Introduction
Renal cell carcinoma (RCC) is the most common
malignant renal tumor originating from tubular epithelioid cells.
In the United States, RCC accounts for 3% of all tumors (1). The tumors are most likely to occur among
males who are ~60 years old (2,3). The most
common clinical manifestations include hematuria, bellyache and a
lateral abdominal mass, but only ~10% of cases present with this
typical triad (4). The incidence rate
of RCC is stably increasing, and one major reason for this is an
increased detection rate due to gradually enhanced diagnostic
imaging capabilities (5,6). Ultrasound and enhanced computed
tomography (CT) are commonly used in the examination and evaluation
of renal masses, owing to their advantages in the detection and
staging of tumors, and in post-operative follow-up. Xp11.2
translocation RCC is an uncommon type of RCC (7,8). This type
of RCC has a more invasive clinical progression in adults with a
worse prognosis compared with other types of RCC. Therefore it is
important to become familiar with the imaging characteristics of
this particular type of RCC (7,8). The
present study reports the case of an Xp11.2 translocation RCC with
egg-shell calcification that was misdiagnosed as renal
angiomyolipoma (AML).
Case report
Three months ago, a left renal mass was identified
in a 20-year-old female by ultrasound examination. The patient
presented with a >3-month history of lower abdominal pain, lower
back pain and drinking-induced abdominal distension, and a ≥1-month
history of frequent urination and hematuria. Three continuous
ultrasound examinations in the subsequent 2 months revealed the
rapid growth of the lesion.
Enhanced CT indicated the possibility of epithelioid
AML. The possibility of renal cancer (Bosniak IV type) could not be
excluded. On June 28th, 2013, the patient was admitted to The First
