Introduction
The past and present
The urinary bladder is an elastic reservoir that is
responsible for the low pressure storage of urine. A continent,
pain free and healthy bladder is crucial for the preservation of a
good quality of life. Numerous conditions disturb the anatomy and
physiology of the urinary bladder, leading to insufficient and
restricted evacuation of urine (1).
Bladder cancer (BC) is the sixth most common cause
of cancer-associated morbidity in the western world: In the UK in
2010, there were 10,300 new cases, and 4,900 cases of BC-associated
mortality (2). It represents the most
common malignancy of the urinary tract, with a median survival rate
following metastasis that rarely surpasses 15 months (3). The vast majority (90%) of bladder
tumours are histologically classified as urothelial cell carcinoma,
these tumours are often further classified as being non-muscle
invasive BC (NMIBC) or muscle invasive BC (MIBC) (4). On initial presentation, 30% of patients
are diagnosed with an MIBC tumour. From the remainder of patients
that had been diagnosed with an NMIBC tumour at presentation, 30%
go on to develop an MIBC during follow-up (5). With the highest susceptibility of
recurrence and progression of any malignancy, patients with BC
require consistent surveillance and close follow-up during and,
more importantly, following therapy. With such high odds of
recurrence, BC has produced the most expensive management protocol
of any malignancy, costing the National Health Service ~£55.39
million in the UK per annum (6). The
gold standard treatment for organ-confined MIBC is radical
cystectomy, with dissection of the pelvic lymph nodes. Regardless,
malignancy is not the sole bladder-associated pathology in which
cystectomy is indicated (7).
Bladder damage occurs in several additional
disorders within the genitourinary (GU) system, including
inflammatory conditions (interstitial cystitis/bladder pain
syndrome), urinary tract infections, nerve damage (neuropathic
bladder) and congenital disorders (spina bifida). In these cases,
the essential task is to re-establish the function of the urinary
bladder. Medical treatments are limited, and thus, surgical
intervention, particularly cystectomy, is often required (8–11).
Since the first BC-associated cystectomy in 1887,
the pursuit to identify the most appropriate replacement voiding
system has been largely problematic (12). At present, the most common method of
bladder replacement or repair involves the use of autologous
segments of gastrointestinal tissue, in order to restore bladder
storage and voiding capacity (13).
This is often achieved through ileal conduit urinary diversion,
orthotopic bladder substitution or continent cutaneous diversion
(Table I) (14,15).
 | Table I.Surgical procedures used post
cystectomy. |
Table I.
Surgical procedures used post
cystectomy.
| Technique | Description |
|---|
| Ileal conduit
urinary diversion | Urine is drained
from the ureters to a loop of small bowel anastomosed to the
abdominal skin surface. It is then collected in an external
appliance. |
| Orthotopic bladder
substitution | This practice
imitates the typical role of the urinary bladder through using a
section of bowel to reconstruct the bladder. |
| Continent cutaneous
diversion | This method uses
the ileocaecal valve which may aid the regulation of urination.
This procedure utilises the bowel, which is deployed to mimic the
urinary bladder. The ureters are attached to the pouch. The pouch
is then brought to the skin as a stoma. |
|
Ureterosigmoidostomy | Possibly the oldest
procedure, here the ureters are attached to the large bowel and
urine exits this way. The anal sphincter provides continence. |
Since the original application of a free tissue
graft in 1917, in which canine bladders were augmented with fascia,
various additional materials have been utilized as free grafts
(16). The ileum, however, is the
most frequently utilized segment in bladder augmentation, due to
its notably simple mobilization, extensive mesentery and
copiousness; features that are particularly appropriate for urinary
tract substitution (17). Although
the use of gastrointestinal segments in bladder reconstruction was
first proposed almost 150 years ago, it remains the gold standard
due to the absence of a superior alternative. This was demonstrated
by Stein et al (18), who
observed excellent long-term survival rates in patients undergoing
radical cystectomy. Whilst radical extirpation of the bladder is
frequently successful, from an oncological perspective, substantial
morbidity is associated with enteric interposition within the GU
tract (Table II) (19–22).
Surgical intervention itself requires procedures on the urinary and
gastrointestinal tracts which are associated with complication
rates of ≤66%. Considering that the vast majority of patients that
require cystectomy and consequent bladder substitution are elderly
and suffer from other co-morbidities, in particular renal
impairment, urologists find themselves fighting an uphill battle in
surgical management post-cystectomy (16).
 | Table II.Complications of current bladder
augmentation procedures through the use of gastrointestinal tissue
in the urinary tract. |
Table II.
Complications of current bladder
augmentation procedures through the use of gastrointestinal tissue
in the urinary tract.
| Complication | Description |
|---|
| Electrolyte
disturbances | Hyperchloreamic,
hypokalaemic metabolic acidosis is the most common complication. A
number of these patients will be required to take life long oral
Sodium bicarbonate as a result. |
| Vitamin
deficiency | Loss of segments of
bowel or stomach can impair vitamin B12/ bile salt/fat/fat soluble
vitamin absorption. Vitamin B12 deficiency can manifest as
peripheral neuropathy. |
| Drug
metabolism | Drugs can be
reabsorbed by the intestinal segments that have been incorporated
into the urinary tract, causing toxicity. Methotrexate, phenytoin
and lithium in particular result in therapeutic changes. Dose
adaptation and close surveillance is often required. |
| Hepatic
metabolism | The intestinal
segments that are used for diversion will drain into the portal
circulation and consequently, into the liver. This abnormal portal
system communication can lead to ammonia toxicity, manifesting as
ammoniagenic encephalopathy. |
| Bone disease | This is generally a
long term complication of diversion which has been demonstrated in
adults with osteomalacia and children with rickets. The
pathological process is complex, but it has been attributed to
chronic acidosis. |
| Cancer | Tumours have been
reported close to the anastomotic sites and include transitional
cell carcinoma, adenocarcinoma, sarcoma and adenomatous
polyps. |
| Surgical | These are numerous
and can manifest early or late. The most common complications are
stomal, including stomal bleeds, parastomal hernias and stomal
stenosis. |
Certain bladder pathologies, in particular
metastatic BC, are managed medically prior to considering radical
cystectomy. This method of organ preservation involves aggressive
treatment through surgery and radiotherapy, often with neo-adjuvant
chemotherapy as the treatment of choice (23). Currently, only multidrug
platinum-based chemotherapeutic regimens have been successful
(24,25), whereas monotherapy treatments have
failed (26,27). The methotrexate, vinblastine,
adriamycin and cisplatin (MVAC) and gemcitabine and cisplatin
(gem-cis) regimens have been the most commonly used
chemotherapeutic treatment regimens. However, the clinical efficacy
of cisplatin-based therapy in BC is currently restricted by the
rapid development of drug resistance in the majority of patients,
frequently leading to therapeutic failure (28). This was demonstrated in a previous
study in which response rates from BC patients receiving
chemotherapeutic drug intervention in phase II trials varied
between 20 and 60%, with the majority of survival rate advantage in
the randomised control trial setting (29,30). This
has resulted in the ever apparent requirement for bladder
reconstruction.
