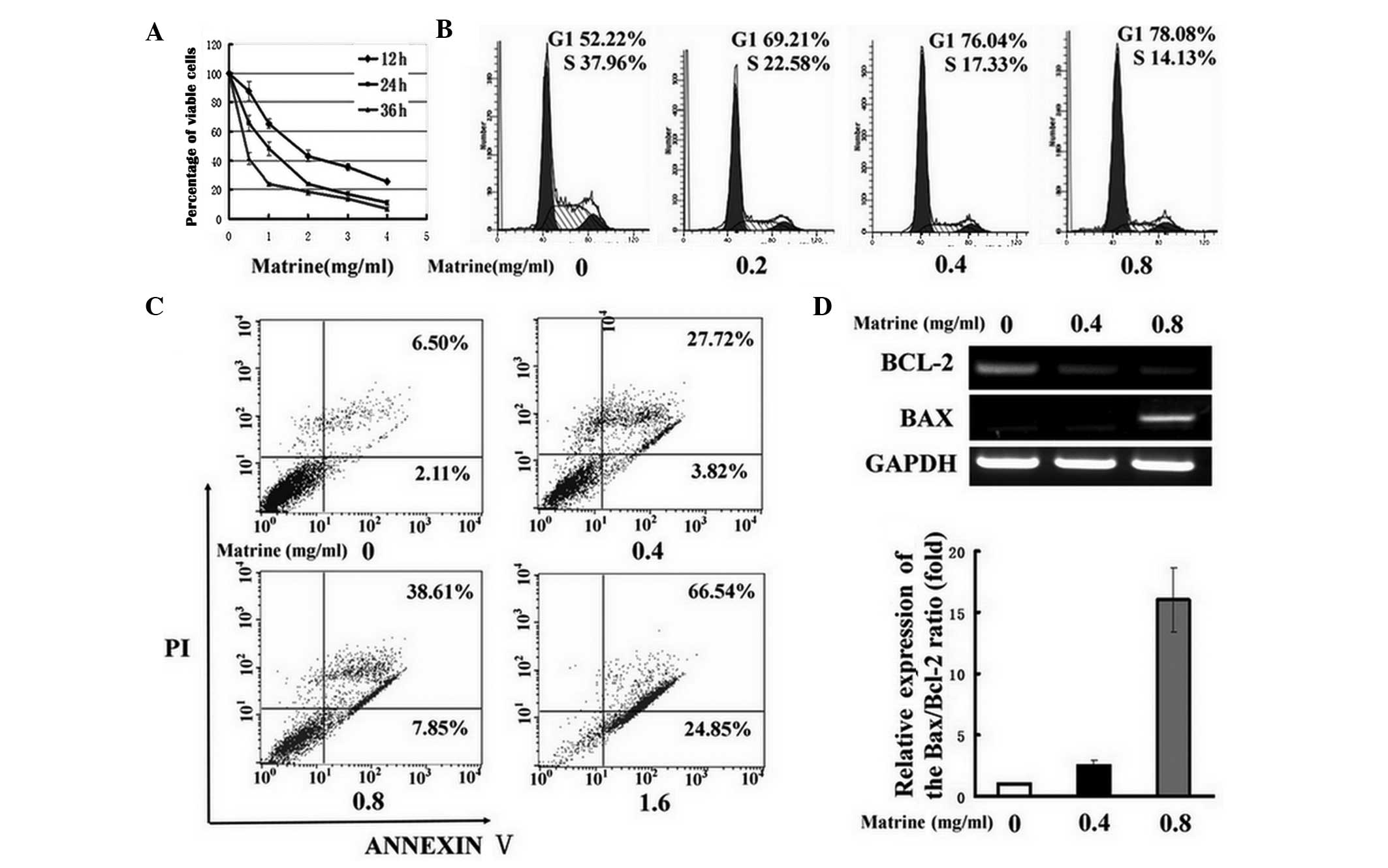 | Figure 1Matrine blocks oval cell cycle
progression and induces apoptosis. (A) The effects of matrine at
different concentrations (0–4 mg/ml) on the regression of WB-F344
cells for 12, 24 or 36 h was examined by the MTT assay. (B) The
cells were incubated with matrine at concentrations of 0.2 (0.2),
0.4 (0.4) and 0.8 mg/ml (0.8) for 24 h, and accumulation of the
G1/G0-phase was detected by flow cytometry. The untreated cells (0)
were used as a control. (C) Apoptotic oval cells were stimulated by
matrine at concentrations of 0.4 (0.4), 0.8 (0.8) and 1.6 mg/ml
(1.6) for 24 h as detected by the Annexin V/PI assay. Using this
assay, the viable (Annexin V−/PI−), early
apoptotic (Annexin V+/PI−), late apoptotic or
necrotic cells (Annexin V+/PI+) were
classified. (D) Bax and Bcl-2 mRNA levels were measured in WB-F344
cells following exposure to matrine at 0.4 (0.4) and 0.8 mg/ml
(0.8) by RT-PCR and then qPCR, and compared with that of untreated
cells (0). GAPDH was used as an internal control. The ΔΔCt value of
the Bax/Bcl-2 ratio in untreated cells was normalized to 1 as a
control. PI, propidium iodide; Bcl2, B-cell lymphoma 2; Bax,
Bcl2-associated X protein; mRNA, messenger RNA; RT-PCR, real-time
polymerase chain reaction; qPCR, quantitative PCR. |
Matrine induces autophagy in WB-F344
cells, which inhibits apoptosis
The present study tested whether matrine induced
autophagy in WB-F344 cells. The changes of autophagosome morphology
were initially visualized with MDC staining, proposed as a tracer
for autophagic vacuoles (11).