Affiliated Hospital of Zhejiang University (Hangzhou, China) for
further evaluation and treatment. There was no history of central
nervous system disease, kidney disease, hypertension or other
chronic diseases, and the patient had no relevant bad habits. Upon
physical examination, there was no swelling in the lower limbs, no
pain on percussion in the kidneys, no pain on palpation of the
bilateral ureteral paths. Bladder insufficiency and dull bladder
percussion were noted. Renal routine examinations revealed a
glomerular filtration rate (MDRD equation) of 126.0 ml/min (normal
range, 80.0–125.0 ml/min), 56 µmol/l creatinine (normal range,
45.0–84.0 µmol/l), 3.5 mmol/l urea (normal range, 2.9–8.2 mmol/l)
and 269 µmol/l uric acid (normal range, 155.0–357.0 µmol/l). Urine
routine examinations revealed occult blood + (1.0) mg/l (normal, 0
mg/l) and glucose ± (1.7) mmol/l (normal, 0 mmol/l). Other
laboratory test indices were all normal.
The patient received three ultrasound examinations
and one enhanced CT kidney examination. The first ultrasound
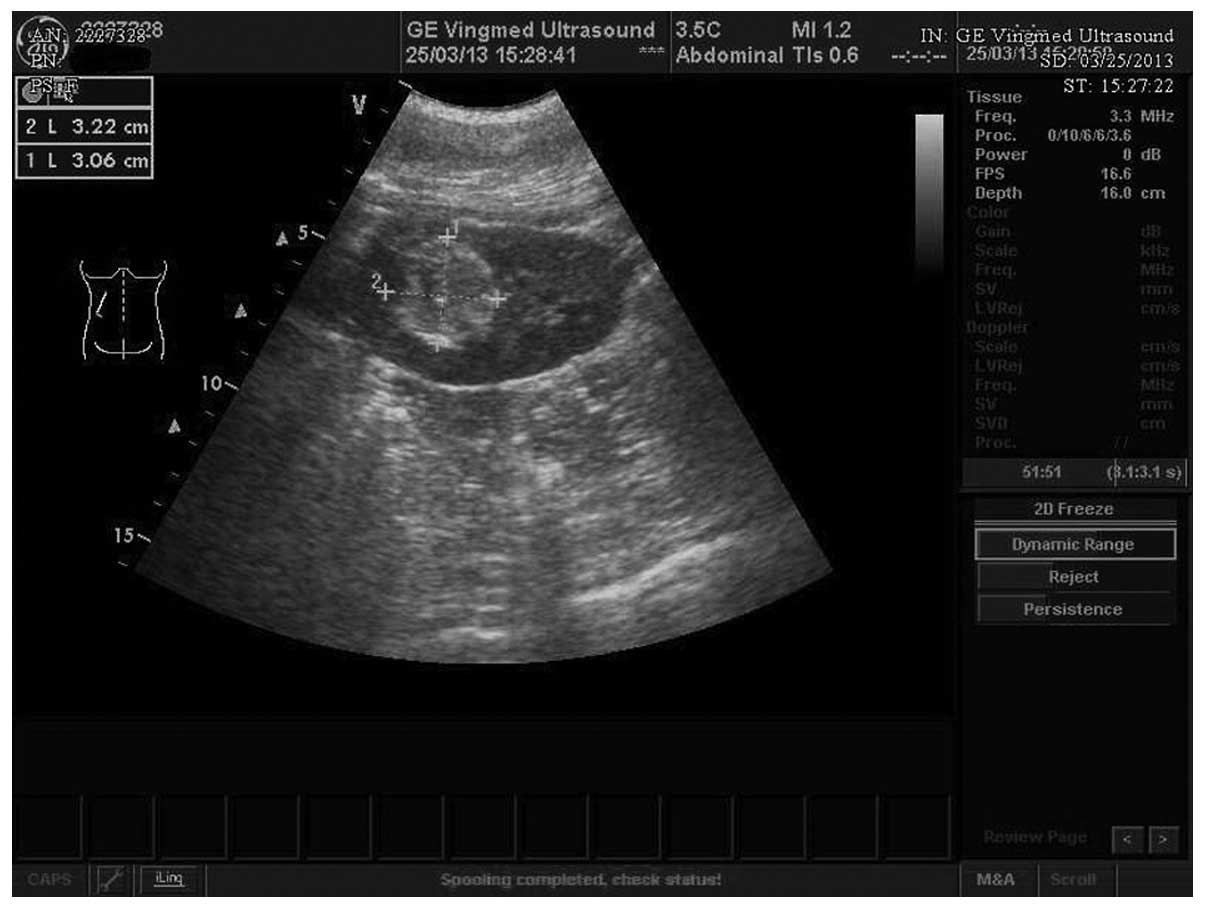
examination confirmed a strong-echo mass with sizes of 3.2×3.1
cm2 in the upper pole of the left kidney on March 25th,
2013 (Fig. 1). Due to the strong-weak
alternating echoes inside and the partial echoless areas observed
locally, AML accompanied with hemorrhage was first considered. The
repeat ultrasound revealed continuous mass with sizes of 3.4×3.0
cm2 in the left kidney on April 22th, 2013. The third ultrasound
revealed the left renal mass with sizes of 3.6×3.0 cm2 on May 23th,
2013, indicating that the tumor was growing rapidly. Next, enhanced
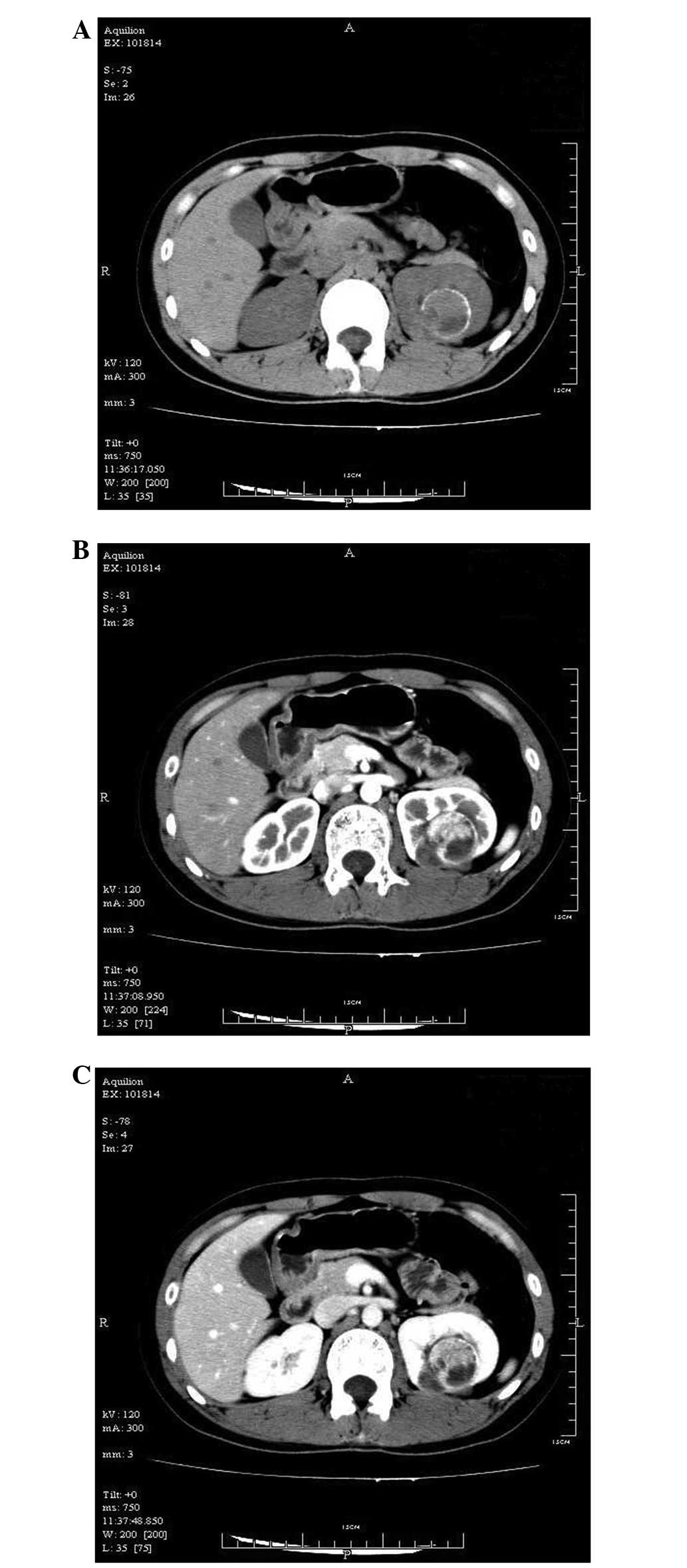
CT was performed to further clarify the nature of the mass. CT
plain scans revealed that the upper pole of the left kidney was