The future
The absence of autologous tissue with parallel
properties to the native bladder has directed numerous studies to
develop alternative methods of bladder substitution, avoiding the
use of bowel tissue altogether. The theory of substituting the
native bladder with a synthetic prosthesis has always been an
attractive prospect in the development of cutting edge technology.
Thus, tissue engineering, cell and stem cell biology, material
science and regenerative medicine are at the forefront of medical
research (31).
The fields of tissue engineering and regenerative
medicine have witnessed substantial progression over the previous
two decades. Various studies have investigated the possibility of
regenerating multilayer urothelium, which led to the first clinical
trial in 2006, in which Atala et al (32) investigated tissue engineered bladders
created from cell seeded grafts. The potential of such novel
findings has underlined the requirement for further advances in
tissue engineering and material science in order to define the
properties required for the ultimate reconstructive material and
method of implantation.
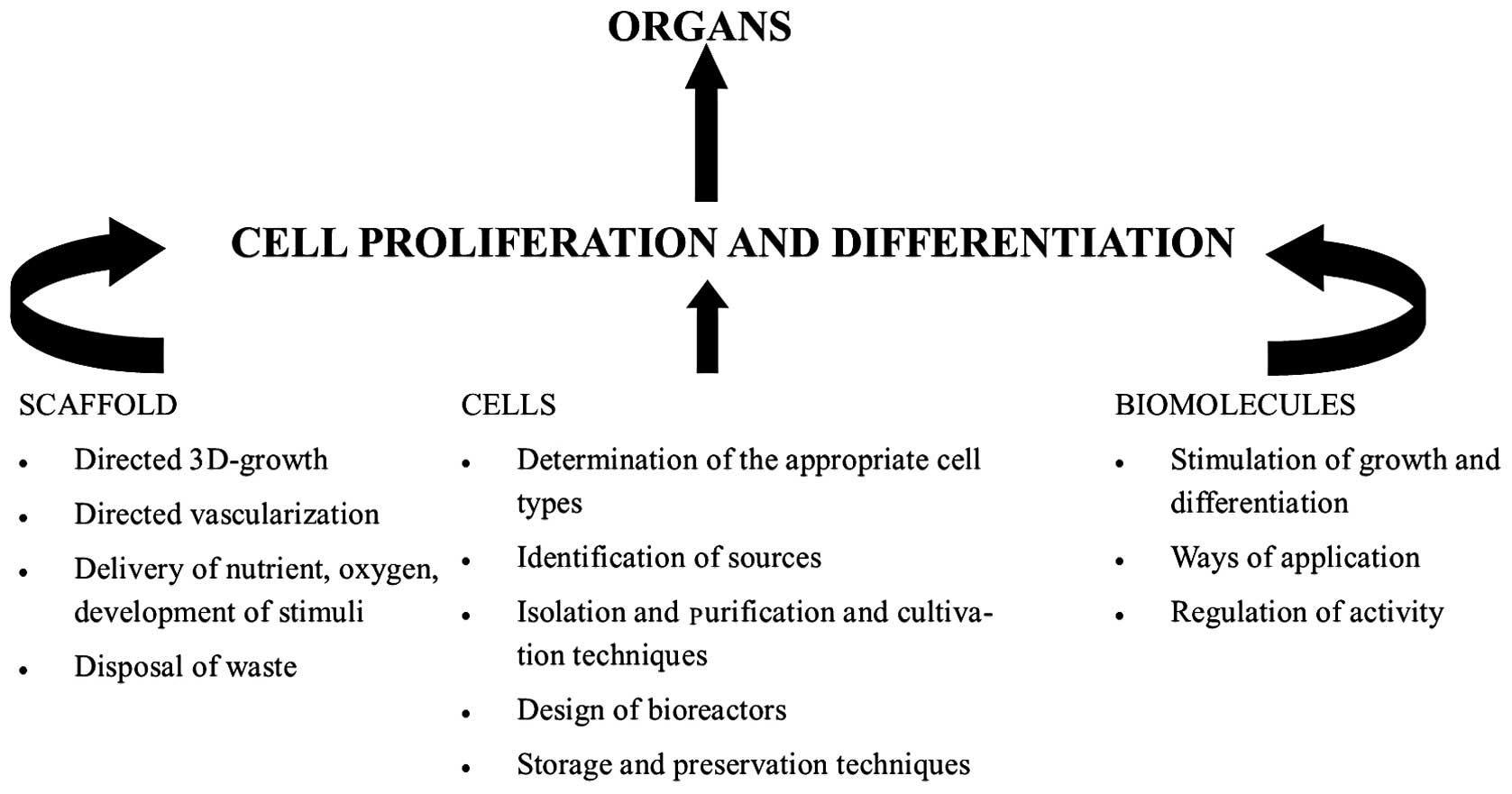
Tissue engineering is the mainstay of regenerative
medicine. It employs the disciplines of cell biology,
transplantation, material science and biomedical engineering,
towards identifying alternatives that can re-establish and preserve
the regular function of damaged tissues and organs (Fig. 1) (33).
Although the human body is outstanding in its ability to repair
damaged tissue, these reparative processes are frequently
restricted to the development of scar tissue. This often proves
detrimental in the function of the bladder (34). The ideal artificial bladder should
possess properties similar to that of the native urinary bladder.
It should possess the ability to store urine at low pressure in a
watertight structure, similar to a mechanical reservoir, and permit
voluntary voiding with minimal reflux. This structure should also
be constructed from inert material and cause minimal complications
in the patient so that long-term renal function is not compromised
(35). Previously published animal
studies have demonstrated promising results in the field of
regenerative medicine, and it represents a possible solution for
the treatment of a number of urological conditions in the future
(31).