MDC-stained punctuate structures, indicative of the formation of
autophagosomes, increased in the cytoplasm of WB-F344 cells
following matrine treatment (Fig.
2A). The cumulative autophagic process was further confirmed by
transmission electron microscopy (Fig.
2B). Next, immunoblotting analysis indicated that the ratio of
LC3-II/β-actin, a specific molecular marker for autophagy detection
(12), was significantly
upregulated; however, the levels of ribosomal S6 protein kinase
(S6K1, also known as p70S6K) phosphorylation, which has been widely
described to reflect the mechanistic target of rapamycin (mTOR)
activity, was downregulated in the treated cells as compared with
the control (Fig. 2C). Altogether,
these findings confirmed that autophagy was stimulated by matrine
in an mTOR-dependent manner in rat oval cells. It is disputed
whether autophagy induction is protective or toxic. 3-MA, a general
inhibitor of autophagy (13), was
used to analyze the role of matrine-induced autophagy in WB-F344
cells. It was presented in Fig. 2D
that the increase of MDC-positive dots induced by matrine was
overcome by the addition of 3-MA. Similarly, immunoblotting
analysis indicated that the increase of the LC3-II/β-actin ratio by
matrine was attenuated following the addition with 3-MA (Fig. 2E). Subsequently, 3-MA cotreatment