occupied, while the margins of the lesion were affected with linear
egg-shell-like calcifications, but with relatively higher density
in the upper lesion and lower density in the lower region (Fig. 2A). The enhanced CT scan revealed that
the upper region of the lesion was significantly enhanced at the
early stage, but that later the enhancement was slightly reduced.
The lower region was not enhanced (Fig.
2B and C).
The enhanced CT clarified the solid components with
a rich blood supply. Since no fat tissues were detected, the
possibility of classic AML was reduced. Despite the probability of
epithelioid AML, the possibility of renal cancer was increased.
Thus, active surgical treatment was implemented.
At first, the patient underwent close conservative
observation, as the lesion was rather small and ultrasonically
manifested as characteristic AML. Since the mass grew rapidly,
enhanced CT was implemented. The possibility of renal cancer could
not be excluded. According to the surgical plan, a partial
nephrectomy was first performed laparoscopically. RCC rather than
AML was confirmed by intraoperative frozen section with the finding
that the tumor was composed of obviously heteromorphic transparent
cells. A radical nephrectomy was subsequently performed, which
showed that tumor was nest-shaped with papillary arrangement,
invasive growth and obvious heteromorphic cells; the cytoplasm was
transparent and blood sinuses were enriched in the stroma.
Immunohistochemistry demonstrated the expression level of CK(pan)
(partly+), Vimentin (+), CK7(focus+), CD10 (+), E-cadherin (−),
CD117 (−), TFE3 (+), HMB45 (−), Melan-A (−). Routine
histopathological and immunohistochemical tests confirmed that the
lesion was an RCC with Xp11.2 translocation. The patient recovered
well post-operatively. During the 12-month follow-up, no recurrence
or metastases were found.
