Introduction
Melanoma is one of the most common tumors to
metastasize to the brain. Leptomeningeal carcinomatosis (LMC) is
defined as a malignant infiltration of the pia mater and the
arachnoid membranes. LMC is one of the most serious complications
occurring in cancer patients. According to the results of an
extensive autopsy study, the incidence of LMC was 5–8% in cancer
patients (1). Since a significant
proportion of these patients exhibited asymptomatic microscopic
disease, the clinical diagnosis of LMC is established in only 2–4%
of the patients diagnosed with LMC at autopsy. LMC is frequently
detected in patients with leukaemia, breast cancer, lymphoma,
melanoma and lung cancer. Among solid tumors, LMC is observed more
frequently in cases of disseminated and progressive disease.
Although a subset of patients, particularly those with lymphoma or
breast cancer, may survive for more than 12 months with a
reasonable quality of life, leptomeningeal metastasis from solid
tumors such as melanoma, is associated with a poor overall
prognosis. The treatment of LMC is palliative and unsatisfactory.
At present, intrathecal chemotherapy is the standard treatment, but
this procedure does not demonstrate a significant increase in
survival, and at best is only palliative (2).
Case report
We present a case of a 44-year-old female who was
diagnosed with cutaneous melanoma on the back in August 2008. She
underwent surgery, and sentinel node testing was negative.
Pathological findings were consistent with nodular melanoma, Clark
III, Breslow thickness 2.62 mm, 9 mitoses/field, non-ulcerated and
without vascular or perineural invasion. No satellite lesions were
found upon pathological examination. The work-up did not show
distant metastases (physical examination, abdominal ultrasound and
chest X-ray). Following surgery, the patient was referred to the
Medical Oncology Department to evaluate whether adjuvant treatment
with interferon was required. The lesion did not show poor
prognostic criteria (Clark <IV, non-ulcerated,
non-lymphovascular invasion); therefore, she did not receive
adjuvant interferon treatment according to Kirkwood's regimen
(3). The patient continued with
routine follow-up every 3 months including a physical examination,
abdominal ultrasound, chest x-ray and blood test with hemogram and
basic biochemistry that included lactate dehydrogenase and
transaminases.
In June 2009, she was admitted to the emergency room
with a persistent headache, predominantly in the frontal area, that
did not respond to anti-inflammatory drugs. A head computed
tomography (CT) and magnetic resonance imaging (MRI) showed a 28-mm
lesion located in the right frontoparietal area with considerable
perilesional edema with subfalcial hernia and compression of the
frontal horn of the right lateral ventricle. All images were
suggestive of metastasis. An MRI of the brain confirmed the CT scan
findings and did not show additional lesions in the cerebral
parenchyma (Fig. 1a and b). Steroid
therapy was commenced (dexamethasone 8 mg tid), and after 1 day of
steroid treatment, the headache almost subsided. The patient
underwent a work-up study with body CT scan and PET-TAC. In all of
these studies, no evidence of extra-cerebral disease was found. The
patient was referred to the Neurosurgical Department to consider
resection of the solitary melanoma brain metastasis.
In September 2009, she underwent resection for brain
metastasis. Pathological examination showed features suggestive of
melanoma metastasis with a resection margin free of tumor. To
reduce the risk of brain dissemination, the patient received whole
brain radiation 15 days after resection.
The patient continued with the routine follow-up,
including body CT and MRI of the brain every 3 months. A PET-TAC
carried out on October 2009 did not show any uptakes suggestive of
malignant disease.
In December 2009, she was admitted to the emergency
room because of an acute confusional state and severe back pain.
The condition was extremely acute and progressive. The pain did not
subside with anti-inflammatory drugs. A fever of 38°C, blood
pressure 150/110, somnolence (Glasgow Coma Scale 13) and positivity
for meningeal signs were noted. Blood testing did not show relevant
findings. A non-traumatic lumbar puncture was performed. The
macroscopical appearance of the cerebrospinal fluid (CSF) was
haematic and cloudy, and the opening pressure was 40 mmHg. The
biochemical analysis of the CSF showed a glucose of 6 mg/dl
(40–70), proteins 265.8 mg/dl (15–40) and lactate 7.1 (0–3). The
cell consisted of leucocytes 38 cells/mm3; neutrophils
2%; lymphocytes 98% and erythrocytes, 1,000 cells/mm3.