induced more significant levels of apoptotic cells compared with
matrine alone, as indicated by the Annexin-V-FITC/PI assay
(Fig. 2F). These results indicated
that suppression of autophagy augmented matrine-induced apoptosis,
indicating that the matrine-stimulated autophagic process serves as
an oval cell survival mechanism.
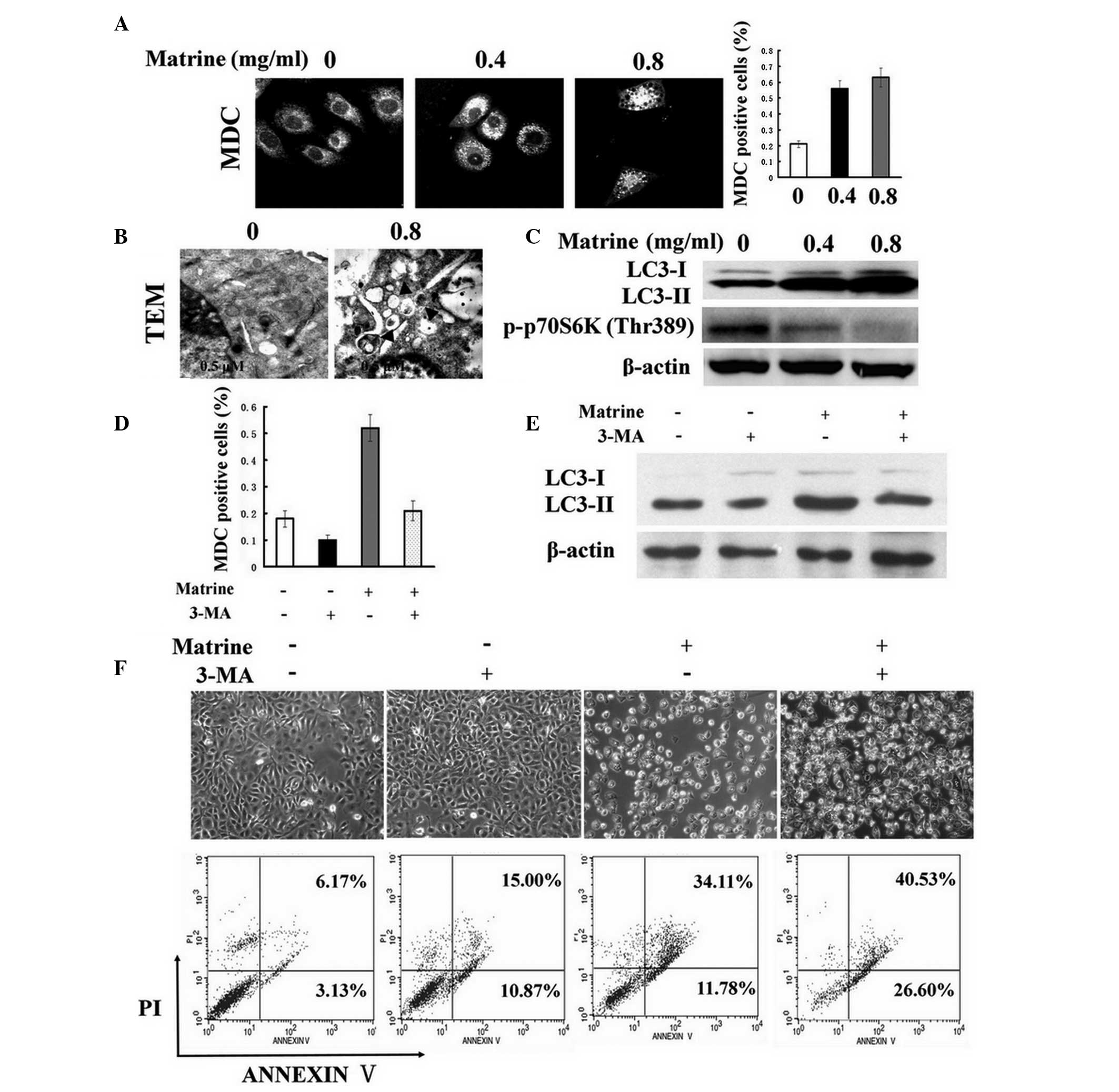 | Figure 2Matrine promotes autophagy in WB-F344
cells, which inhibits apoptosis. (A) Following exposure to matrine
at 0.4 (0.4) and 0.8 mg/ml (0.8) for 24 h, MDC staining dots were
accumulated in the treated cells, in contrast to the control (0).
Representative confocal microscopic images (magnification, ×600)
were obtained. A significant difference between MDC-positive cells
(%) in matrine-treated cells and untreated cells was present. (B)
Representative electron microscopic images were obtained from
matrine-treated cells at 0.8 mg/ml. The typical autophagosomes or
autolysosomes are denoted by arrows (original magnification,
×10,000). (C) Western blot analysis of LC3-II and p-p70S6K (Thr389)
in the cells subsequent to exposure to matrine at 0.4 (0.4) and 0.8
mg/ml (0.8) for 24 h. β-actin was employed as a protein loading
control. (D) The cells were treated with matrine for 24 h in the
presence or absence of 5 mM 3-MA. MDC-positive cells (%) are