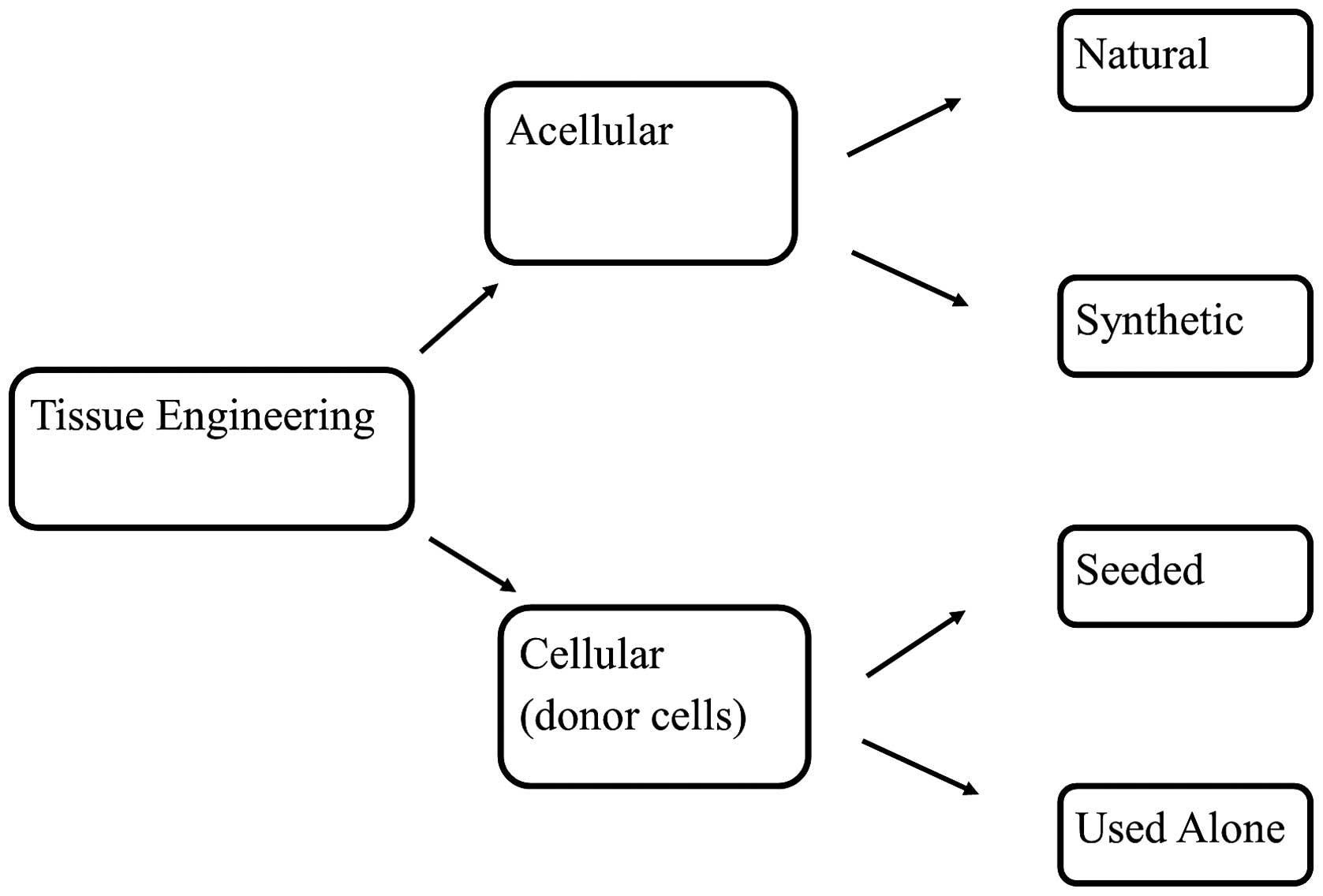
Tissue engineering strategies vary, and currently,
studies are being orientated in two directions: Firstly, to
identify the most appropriate type of stem cell for regeneration
and to proficiently incorporate it into bladder cells; secondly, to
determine the most appropriate material and technique of embedding
these cells using tissue engineered grafts (Fig. 2) (36,37). The
selected grafts must exhibit all the qualities of the native
tissue, acting ultimately as microenvironments for the implanted
cells to prosper (38).
Biomaterials in bladder regeneration
There are distinct benefits to using biocompatible
material in regenerative medicine for the purpose of cell delivery
vehicles, and for bearing the physical maintenance required for
tissue replacement (39). Scaffolds
are constructs that are designed to direct tissue development and
the growth of cells during the process of healing (40). Bladder replacements should therefore
provide provisional mechanical support, adequate to endure forces
exerted from neighbouring structures, whilst maintaining a
potential zone for tissue development. Biomaterials used for
bladder replacements should possess the ability to be easily
manipulated into a hollow, spherical configuration. Furthermore,
the biomaterials should possess the ability to biodegrade for
complete tissue development, without causing inflammation.
Autologous tissue has been experimented on for bladder repair since
the early 1980s (41). The use of
omentum, pericardium, stomach and skin has been attempted with
limited success (42–45). It was the lack of watertight
properties that led to the failure of these materials. It is clear
that the anatomical and physiological properties of the urinary
bladder are not easily substituted.
Biomaterials can be divided into 3 main categories:
i) Naturally derived matrices, including collagen; ii) acellular
tissue matrices, including bladder submucosa; and iii) synthetic
matrices, including poly lactic-co-glycolic acid (PLGA) (46).
Naturally derived matrices
Collagen is considered to be the most ubiquitous
protein in the human body, and it is often used alongside alginate
as a natural matrix. It is useful in tissue engineering, as it
possesses the ability to be easily manipulated and does not provoke
an immune response (47). Through the
use of innovative inkjet technology, it has been possible to use
bio-printing to create a naturally derived 3D construct, with a
precise arrangement of growth factors and other cellular
components, into a patient-specific scaffold (48).
Acellular tissue matrices
Decellularised matrices are the most commonly used
naturally derived urological matrices. They are usually harvested
from autologous, allogenic or xenogenic tissue (49,50).
Chemical or mechanical processing decellularises the matrix,
removing all cellular components and leaving a natural platform for
tissue development (51). The most
common origin of decellularised matrices is tissue harvested from
the bladder or small intestinal mucosa (52). Once implanted, the matrices eventually
degrade and are ultimately replaced by an extracellular matrix. The
matrices provide cells with a structural support that can dictate
the tissue structure. Bladder acellular matrix may be the most
extensively used scaffold in tissue engineering. It has been
demonstrated that the cells have the ability to induce the ingrowth
of urothelium, smooth muscle, endothelium and nerve cells (53). Perhaps the greatest advantage of the
use of acellular matrices is the ability to provide a method of
neovascularisation following graft insertion, promoting graft
survival. Kikuno et al (54)
demonstrated that nerve growth factor and vascular endothelial
growth factor enhanced bladder acellular matrix grafting in
neurogenic rat bladders through increased angiogenesis and
neurogenesis (55).