CT of the head showed post-surgical changes and possible bleeding
(Fig. 2a and b). The MRI showed a
right frontal area of malacia related to the previous metastasis
surgery with 19×13 mm of transversal and anteroposterior diameters.
Various changes surrounding this area were observed, suggestive of
post-radiotherapy gliosis. In T1-weighted images (T1WI) in this
malacic area a hyperintensity was observed that was potentially
related to haemorrhage. In convexity, in the posterior parasagital
region and particularly in the frontal area, a sulcus
hyperintensity was described. The findings were suggestive of a
subarachnoid haemorrhage (SAH) and/or meningeal carcinomatosis. the
third ventricle was enlarged with periventricular hyperintensity
consistent with early acute hydrocephaly (Fig. 2c). Interpretation of these findings
by radiologists was difficult due to the severe agitation of the
patient even after administration of intensive sedative
medications.
Based on the MRI images a possible diagnosis was SAH
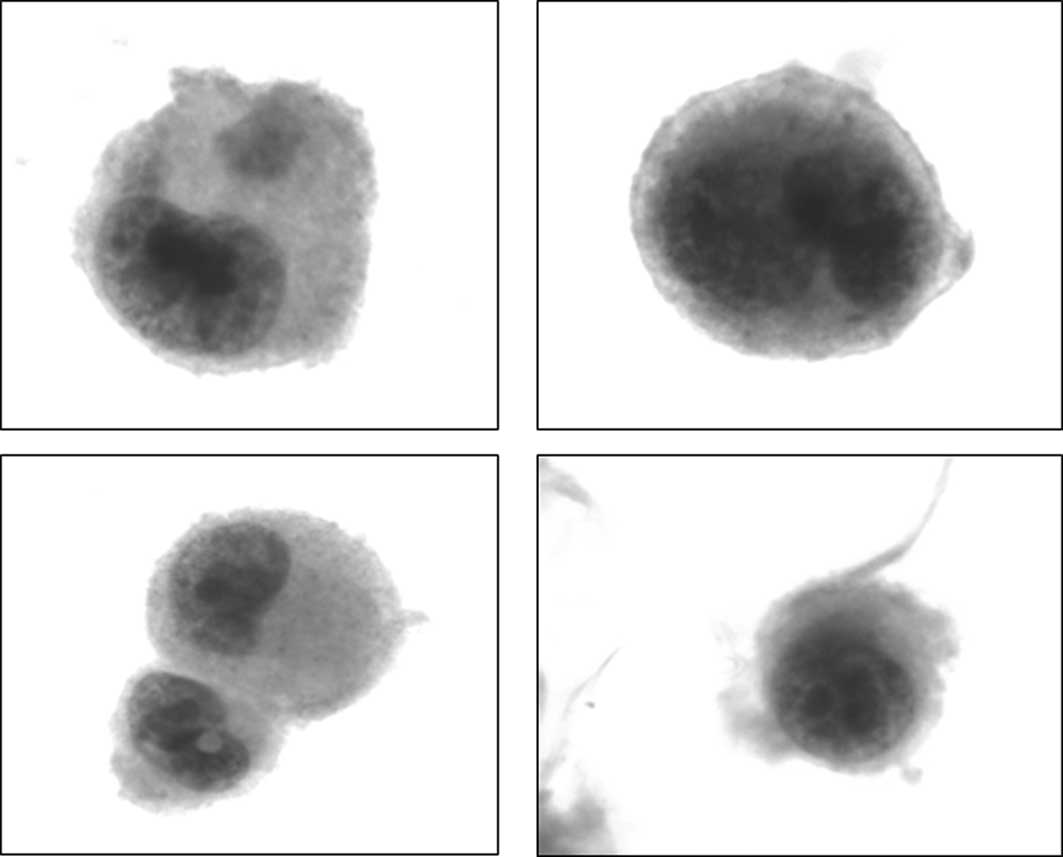
and/or meningeal infiltration. The cytological examination of the
CSF showed neoplastic epithelial cells consistent with metastatic
melanoma cells. Immunohistochemical analysis was not performed due
to the small number of atypical cells found (Fig. 3). Treatment of high doses of
corticoids did not show any improvement, and the patient developed
a progressive confusional syndrome. Intrathecal chemotherapy was
not possible due to her poor functional status. The patient
succumbed 1 week after admission.