presented as the mean ± standard error of three independent
experiments, and a significant difference was found between the
combination group (Matrine + 3-MA) and the matrine group (Matrine)
at the level of P<0.05. (E) Immunoblotting results represent the
decrease of LC3-II levels under matrine treatment in combination
with 3-MA (Matrine + 3-MA), as compared with matrine alone. (F)
Representative light microscopic images (magnification, ×100) and
Annexin V/PI analysis revealed that apoptosis was increased in the
combined group (Matrine + 3-MA) as compared with matrine alone
(Matrine). LC3, microtubule-associated protein 1 light chain 3; PI,
propidium iodide; TEM, transmission electron microscopy; MDC,
monodansylcadaverine; 3-MA, 3-methyladenine. |
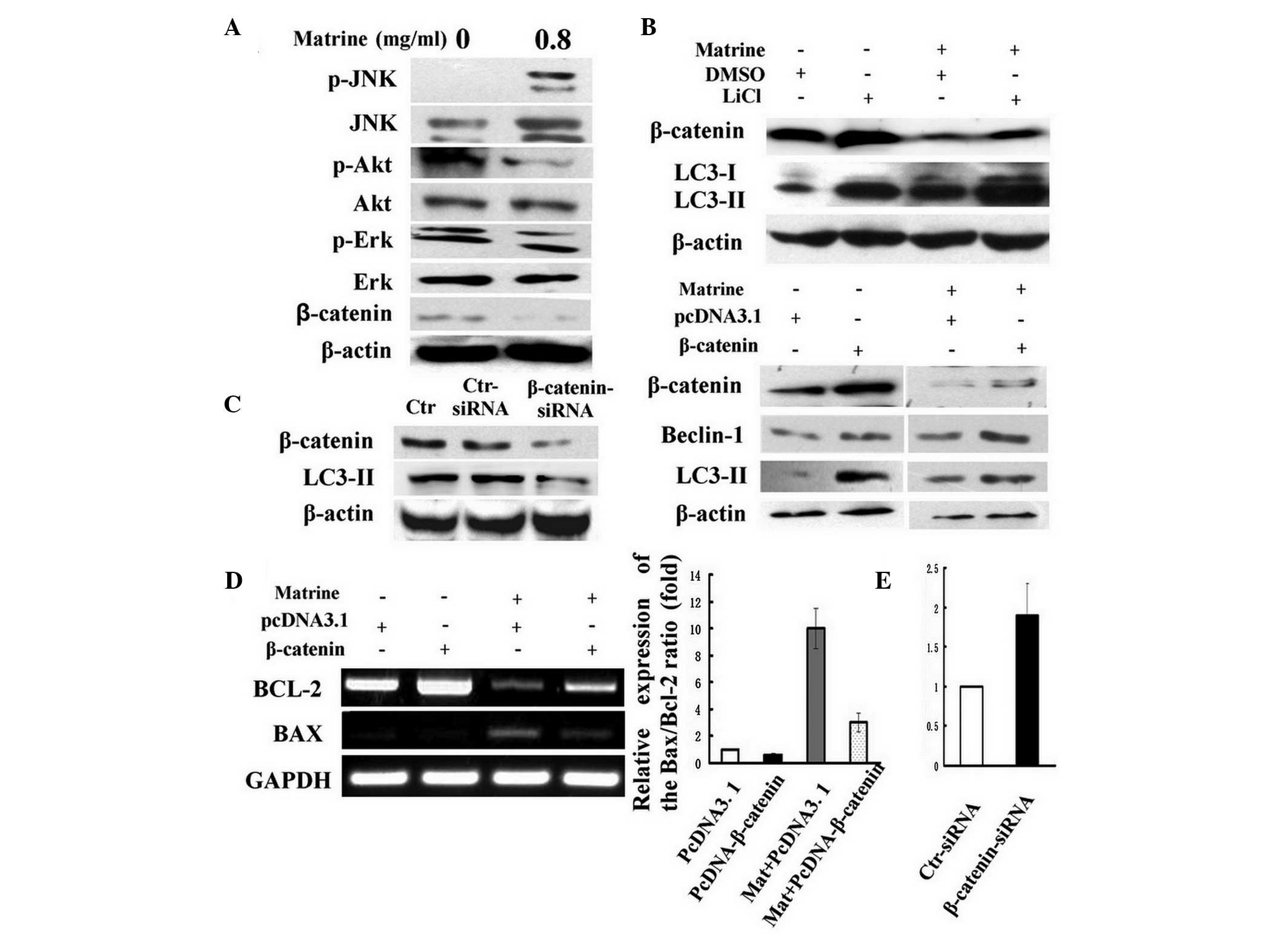
β-catenin is involved in matrine-induced
autophagy and apoptosis in WB-F344 cells
Accumulating data indicate that Akt, ERK, JNK and
Wnt/β-catenin signaling pathways have significant roles in
apoptosis or autophagy (14). As
shown in Fig. 3A, immunoblotting
analysis revealed that matrine treatment suppressed the
phosphorylation of Akt and ERK, but significantly increased the
phosphorylation of JNK. β-catenin, the major downstream effector of
the canonical Wnt signaling pathway, was attenuated by matrine in
WB-F344 cells.