However, in general, these materials possess various
drawbacks depending on their graft origin. Autologous materials
have proven difficult to reproduce as a result of increased patient
morbidity associated with the graft harvest (56). The increased cost in allograft and
xenograft material production, in addition to the risk of disease
transmission and varied mechanical strength, is crucial in their
limited clinical application (56,57).
Furthermore, the use of natural decellularised matrices requires
tissue that exhibits no principal pathological change, and is
therefore unfeasible in certain patients (58,59). In
addition, the aggressive decellularisation and sterilisation
protocols used can denature proteins in the extracellular matrix,
damaging the physiological environment (60).
Synthetic matrices
The use of synthetic material in patients in which
the native bladder has undergone pathological change, such as BC,
is hypothesised to be the ideal solution. The most commonly used
materials in experimental studies and clinical trials are Teflon,
silicon and collagen matrices (61).
However, cell and tissue incompatibilities appear to be the major
restrictions of these materials, leading to the development of
other synthetic polymers, such as PLGA (62–65). These
materials were designed specifically to possess adequate structural
and biological properties, which can be manipulated for optimal
cell proliferation and differentiation (66). In addition, these scaffolds are safe,
possess controlled properties of degradation rate and strength, are
readily available and possess the ability to carry vital growth
factors, such as vascular endothelial growth factor, for
neovascularisation (67–69). Indeed, the most attractive property of
PLGA compared with older polymers, such as Teflon, is its
resorbable biodegradability. This eliminates the disadvantages of
infection, calcification and unfavourable connective tissue
responses observed in older polymers (70). When using polymers, tissue recognition
is difficult, and often requires immunosuppressive therapy.
However, it has now been demonstrated that novel biological
factors, including adhesive proteins such as collagen and
fibronectin and growth factors such as basic fibroblast growth
factor, epidermal growth factor and insulin, are able to enhance
cell recognition (71,72).
The failure of certain materials in clinical cases
has been extensively reported, including plastic moulds (73,74),
gelatin sponges (75,76), Japanese paper produced from the rice
paper plant (Tetrapanax papyrifer) (77,78) and
bovine pericardium (79,80).
Nanotechnology, which arose in the last decade of
the 20th century, has been used in the formation of matrices that
can be composed of natural and synthetic materials. The use of
nanotechnology permits the development of a graft that can be
manipulated to produce characteristics with definitive properties
in order to promote optimal cell proliferation and tissue
differentiation (53). A previous
study investigated the effect of surface roughness on the
interaction with matrix proteins (81). Vitronectin absorbance improved by 20%
with the addition of nanotechnology-induced surface roughness in
the synthetic materials compared with the nanosmooth surface.
Similarly, enhanced adhesion and proliferation of urothelial cells
has been demonstrated with increased synthetic surface roughness
(82).
The use of unseeded and cell-seeded matrices
in bladder regeneration
Although there have been studies illustrating the
use of unseeded matrices in bladder tissue engineering, the results
of these studies have been inconclusive. The normal regeneration of
the urothelium layer has been demonstrated in unseeded grafts used
for cystoplasty, however, the muscular layer did not develop
(83). By contrast, the cell-seeded
approach has produced more positive and consistent results in
bladder reconstruction, and has been the most extensively
investigated strategy in reconstruction. This approach involves
seeding of a scaffold with autologous patient-derived cells, which
is then transplanted back into the patient to accomplish
regeneration (32).
In a study conducted on beagle dogs following
subtotal cystectomy, polyglycolic acid acellular matrices were
seeded with urothelium and smooth muscle cells. These dogs
demonstrated a 95% increase in pre-operative bladder capacity
compared with dogs treated with an unseeded matrix, indicating a
46% increase in pre-operative bladder capacity (84).
The study by Atala et al (32) was crucial for the first
laboratory-created organ to be transplanted into the human body.
The authors demonstrated an increase in bladder compliance and
capacity, and a reduction in end filling pressure in 7 patients
with neurogenic bladder (myelo meningocele). These patients
underwent cystoplasty created from autologous cells seeded on
collagen-polyglycolic acid scaffolds. This small clinical study
established the possibility of using tissue-engineered substitutes
for organ replacements in humans, circumventing the complications
of intestinal substitutes (32).
Stem cells in bladder regeneration
Historically, tissue engineering has depended on
autologous cells from the host organ. However, physiologically
normal tissue may not always be available for harvest to elaborate
a regenerative cystoplasty, this has led to the use of stem cells
as an alternative to restore urinary bladder function. The aim of
using stem cells as a therapeutic option is to achieve adequate
differentiation into urothelial cells and smooth muscle cells, so
that the normal histological structure is maintained for use as a
potential resource for cell-based therapy in urology (85).
Embryonic stem cells are a group of pluripotent stem
cells and exhibit two valuable attributes, including the capability
to undergo self-renewal and the capability to differentiate into
numerous specialised cell types. The in vivo benefits of
these have been demonstrated previously (86,87),
although the exact culture conditions which would enable controlled
differentiation are yet to be identified. However, their potential
for tumorigenicity, the prospect of immune rejection and the
ethical dilemmas associated with embryonic stem cells have limited
their clinical application (85).
Adult stem cells are the most extensively
investigated cell types in stem cell biology, regardless,
progression has been slow. Despite this, study of adult stem cells
is ongoing due to their extensive potential to be applied as
therapies in a vast array of disorders. The most promising source
of adult stem cells is adult bone marrow. The use of autologous
adult stem cells avoids the obstacles associated with an immune
response (88,89), which is useful for autologous and
tissue-specific regenerative therapies. Within the past two
decades, stem cells have been identified throughout the tissues of
the body, not just the bone marrow (90). It has been indicated that stem cells
may function as primary repair entities for the particular organ in
which they reside. These tissue-specific progenitors are now the
subject of numerous different areas of research (88).
Mesenchymal stem cells (MSCs) are derived from the
bone marrow stroma. MSCs have been demonstrated to differentiate
in vitro into a variety of tissue types, including smooth
muscle, urothelium and endothelial cells. It is for this reason
that bone marrow-derived MSCs are an attractive candidate for
bladder tissue regeneration (91).