Discussion
Whereas brain metastasis can be a complication
associated with various types of solid tumors, such as small-cell
lung cancer and melanoma, leptomeningeal dissemination (LMD) is a
relatively infrequent complication of solid tumors. Again, the most
common solid malignancies associated with brain metastasis are
breast cancer, small-cell lung cancer and melanoma. The majority of
patients with leptomeningeal metastasis have advanced disease, and
it rarely occurs as an initial or unique presentation.
We presented a case of a patient with a solitary
brain metastasis of melanoma previously treated with surgery.
Numerous trials have served as level I evidence supporting the role
of surgery in patients with single brain metastasis, particularly
for recursive partitioning analysis class I. Some authors support
the combination of surgery followed by radiosurgery and whole-brain
radiation as one of the treatment modalities that achieves the
longest progression-free survival (4) (Table
I). However, in our patient, resection was followed by
whole-brain radiation instead of whole-brain radiation alone
(5). Surgery of brain metastases
is, however, not free of complications. At present, there is
discussion whether surgery for brain metastasis causes meningeal
dissemination. Resection of metastatic posterior fossa lesions
(MPLFs) is often cited as a risk factor for LMD, but a review of
the literature suggests the need for further evidence (6–8).
 | Table IPatient survival when one or more
treatments were administered for brain metastases.a |
Table I
Patient survival when one or more
treatments were administered for brain metastases.a
| Treatment | No. | % | Median survival
(months) |
|---|
| None | 83 | 23.3 | 2.04 |
| WBRT alone | 100 | 28.2 | 3.98 |
| RS alone | 26 | 7.3 | 9.87 |
| Surgery alone | 36 | 10.1 | 8.16 |
| WBRT + RS | 20 | 5.6 | 9.44 |
| Surgery + WBRT | 58 | 16.3 | 8.81 |
| Surgery + RS | 20 | 5.6 | 13.75 |
| Surgery + WBRT +
RS | 12 | 3.4 | 10.20 |
One recent publication demonstrated the risk of
meningeal dissemination with resection of MPLFs compared to
radiosurgery. Suki et al found an increased risk of LMD in
patients treated with surgery compared to patients treated with
radiosurgery, although the difference did not reach statistical
significance (9). The slow CSF flow
in the posterior fossa region of the brain that promotes the
deposition of circulating cells, and the subarachnoid space and
cisterna magna in this region, which may offer a nidus for
malignant cells, may increase the risk of LMD following resection
of tumors in this region (10).
Conversely, the absence of such a CSF-containing space over the
hemispheres may explain the low incidence of LMD in cases of
supratentorial lesions. There is no formal estimate of the minimum
number of tumor cells in the CSF that are sufficient to cause LMD.
Extrapolating from animal models, in which the introduction of
3,000 tumor cells has resulted in rapid death from LMD, the number
is likely to be relatively small (11). Van der Ree et al published a
review of the records of all the patients operated on for brain
metastasis between January 1990 and August 1995 in their centre.
The authors included 28 patients with melanoma brain metastasis who
underwent surgery for intracranial lesions. Their results showed
that 9 patients (33%) developed meningeal metastasis 2–13 months
after surgery, which included 6 of the 9 patients operated on for
posterior fossa metastasis (12).
In contrast, DeAngelis et al found that 38% of patients
developed leptomeningeal metastasis following surgery for
cerebellar metastasis, while only 4.7% of the patients were treated
for a supratentorial metastasis (13).