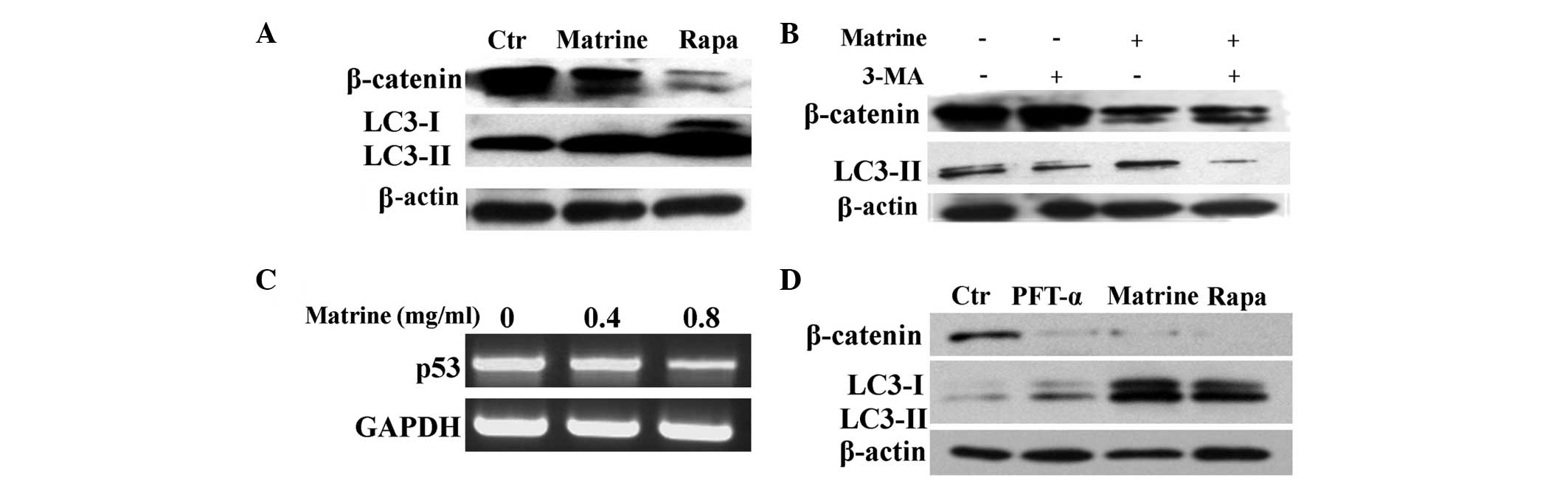
 | Figure 3The wnt/β-catenin pathway is involved
in matrine-induced autophagy and apoptosis. (A) The cells were
treated with matrine at 0.8 mg/ml (0.8) for 24 h. Immunoblotting
results represent the levels of p-JNK, p-Akt, p-ERK and β-catenin
in WB-F344 cells. (B) In the upper panels, the cells were treated
with matrine at 0.8 mg/ml for 24 h in the presence or absence of 20
mM LiCl. Control cells were treated with dimethylsulfoxide.
Immunoblotting analysis of the increase in LC3-II levels in
matrine-treated cells cotreated with LiCl. In the lower panels, the
cells were transiently transfected with constitutively active
β-catenin plasmid. The pCDNA3.1 plasmid was used as a control.
After 24 h, the transfected cells were incubated with matrine at
0.8 mg/ml for 24 h. Immunoblotting analysis of the increase of
LC3-II and beclin-1 levels in matrine-treated cells transfected
with β-catenin overexpression plasmid. (C) β-catenin was suppressed
by siRNA in the WB-F344 cells and β-catenin loss led to a decline
in LC3-II levels as indicated by immunoblotting. The scrambled
siRNA was used as a control. (D) RT-PCR and qPCR analysis of the
Bax/Bcl-2 ratio in matrine-treated cells transfected with β-catenin
overexpression plasmid. The ΔΔCt value of the Bax/Bcl-2 ratio in
untreated cells was normalized to 1 as a control. (E) qPCR analysis
of the Bax/Bcl-2 ratio in WB-F344 cells transfected with β-catenin
siRNA. The scrambled siRNA was used as a control. p-JUN,
phosphorylated-c-Jun N-terminal kinase JNK; ERK, mitogen-activated
protein kinase 1; LiCl, LC3, microtubule-associated protein 1 light
chain 3; siRNA, small interfering RNA; RT-PCR, real-time polymerase
chain reaction; qPCR, quantitative PCR; Bcl-2, B-cell lymphoma 2;
Bax, Bcl2-associated X protein; Mat, matrine; Ctr, untreated
control cells. |
The Wnt/β-catenin pathway has a crucial role in stem
cell development and renewal. Thus, the role of β-catenin protein
in matrine-induced autophagy and apoptosis was analyzed next. LiCl,
activating Wnt signaling selectively via the β-catenin/T-cell
factor pathway (15), led to an
increase in the ratio of LC3-II/β-actin, which was enhanced upon
the addition of matrine (Fig. 3B).