Chung et al (92) reported
positive results in healthy rat bladders following the introduction
of porcine small intestinal submucosa seeded with MSCs from rat
bone marrow. These rats exhibited normal urinary bladder
architecture, and histological analysis demonstrated
well-differentiated urothelial and smooth muscle cells compared
with control experiments using unseeded intestinal submucosa
(92).
Amniotic fluid-derived stem (AFS) cells,
adipose-derived stem cells, and stem cells isolated from hair have
all demonstrated the capability of differentiating into different
urinary bladder cells in vitro (52,93,94). AFS
cells were initially isolated for therapeutic use in 2007 and
represent a minor subset of cells originating from the placenta and
amniotic fluid (95). AFS cells have
been demonstrated to expand extensively, doubling every 36 h
(96). They have the advantage over
embryonic stem cells in that they do not form tumours in
vivo (95). AFS cells possess a
differentiation potential between that of adult stem cells and
embryonic stem cells and can be obtained from routine clinical
amniocentesis, prenatal chorionic villus biopsies and placental
biopsies performed after birth (95).
Since their identification, these cells have been demonstrated to
differentiate into functioning cell types of numerous different
organs and to prevent bladder hypertrophy in cryo-injured mouse
bladders in vitro via the regulation of post-injury bladder
remodelling (97). AFS cells possess
a wide range of potential applications in the field of regenerative
medicine, and may in theory supply the vast majority of the UK with
suitable genetic matches for transplantation (98–100).
Stem cells are currently employed in tissue
engineering using two main methods, including implantation of the
stem cells in vivo without pre-differentiation and induction
of stem cell differentiation towards the bladder in vitro
followed by its implantation in vivo (101). The first method is often employed
when a section of the bladder requires reconstruction, in
comparison with the second method that is used when de novo
whole bladder reconstruction is required.
Innervation is vital in bladder reconstruction. The
autonomic nervous system controls the function of the bladder, thus
a poorly functioning neuronal network can lead to significant
bladder dysfunction (102). It has
been indicated that seeding Schwann cells and neurotrophic factors
may encourage nerve innervation of the urinary bladder (103).
Several bladder malformations exist, which are often
identified prenatally with sonography. The future prenatal
management of patients with bladder disease has emerged as a
notable possibility (104). The
potential to have readily available urological tissue at birth for
a one-stage reconstruction following prenatal ultrasound-guided
bladder biopsy is of note as this would present a significant
advancement for the treatment of congential urinary tract
abnormalities as cells could be harvested and grown in
transplantable tissue during gestation. This has been successfully
demonstrated in foetal lambs (105).
Chondrocytes obtained from hyaline and elastic cartilage were
harvested from fetal lambs, expanded in vitro and seeded
onto biodegradable scaffolds. The scaffolds were then implanted as
replacement tracheal tissue in fetal lambs. Strucural support and
patency of the tissue engineered cartilage was maintained and all
lambs that were allowed to reach term were able to breathe
spontaneously. Thus, this may be useful for in utero repair
of congenital tracheal abnormalities, such as tracheal atresia and
agenesis (106,107).
Conclusion and perspectives
Existing data indicates a forthcoming role of tissue
engineering disciplines in the management of urological diseases.
With the extensive advancements within the field in previous years,
the future of urology appears positive. However, the current
knowledge of bladder reconstruction is insufficient in order to use
it as a clinical standard in mainstream urological practice.
Additional in vitro studies are required to make this
possible. The most significant obstacle in material science is the
equilibrium between the creation of biomaterials that are patient-
and disease-specific and fit for purpose and a cost-effective
manufacturing process. The primary objective in the field of cell
biology and transplantation is not solely to control stem cell
differentiation in vivo but also to be able to manage their
growth following transplantation. The most significant aspect of
cell type selection is the degree of regenerative potential; the
more regenerative the cell is, the more likely it is to survive
when implanted in vivo. The survival of artificial tissue
following transplantation has been a significant obstacle thus far
in current tissue engineering strategies. Other factors other than
cell type selection are also important, however these factors often
depend on the ability for the tissue to maintain initial growth and
survive. Thus, as the tissue grows a healthy self-sustained process
of self repair is required, however, this will vary depending on
the cell type used.
References
|
1
|
Walters MD and Weber AM: Anatomy of the
lower urinary tract, rectum and pelvic floor. In: Urogynecology and
Pelvic Reconstructive SurgeryWalters MD and Karram MM: 2nd. Mosby,
St. Louis, MO: pp. 3–13. 2000
|
|
2
|
Cancer Research UK, . Bladder Cancer
Survival Statistics. https://www.cancerresearchuk.org/cancer-info/cancerstats/?=5441Accessed.
April 10–2015
|
|
3
|
BMJ, . Best practise: Epidemiology.
http://bestpractice.bmj.com/best-practice/monograph/980/basics/epidemiology.htmlAccessed.
April 11–2015
|
|
4
|
Cancer Research UK, . Types of bladder
cancer. http://cancerhelp.cancerresearchuk.org/type/bladder-cancer/about/types-of-bladder-cancer#spreadAccessed.
April 11–2015
|
|
5
|
Witjes JA, Compérat E, Cowan NC, et al:
EAU guidelines on muscle-invasive and metastatic bladder cancer:
Summary of the 2013 guidelines. Eur Urol. 65:778–792. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sievert KD, Amend B, Nagele U, Schilling
D, Bedke J, Horstmann M, Hennenlotter J, Kruck S and Stenzl A:
Economic aspects of bladder cancer: What are the benefits and
costs? World J Urol. 27:295–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee RK, Abol-Enein H, Artibani W, et al:
Urinary diversion after radical cystectomy for bladder cancer:
Options, patient selection, and outcomes. BJU Int. 113:11–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hautmann S, Felix-Chun KH, Currlin E, et
al: Cystectomy for indications other than bladder cancer. Urologe
A. 43:172–177. 2004.(In German). PubMed/NCBI
|
|
9
|
Evans B, Montie JE and Gilbert SM:
Incontinent or continent urinary diversion: How to make the right
choice. Curr Opin Urol. 20:421–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Westney OL: The neurogenic bladder and
incontinent urinary diversion. Urol Clin North Am. 37:581–592.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andersen AV, Granlund P, Schultz A,
Talseth T, Hedlund H and Frich L: Long-term experience with
surgical treatment of selected patients with bladder pain
syndrome/interstitial cystitis. Scand J Urol Nephrol. 46:284–289.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poole-Wilson DS and Barnard RJ: Total
cystectomy for bladder tumours. Br J Urol. 43:16–24. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cody JD, Nabi G, Dublin N, McClinton S,
Neal DE, Pickard R and Yong SM: Urinary diversion and bladder
reconstruction/replacement using intestinal segments for
intractable incontinence or following cystectomy. Cochrane Database
Syst Rev. 15:CD0033062012.
|
|
14
|
Parekh DJ and Donat SM: Urinary diversion:
Options, patient selection, and outcomes. Semin Oncol. 34:98–109.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Basic DT, Hadzi-Djokic J and Ignjatovic I:
The history of urinary diversion. Acta Chir Iugosl. 54:9–17. 2007.