The present case report described a patient who
developed LMC 3 months after solitary brain resection in the
anterior fossa. She also presented with meningeal signs, and an MRI
showed images suggestive of SAH and/or LMC. The CFS analysis showed
malignant as well as red blood cells. It is well known that
melanoma meningeal lesions bleed, explaining the association
between LMC and SAH. The relationship between SAH and
leptomeningeal metastases was previously reported in only 5 cases
in the literature. The diagnosis of SAH secondary to neoplastic
seeding is based on CSF studies and on neuroimaging evaluations,
which are confirmed by autopsy or surgery (14). In a retrospective evaluation of 120
patients treated for leptomeningeal metastases, Lossos et al
reported that 3 patients had spontaneous SAH in the absence of a
bleeding tendency. Resection of an intraparenchymal posterior fossa
tumor antedated the development of subarachnoid seeding in 3 of the
5 patients (15).
Taking into account the radiological findings, the
CSF examination and the previous surgery for melanoma brain
metastasis, we concluded that our patient presented with an SAH
secondary to LMC. We attributed this complication to LMC since the
MRI did not show aneurisms or any other type of malformation. The
patient outcome was unforeseen, particularly when we consider that
the PET-TAC did not show any uptakes suggestive of cancer in other
locations. Although the risk of LMC was low following brain
metastasis resection due to its location, we suggest that there was
a clear relationship between the surgery and LMC. In addition, this
patient suffered an acute meningeal syndrome which may have been
secondary to the SAH according to the haemorrhagic CSF and MRI
findings. This complication, as is well known, is not a rare
consequence of melanoma meningeal dissemination.
References
|
1
|
Pentheroudakis G and Pavlidis N:
Management of leptomeningeal malignancy. Expert Opin Pharmacother.
6:1115–1125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruno MK and Raizer J: Leptomeningeal
metastases from solid tumours (meningeal carcinomatosis). Cancer
Treat Res. 125:31–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tarhini AA and Kirkwood JM: Clinical and
immunologic basis of interferon therapy in melanoma. Ann NY Acad
Sci. 1182:47–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raizer J, Hwu W, Panageas K, et al: Brain
and leptomeningeal metastases from cutaneous melanoma: survival
outcomes based on clinical features. Neuro Oncol. 10:199–207. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sloan A, Nock C, Estein D, et al:
Diagnosis and treatment of melanoma brain metastasis: a literature
review. Cancer Control. 3:248–254. 2009.PubMed/NCBI
|
|
6
|
Chamberlain MC and Johnston SK: Neoplastic
meningitis: survival as a function of cerebrospinal fluid cytology.
Cancer. 115:1941–1946. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vellin JF, Achim V, Sinardet D, et al:
Rapidly developing leptomeningeal carcinomatosis following anterior
skull base surgery: a case report. Auris Nasus Larynx. 34:565–567.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu H, Mitsumori M, Nagata Y, et al:
Meningeal carcinomatosis in patients with breast cancer: report of
8 patients. Breast Cancer. 8:74–78. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suki D, Abouassi H, Patel A, et al:
Comparative risk of leptomeningeal disease after resection or
stereotactic radiosurgery for solid tumor metastasis to the
posterior fossa. J Neurosurg. 108:248–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grossman SA and Krabak MJ: Leptomeningeal
carcinomatosis. Cancer Treat Rev. 25:103–119. 1999. View Article : Google Scholar
|
|
11
|
Norris LK, Grossman SA and Olivi A:
Neoplastic meningitis following surgical resection of isolated
cerebellar metastasis: a potentially preventable complication. J
Neurooncol. 32:215–233. 1997. View Article : Google Scholar
|
|
12
|
Van der Ree T, Dippel D, Avezaart C,
Sillevis Smitt PA, Vecht CJ and van den Bent MJ: Leptomeningeal
metastasis after surgical resection of brain metastases. J Neurol
Neurosurg Psychiatry. 66:225–227. 1999.PubMed/NCBI
|
|
13
|
De Angelis LM, Mandell LR, Thaler HT, et
al: The role of postoperative radiotherapy after resection of
single brain metastasis. Neurosurgery. 24:798–805. 1989.PubMed/NCBI
|
|
14
|
Chang C, Kuwana N and Ito S: Spinal
leptomeningeal metastases of giant cell glioblastoma associated
with subarachnoid haemorrhage: case report. J Clin Neurosci.
8:56–59. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lossos A and Siegal T: Spinal subarachnoid
haemorrhage associated with leptomeningeal metastases. J
Neurooncol. 12:167–171. 1992. View Article : Google Scholar
|