Similarly, upregulation of beclin-1, the angiotensinogen (Atg)6
mammalian analogue, and the LC3-II/β-actin ratio, was further
received in WB-F344 cells transiently transfected with the
β-catenin overexpression plasmid, indicating that β-catenin
activation stimulated autophagy (Fig.
3B). Additionally, the decline in Bcl-2/Bax ratio under matrine
treatment was reversed by β-catenin overexpression in contrast to
the control plasmid (Fig. 3D). In
addition, it was tested whether inactivation of β-catenin inhibits
autophagy activation in WB-F344 cells. For this purpose, RNA
interference was used to deplete the β-catenin protein. The amount
of β-catenin protein and the ratio of LC3-II/β-actin in the cells
were reduced by the specific siRNA duplex as compared to the
control siRNA (Fig. 3C).
Furthermore, the Bax/Bcl-2 ratio was increased in WB-F344 cells
transfected with β-catenin siRNA (Fig.
3E). These results indicated that β-catenin is involved in
matrine-induced autophagy and apoptosis, possibly through the
Bax/Bcl-2 regulation.
β-catenin is modulated by autophagy and
p53 in WB-F344 cells
It has been recently reported that β-catenin is
downregulated by autophagy (14).
As expected, β-catenin was significantly attenuated by matrine and
rapamycin (Fig. 4A), a foregone
chemical agonist of autophagy (16). However, 3-MA partially reversed the
inhibitory effect of β-catenin levels by matrine (Fig. 4B), indicating that β-catenin
degradation was negatively modulated by matrine-induced autophagy.
Additionally, the Wnt/β-catenin pathways can be modulated by the
tumor suppressor p53, which can turn autophagy on or off (17). Next, it was investigated whether
p53 was implicated in the β-catenin signal transduction via
increased autophagy in liver oval cells. RT-PCR revealed that
matrine attenuated the levels of p53 (Fig. 4C). Subsequently, PFT-α, a
pharmacological inhibitor of p53 (18), was used to quantify the effect of
p53 levels on Wnt signaling and autophagy. PFT-α caused a
significant decline of β-catenin levels, but exerted little or no
effect on the LC3-II/β-actin ratio. While β-catenin levels
decreased, an increase in the LC3-II/β-actin ratio was detected
following exposure to rapamycin and matrine (Fig. 4D). These results indicated that p53
interfered with the β-catenin signaling pathway, possibly not via
autophagy in WB-F344 cells. β-catenin degradation may be separately
modulated by autophagy and p53.
Discussion
Matrine has been shown to exhibit the ability to
promote hepatocytic differentiation of rat liver oval cells, one of
the origins of HCC, and thereby, to have the potential to be
applied in HCC prevention. Besides hepatocytic differentiation,
matrine was identified to inhibit rat oval cell proliferation,
possibly not via autophagic cell death, but via apoptosis. In rat
hepatic oval cells, blockage of the cell cycle at the G0/G1-phase
and stimulation of apoptosis was presented following exposure to
matrine.
Morphological observations revealed that autophagy
was stimulated under matrine treatment. Results on LC3 expression
and mTOR inhibition further confirmed these observations. In
matrine-treated C6 glioma cells, besides apoptosis, autophagy has
been regarded as an additional process of cell death (6). Conversely, other studies have
demonstrated that autophagy protects against apoptosis and serves
as a cell survival mechanism (19–24).
The present study identified that chemical inhibition of autophagy
enhanced matrine-induced apoptosis, indicating that autophagy
functions as a mechanism of cell survival under matrine treatment.
Additionally, it was demonstrated that the activation of β-catenin
promotes autophagy in rat hepatic oval cells. The activation of the
Wnt/β-catenin signaling pathway has been reported to account for
cell survival and proliferation in vitro (25). An aberrant activation of β-catenin,
which has a role in cell survival or stem cell renewal, was
detected in hepatic adenoma and HCC (26). It is conceivable that
β-catenin-induced autophagy conduces to oval cell proliferation and
adaptation.
A pathway contributing to apoptosis involves the
Wnt/catenin signaling cascade (27). Matrine treatment enhanced β-catenin
degradation and the Bax/Bcl-2 ratio in rat liver oval cells,
indicating that β-catenin may be associated with the induction of
apoptosis by matrine. Subsequently, β-catenin inactivation by RNA
interference upregulated the Bax/Bcl-2 ratio, whereas β-catenin
activation reversed the stimulative effect of the Bax/Bcl-2 ratio
by matrine. Possibly, β-catenin loss is essential for
matrine-induced stem cell apoptosis. Furthermore, the present study
demonstrated that β-catenin inactivation was downregulated by the
induction of autophagy by matrine. Under metabolic stress,
autophagy induction was previously observed to negatively regulate
the Wnt signaling cascade through accelerating segment polarity
protein dishevelled homolog-2 turnover (28). It appears that autophagy-mediated
β-catenin inactivation induces rat oval cell apoptosis. The
canonical Wnt signaling possibly functions as a crosstalk between
autophagy and apoptosis in rat hepatic oval cells.