View Article : Google Scholar
|
|
16
|
Neuhof H: Facial transplantation into
visceral defects: An experimental and clinical study. Surg Gynecol
Obstet. 25:3831917.
|
|
17
|
Moon A, Vasdev N and Thorpe AC: Continent
urinary diversion. Indian J Urol. 29:303–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stein JP, Lieskovsky G, Cote R, et al:
Radical cystectomy in the treatment of invasive bladder cancer:
Long-term results in 1,054 patients. J Clin Oncol. 19:666–675.
2001.PubMed/NCBI
|
|
19
|
Mills RD and Studer UE: Metabolic
consequences of continent urinary diversion. J Urol. 161:1057–1066.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimko MS, Tollefson MK, Umbreit EC,
Farmer SA, Blute ML and Frank I: Long-term complications of conduit
urinary diversion. J Urol. 185:562–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hautmann RE: Urinary diversion: Ileal
conduit to neobladder. J Urol. 169:834–842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hyndman ME, Kaye D, Field NC, et al: The
use of regenerative medicine in the management of invasive bladder
cancer. Adv Urol. 2012:6536522012.PubMed/NCBI
|
|
23
|
Hussain SA, Moffitt DD, Glaholm JG, Peake
D, Wallace DM and James ND: A phase I-II study of synchronous
chemoradiotherapy for poor prognosis locally advanced bladder
cancer. Ann Oncol. 12:929–935. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harker WG, Meyers FJ, Freiha FS, Palmer
JM, Shortliffe LD, Hannigan JF, McWhirter KM and Torti FM:
Cisplatin, methotrexate, and vinblastine (CMV): An effective
chemotherapy regimen for metastatic transitional cell carcinoma of
the urinary tract. A Northern California Oncology Group study. J
Clin Oncol. 3:1463–1470. 1985.PubMed/NCBI
|
|
25
|
Sternberg CN, Yagoda A, Scher HI, et al:
Preliminary results of M-VAC (methotrexate, vinblastine,
doxorubicin and cisplatin) for transitional cell carcinoma of the
urothelium. J Urol. 133:403–407. 1985.PubMed/NCBI
|
|
26
|
Saxman SB, Propert KJ, Einhorn LH,
Crawford ED, Tannock I, Raghavan D, Loehrer PJ Sr and Trump D:
Long-term follow up of a phase III intergroup study of cisplatin
alone or in combination with methotrexate, vinblastine, and
doxorubicin in patients with metastatic urothelial carcinoma: A
cooperative group study. J Clin Oncol. 15:2564–2569.
1997.PubMed/NCBI
|
|
27
|
Waxman J and Barton C: Carboplatin-based
chemotherapy for bladder cancer. Cancer Treat Rev. 19(Suppl C):
S21–S25. 1993.
|
|
28
|
Stewart DJ: Mechanisms of resistance to
cisplatin and carboplatin. Crit Rev Oncol Hematol. 63:12–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hussain SA, Stocken DD, Riley P, et al: A
phase I/II study of gemcitabine and fractionated cisplatin in an
outpatient setting using a 21-day schedule in patients with
advanced and metastatic bladder cancer. Br J Cancer. 91:844–849.
2004.PubMed/NCBI
|
|
30
|
James ND, Hussain SA, Hall E, et al:
BC2001 Investigators: Radiotherapy with or without chemotherapy in
muscle-invasive bladder cancer. N Engl J Med. 366:1477–1488. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Atala A: Tissue engineering for bladder
substitution. World J Urol. 18:364–370. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Atala A, Bauer SB, Soker S, Yoo JJ and
Retik AB: Tissue-engineered autologous bladders for patients
needing cystoplasty. Lancet. 367:1241–1246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aboushwareb T, McKenzie P, Wezel F,
Southgate J and Badlani G: Is tissue engineering and biomaterials
the future for lower urinary tract dysfunction (LUTD)/pelvic organ
prolapse (POP)? Neurourol Urodyn. 30:775–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baskin LS, Sutherland RS, Thomson AA,
Nguyen HT, Morgan DM, Hayward SW, Hom YK, DiSandro M and Cunha GR:
Growth factors in bladder wound healing. J Urol. 157:2388–2395.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Korossis S, Bolland F, Ingham E, Fisher J,
Kearney J and Southgate J: Review: Tissue engineering of the
urinary bladder: considering structure-function relationships and
the role of mechanotransduction. Tissue Eng. 12:635–644. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Petrovic V, Stankovic J and Stefanovic V:
Tissue engineering of the urinary bladder: Current concepts and
future perspectives. ScientificWorldJournal. 11:1479–1488. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shokeir AA, Harraz AM and El-Din AB:
Tissue engineering and stem cells: Basic principles and
applications in urology. Int J Urol. 17:964–973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wood D and Southgate J: Current status of
tissue engineering in urology. Curr Opin Urol. 18:564–569. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Furth ME, Atala A and Van Dyke ME: Smart
biomaterials design for tissue engineering and regenerative
medicine. Biomaterials. 28:5068–5073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chan BP and Leong KW: Scaffolding in
tissue engineering: General approaches and tissue-specific
considerations. Eur Spine J. 17(Suppl 4): S467–S479. 2008.
|
|
41
|
Davis NF, Callanan A, McGuire BB, Mooney
R, Flood HD and McGloughlin TM: Porcine extracellular matrix
scaffolds in reconstructive urology: An ex vivo comparative study
of their biomechanical properties. J Mech Behav Biomed Mater.
4:375–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goldstein MB, Dearden LC and Gualtieri V:
Regeneration of subtotally cystectomized bladder patched with
omentum: An experimental study in rabbits. J Urol. 97:664–668.