Autophagy and apoptosis pathways are usually
regulated by several common factors, including Beclin-1 and Atg5
(29,30); however, the underlying mechanisms
remain to be elucidated. β-catenin activation was indicated to
enhance LC3-II levels, but reduce the Bax/Bcl-2 ratio in oval
cells, whereas inactivation of the Wnt cascade decreased LC3-II
levels, but increased the Bax/Bcl-2 ratio, indicating that
β-catenin is linked to matrine-induced autophagy and apoptosis,
possibly via the Bax/Bcl-2 proteins. The anti-apoptotic protein
Bcl-2 can also inhibit autophagy by a direct interaction with
beclin-1 (31). The intersection
between autophagy and apoptosis is presumably cross-linked via
β-catenin, which conjugates or dissociates Bcl-2 from the complex
with beclin-1 through a mechanism that remains to be
elucidated.
It has been previously reported that p53 can
adversely modulate the Wnt/β-catenin signaling (32). The present study demonstrated that
β-catenin inactivation and p53 loss was detected concurrently in
matrine-treated cells. Thus, this elimination of β-catenin is
likely to be exerted by the autophagy process induction by p53
inactivation. However, chemical inhibition of p53 decreased
β-catenin accumulation, but failed to affect the autophagic
activities. Therefore, it is likely that p53 did not exert its
effect via the autophagic flux. The existence of parallel pathways,
a canonical cascade involving components of the Wnt pathway and a
second mechanism involving seven in absentia homolog 1,
Siah-interacting protein and Ebi (32), may contribute to β-catenin
degradation by p53 in oval cells.
In conclusion, matrine-induced apoptosis, which is
involved in β-catenin inactivation, contributes to oval cell
suppression, while β-catenin activation is associated with the
autophagic flux. The canonical Wnt pathway, which can be regulated
by autophagy and p53, is likely to be a novel intersection between
autophagy and apoptosis in hepatic stem cells.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30772859). The authors would like
to thank Professor Ze Bin Mao for his professional support and
help.
References
|
1
|
Luo C, Zhu Y, Jiang T, et al: Matrine
induced gastric cancer MKN45 cells apoptosis via increasing
pro-apoptotic molecules of Bcl-2 family. Toxicology. 229:245–252.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Long Y, Lin XT, Zeng KL and Zhang L:
Efficacy of intramuscular matrine in the treatment of chronic
hepatitis B. Hepatobiliary Pancreat Dis Int. 3:69–72.
2004.PubMed/NCBI
|
|
3
|
Guo D, Chen NN, Zhou P, Pan B and Hou LB:
Suppressive effect of matrine on cell growth and decreases
beta-catenin-dependent transcriptional activity in hepatoma cell
line Hep3B. Journal of Chinese Medicinal Materials. 33:778–781.
2010.(In Chinese).
|
|
4
|
Zhang Y, Zhang H, Yu P, et al: Effects of
matrine against the growth of human lung cancer and hepatoma cells
as well as lung cancer cell migration. Cytotechnology. 59:191–200.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang LP, Jiang JK, Tam JW, et al: Effects
of Matrine on proliferation and differentiation in K-562 cells.
Leuk Res. 25:793–800. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang S, Qi J, Sun L, et al: Matrine
induces programmed cell death and regulates expression of relevant
genes based on PCR array analysis in C6 glioma cells. Mol Biol Rep.
36:791–799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coleman WB, Wennerberg AE, Smith GJ and
Grisham JW: Regulation of the differentiation of diploid and some
aneuploid rat liver epithelial (stemlike) cells by the hepatic
microenvironment. Am J Pathol. 142:1373–1382. 1993.PubMed/NCBI
|
|
8
|
Coleman WB, Smith GJ and Grisham JW:
Development of dexamethasone-inducible tyrosine aminotransferase
activity in WB-F344 rat liver epithelial stemlike cells cultured in
the presence of sodium butyrate. J Cell Physiol. 161:463–469. 1994.
View Article : Google Scholar
|
|
9
|
Couchie D, Holic N, Chobert MN, Corlu A
and Laperche Y: In vitro differentiation of WB-F344 rat liver
epithelial cells into the biliary lineage. Differentiation.