1967.PubMed/NCBI
|
|
43
|
Andretto R, Gonzales J, Guidugli Netro J,
de Miranda JF and Antunes AM: Experimental cystoplasty in dogs
using preserved equine pericardium. AMB Rev Assoc Med Bras.
27:153–154. 1981.(In Portuguese). PubMed/NCBI
|
|
44
|
Nguyen DH and Mitchell ME: Gastric bladder
reconstruction. Urol Clin North Am. 18:649–657. 1991.PubMed/NCBI
|
|
45
|
Draper JW and Stark RB: End results in the
replacement of mucous membrane of the urinary bladder with
thick-split grafts of skin. Surgery. 39:434–440. 1956.PubMed/NCBI
|
|
46
|
Atala A: Tissue engineering of human
bladder. Br Med Bull. 97:81–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dahms SE, Piechota HJ, Dahiya R, Lue TF
and Tanagho EA: Composition and biomechanical properties of the
bladder acellular matrix graft: Comparative analysis in rat, pig
and human. Br J Urol. 82:411–419. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mahfouz W, Elsalmy S, Corcos J and Fayed
AS: Fundamentals of bladder tissue engineering. Afr J Urol.
19:51–57. 2013. View Article : Google Scholar
|
|
49
|
Yoo JJ, Meng J, Oberpenning F and Atala A:
Bladder augmentation using allogenic bladder submucosa seeded with
cells. Urology. 51:221–225. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sutherland RS, Baskin LS, Hayward SW and
Cunha GR: Regeneration of bladder urothelium, smooth muscle, blood
vessels and nerves into an acellular tissue matrix. J Urol.
156:571–577. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Crapo PM, Gilbert TW and Badylak SF: An
overview of tissue and whole organ decellularization processes.
Biomaterials. 32:3233–3243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Langer R and Vacanti JP: Tissue
engineering. Science. 260:920–926. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brehmer B, Rohrmann D, Becker C, Rau G and
Jakse G: Different types of scaffolds for reconstruction of the
urinary tract by tissue engineering. Urol Int. 78:23–29. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kikuno N, Kawamoto K, Hirata H, et al:
Nerve growth factor combined with vascular endothelial growth
factor enhances regeneration of bladder acellular matrix graft in
spinal cord injury-induced neurogenic rat bladder. BJU Int.
103:1424–1428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Atala A: Recent developments in tissue
engineering and regenerative medicine. Curr Opin Pediatr.
18:167–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Orabi H, Bouhout S, Morissette A, Rousseau
A, Chabaud S and Bolduc S: Tissue engineering of urinary bladder
and urethra: Advances from bench to patients.
ScientificWorldJournal. 2013:1545642013.PubMed/NCBI
|
|
57
|
Brown AL, Farhat W, Merguerian PA, Wilson
GJ, Khoury AE and Woodhouse KA: 22 week assessment of bladder
acellular matrix as a bladder augmentation material in a porcine
model. Biomaterials. 23:2179–2190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Subramaniam R, Hinley J, Stahlschmidt J
and Southgate J: Tissue engineering potential of urothelial cells
from diseased bladders. J Urol. 186:2014–2020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chung SY: Bladder tissue-engineering: a
new practical solution? Lancet. 367:1215–1216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Atala A: Autologous cell transplantation
for urologic reconstruction. J Urol. 159:2–3. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Matoka DJ and Cheng EY: Tissue engineering
in urology. Can Urol Assoc J. 3:403–408. 2009.PubMed/NCBI
|
|
62
|
Bogash M, Kohler FP, Scott RH and Murphy
JJ: Replacement of the urinary bladder by a plastic reservoir with
mechanical valves. Surg Forum. 10:900–903. 1960.PubMed/NCBI
|
|
63
|
Bona AV and De Gresti A: Partial
substitution of the bladder wall with teflon tissue. (Preliminary
and experimental note on the impermeability and tolerance of the
prosthesis). Minerva Urol. 18:43–47. 1966.(In Italian). PubMed/NCBI
|
|
64
|
Kelâmi A, Dustmann HO, Lüdtke-Handjery A,
Cárcamo V and Herlld G: Experimental investigations of bladder
regeneration using teflon-felt as a bladder wall substitute. J
Urol. 104:693–698. 1970.PubMed/NCBI
|
|
65
|
Rohrmann D, Albrecht D, Hannappel J,
Gerlach R, Schwarzkopp G and Lutzeyer W: Alloplastic replacement of
the urinary bladder. J Urol. 156:2094–2097. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Baker SC, Rohman G, Southgate J and
Cameron NR: The relationship between the mechanical properties and
cell behaviour on PLGA and PCL scaffolds for bladder tissue
engineering. Biomaterials. 30:1321–1328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Pattison MA, Wurster S, Webster TJ and
Haberstroh KM: Three dimensional, nano-structured PLGA scaffolds
for bladder tissue replacement applications. Biomaterials.
26:2491–2500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pariente JL, Kim BS and Atala A: In vitro
biocompatibility assessment of naturally derived and synthetic
biomaterials using normal human urothelial cells. J Biomed Mater
Res. 55:33–39. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen FM, Zhang M and Wu ZF: Toward
delivery of multiple growth factors in tissue engineering.
Biomaterials. 31:6279–6308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ulery BD, Nair LS and Laurencin CT:
Biomedical applications of biodegradable polymers. J Polym Sci B
Polym Phys. 49:832–864. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Harrington DA, Sharma AK, Erickson BA and
Cheng EY: Bladder tissue engineering through nanotechnology. World
J Urol. 26:315–322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Elbert DL and Hubbell JA: Surface
treatments of polymers for biocompatibilityAnnual Review of
Materials Science. Vol 26. Annual Reviews. Palo Alto, CA: pp.
365–394. 1996
|
|
73
|
Bohne AW and Urwiller KL: Experience with
urinary bladder regeneration. J Urol. 77:725–732. 1957.PubMed/NCBI
|
|
74
|
Portilla Sanchez R, Blanco FL, Santamarina
A, Casals Roa J, Mata J and Kaufman A: Vesical regeneration in the
human after total cystectomy and implantation of a plastic mould.
Br J Urol. 30:180–188. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tsuji I, Kuroda K, Fujieda J, Shiraishi Y
and Kunishima K: Clinical experiences of bladder reconstruction
using preserved bladder and gelatin sponge bladder in the case of
bladder cancer. J Urol. 98:91–92. 1967.PubMed/NCBI
|
|
76
|
Orikasa S and Tsuji I: Enlargement of
contracted bladder by use of gelatin sponge bladder. J Urol.
104:107–110. 1970.PubMed/NCBI
|
|
77
|
Taguchi H, Ishizuka E and Saito K:
Cystoplasty by regeneration of the bladder. J Urol. 118:752–756.
1977.PubMed/NCBI
|
|
78
|
Fujita K: The use of resin-sprayed thin
paper for urinary bladder regeneration. Invest Urol. 15:355–357.
1978.PubMed/NCBI
|
|
79
|
Moon SJ, Kim DH, Jo JK, et al: Bladder
reconstruction using bovine pericardium in a case of enterovesical
fistula. Korean J Urol. 52:150–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pokrywczynska M, Adamowicz J, Sharma AK
and Drewa T: Human urinary bladder regeneration through tissue
engineering - an analysis of 131 clinical cases. Exp Biol Med
(Maywood). 239:264–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Shakhssalim N, Dehghan MM, Moghadasali R,
Soltani MH, Shabani I and Soleimani M: Bladder tissue engineering
using biocompatible nanofibrous electrospun constructs: Feasibility
and safety investigation. Urol J. 9:410–419. 2012.PubMed/NCBI
|
|
82
|
Chun YW, Khang D, Haberstroh KM and
Webster TJ: The role of polymer nanosurface roughness and submicron
pores for improving bladder urothelial cell density and inhibiting
calcium oxalate stone formation. Nanotechnology. 20:0851042009.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Probst M, Dahiya R, Carrier S and Tanagho
EA: Reproduction of functional smooth muscle tissue and partial
bladder replacement. Br J Urol. 79:505–515. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Oberpenning F, Meng J, Yoo JJ and Atala A:
De novo reconstitution of a functional mammalian urinary bladder by
tissue engineering. Nat Biotechnol. 17:149–155. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Aboushwareb T and Atala A: Stem cells in
urology. Nat Clin Pract Urol. 5:621–631. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lamba DA, Gust J and Reh TA:
Transplantation of human embryonic stem cell derived photoreceptors
restores some visual function in Crx-deficient mice. Cell Stem
Cell. 4:73–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yang D, Zhang ZJ, Oldenburg M, Ayala M and
Zhang SC: Human embryonic stem cell-derived dopaminergic neurons
reverse functional deficit in parkinsonian rats. Stem Cells.
26:55–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ballas CB, Zielske SP and Gerson SL: Adult
bone marrow stem cells for cell and gene therapies: Implications
for greater use. J Cell Biochem Suppl. 38(S38): 20–28.
2002.PubMed/NCBI
|
|
89
|
Gonzalez MA and Bernad A: Characteristics
of adult stem cells. Adv Exp Med Biol. 741:103–120. 2012.PubMed/NCBI
|
|
90
|
Spradling A, Drummond-Barbosa D and Kai T:
Stem cells find their niche. Nature. 414:98–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
da Silva Meirelles L, Chagastelles PC and
Nardi NB: Mesenchymal stem cells reside in virtually all post-natal
organs and tissues. J Cell Sci. 119:2204–2213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chung SY, Krivorov NP, Rausei V, et al:
Bladder reconstitution with bone marrow derived stem cells seeded
on small intestinal submucosa improves morphological and molecular
composition. J Urol. 174:353–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zuk PA, Zhu M, Ashjian P, et al: Human
adipose tissue is a source of multipotent stem cells. Mol Biol
Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Drewa T: Using hair-follicle stem cells
for urinary bladder-wall regeneration. Regen Med. 3:939–944. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
de Coppi P, Bartsch G Jr..Siddiqui MM, et
al: Isolation of amniotic stem cell lines with potential for
therapy. Nat Biotechnol. 25:100–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Mosquera A, Fernández JL, Campos A,
Goyanes VJ, Ramiro-Díaz J and Gosálvez J: Simultaneous decrease of
telomere length and telomerase activity with ageing of human
amniotic fliuds cells. J Med Genet. 36:494–496. 1999.PubMed/NCBI
|
|
97
|
de Coppi P, Callegari A, Chiavegato A, et
al: Amniotic fluid and bone marrow derived mesenchymal stem cells
can be converted to smooth muscle cells in the cryo-injured rat
bladder and prevent compensatory hypertrophy of surviving smooth
muscle cells. J Urol. 177:369–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Perin L, Sedrakyan S, Giuliani S, et al:
Protective effect of human amniotic fluid stem cells in an
immunodeficient mouse model of acute tubular necrosis. PLoS One.
5:e93572010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Moorefield EC, McKee EE, Solchaga L,
Orlando G, Yoo JJ, Walker S, Furth ME and Bishop CE: Cloned, CD117
selected human amniotic fluid stem cells are capable of modulating
the immune response. PLoS One. 6:e265352011. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Weber B, Emmert MY, Behr L, et al:
Prenatally engineered autologous amniotic fluid stem cell-based
heart valves in the fetal circulation. Biomaterials. 33:4031–4043.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tian H, Bharadwaj S, Liu Y, Ma PX, Atala A
and Zhang Y: Differentiation of human bone marrow mesenchymal stem
cells into bladder cells: Potential for urological tissue
engineering. Tissue Eng Part A. 16:1769–1779. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Fowler CJ, Griffiths D and de Groat WC:
The neural control of micturition. Nat Rev Neurosci. 9:453–466.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Adamowicz J, Drewa T, Tworkiewicz J,
Kloskowski T, Nowacki M and Pokrywczyńska M: Schwann cells - a new
hope in tissue engineered urinary bladder innervation A method of
cell isolation. Cent European J Urol. 64:87–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hindryckx A and De Catte L: Prenatal
diagnosis of congenital renal and urinary tract malformations.
Facts Views Vis Obgyn. 3:165–174. 2011.PubMed/NCBI
|
|
105
|
Koh CJ and Atala A: Tissue engineering,
stem cells, and cloning: Opportunities for regenerative medicine. J
Am Soc Nephrol. 15:1113–1125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Fuchs JR, Terada S, Ochoa ER, Vacanti JP
and Fauza DO: Fetal tissue engineering: In utero tracheal
augmentation in an ovine model. J Pediatr Surg. 37:1000–1006. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Fuchs JR, Hannouche D, Terada S, Vacanti
JP and Fauza DO: Fetal tracheal augmentation with cartilage
engineered from bone marrow-derived mesenchymal progenitor cells. J
Pediatr Surg. 38:984–987. 2003. View Article : Google Scholar : PubMed/NCBI
|