69:209–215. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsao MS and Grisham JW: Hepatocarcinomas,
cholangiocarcinomas, and hepatoblastomas produced by chemically
transformed cultured rat liver epithelial cells. A light- and
electron-microscopic analysis. Am J Pathol. 127:168–181. 1987.
|
|
11
|
Biederbick A, Kern HF and Elsasser HP:
Monodansylcadaverine (MDC) is a specific in vivo marker for
autophagic vacuoles. Eur J Cell Biol. 66:3–14. 1995.PubMed/NCBI
|
|
12
|
Kabeya Y, Mizushima N, Ueno T, et al: LC3,
a mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caro LH, Plomp PJ, Wolvetang EJ, Kerkhof C
and Meijer AJ: 3-Methyladenine, an inhibitor of autophagy, has
multiple effects on metabolism. Eur J Biochem. 175:325–329. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krajarng A, Nakamura Y, Suksamrarn S and
Watanapokasin R: alpha-Mangostin induces apoptosis in human
chondrosarcoma cells through downregulation of ERK/JNK and Akt
signaling pathway. J Agric Food Chem. 59:5746–5754. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura T, Sano M, Songyang Z and
Schneider MD: A Wnt- and beta-catenin-dependent pathway for
mammalian cardiac myogenesis. Proc Natl Acad Sci USA.
100:5834–5839. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bjornsti MA and Houghton PJ: The TOR
pathway: a target for cancer therapy. Nat Rev Cancer. 4:335–348.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Levine B and Abrams J: p53: The Janus of
autophagy? Nat Cell Biol. 10:637–639. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bassi L, Carloni M, Fonti E, Palma de la
Pena N, Meschini R and Palitti F: Pifithrin-alpha, an inhibitor of
p53, enhances the genetic instability induced by etoposide (VP16)
in human lymphoblastoid cells treated in vitro. Mutat Res.
499:163–176. 2002. View Article : Google Scholar
|
|
19
|
Lee SB, Tong SY, Kim JJ, Um SJ and Park
JS: Caspase-independent autophagic cytotoxicity in
etoposide-treated CaSki cervical carcinoma cells. DNA Cell Biol.
26:713–720. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harhaji-Trajkovic L, Vilimanovich U,
Kravic-Stevovic T, Bumbasirevic V and Trajkovic V: AMPK-mediated
autophagy inhibits apoptosis in cisplatin-treated tumor cells. J
Cell Mol Med. 13:3644–3654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishikawa T, Tsuno NH, Okaji Y, et al:
Inhibition of autophagy potentiates sulforaphane-induced apoptosis
in human colon cancer cells. Ann Surg Oncol. 17:592–602. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abedin MJ, Wang D, McDonnell MA, Lehmann U
and Kelekar A: Autophagy delays apoptotic death in breast cancer
cells following DNA damage. Cell Death Differ. 14:500–510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie BS, Zhao HC, Yao SK, et al: Autophagy
inhibition enhances etoposide-induced cell death in human hepatoma
G2 cells. Int J Mol Med. 27:599–606. 2011.PubMed/NCBI
|
|
24
|
Guo L, Xie B and Mao Z: Autophagy in
premature senescent cells is activated via AMPK pathway. Int J Mol
Sci. 13:3563–3582. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kato H, Gruenwald A, Suh JH, et al: The
Wnt/{beta}-catenin pathway in podocytes integrates cell adhesion,
differentiation and survival. J Biol Chem. 286:26003–26015.
2011.
|
|
26
|
Thompson MD and Monga SP: WNT/beta-catenin
signaling in liver health and disease. Hepatology. 45:1298–1305.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moon RT, Bowerman B, Boutros M and
Perrimon N: The promise and perils of Wnt signaling through
beta-catenin. Science. 296:1644–1646. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao C, Cao W, Bao L, et al: Autophagy
negatively regulates Wnt signalling by promoting Dishevelled
degradation. Nat Cell Biol. 12:781–790. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li M, Jiang X, Liu D, Na Y, Gao GF and Xi
Z: Autophagy protects LNCaP cells under androgen deprivation
conditions. Autophagy. 4:54–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pattingre S, Tassa A, Qu X, et al: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sadot E, Geiger B, Oren M and Ben-Ze’ev A:
Down-regulation of beta-catenin by activated p53. Mol Cell Biol.
21:6768–6781. 2001. View Article : Google Scholar : PubMed/NCBI
|