Discussion
Renal carcinoma associated with Xp11.2
translocations/TFE3 gene fusions is a rare RCC subtype, according
to the 2004 World Health Organization tumor classification
(7). The clinical manifestations and
laboratory tests for this tumor are not specific, and in the
majority of cases, indicate incidental asymptomatic renal masses
(8). Compared with regular RCCs,
Xp11.2 translocation RCC usually occurs among children and young
adults, particularly females (8,9). Compared
with other RCCs, its clinical process is more invasive with a
poorer prognosis (10). Furthermore,
the imaging manifestations of Xp11.2 translocation RCC are diverse,
including hemorrhage, necrosis, cystic degeneration and
calcification (8,11–15).
Therefore, the pre-operative diagnosis of this entity is
occasionally difficult, as in the present case.
Due to its safety and accessibility, ultrasound
examination is used as a general means for screening renal masses.
Ultrasound will differentiate the components of a lesion (e.g.
calcification, necrosis, hemorrhage or fat) by displaying the
differences between transmitted waves. Ultrasound is fairly
accurate for the diagnosis of renal AML, and the typical
manifestations are markedly higher echoes accompanied with rear
acoustic shadowing, as it is mixed with several tissue components
(e.g. fats) and contains rich vascular nets (16,17). In
the present case, however, the lesion was hyperechoic, and was
combined with the sound shadow behind the marginal calcification,
all of which simulated the ultrasonic manifestations of a typical
AML. Moreover, the necrotic cystic degeneration was reasonably
explained as hemorrhage. Due to the limitations of the ultrasound
machine, the blood supply of the mass was not observed. The first
ultrasound examination was completed by a urinary ultrasound expert
with >30 years of experience, and thus, the misdiagnosis of this
case deserves serious attention.
CT scans with high-density resolution aid in the
differentiation between tissue components, such as calcification,
hemorrhage, soft tissues, fats, cystic degeneration or necrosis. In
the present case, the different components of the mass were
displayed clearly by enhanced CT, including the marginal
calcification, the solid components with rich blood supply and the
unenhanced necrotic region. Since no fat was detected, the
diagnosis was targeted around epithelioid AML and renal cancer.
Based on previous findings, calcification inside a mass indicates a
high probability of malignant lesions, while egg-shell
calcification usually occurs in benign lesions (simple cysts)
(18,19). In the present case, the imaging
manifestations on the CT plain scan mimicked a cyst with
calcification and chronic hematoma, but the enhanced scan showed
marked enhancement in the tissues within the tumor, thus revealing
the essence of the tumor. Therefore, a simple CT plain scan is
inadequate for the evaluation of renal lesions accompanied with
egg-shell calcification, and further evaluation of components
inside the lesion is essential. AML is occasionally difficult to
differentiate from RCC; usually AML contains a varying amount of
fats, which is a key differential point (20–22). In
the present case, the egg-shell calcification partly misguided the
diagnosis, but two possible diagnoses were provided, which ensured
that a reasonable surgical therapy was applied for the misdiagnosed
patient.
In general, this case of Xp11.2 translocation RCC
with egg-shell calcification was ultrasonically manifested as AML.
However, the enhanced CT scan revealed egg-shell calcification and
a rich-blood-supply mass without fat. This study suggests that when
an ultrasound indicates AML, the differential diagnosis should
include the possibility of renal cancer accompanied with egg-shell
calcification. When a CT plain scan shows a high-density cyst with
egg-shell calcification, the differential diagnosis should also
include the possibility of renal cancer. Enhanced CT will aid in
the complete evaluation of the nature of a renal mass with
egg-shell calcification.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics. CA Cancer J Clin. 57:43–66. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luciani LG, Cestari R and Tallarigo C:
Incidental renal cell carcinoma-age and stage characterization and
clinical implications: Study of 1092 patients (1982-1997). Urology.
56:58–62. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aron M, Nguyen MM, Stein RJ and Gill IS:
Impact of gender in renal cell carcinoma: An analysis of the SEER
database. Eur Urol. 54:133–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zweizig SL: Cancer of the kidney. Clin
Obstet Gynecol. 45:884–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hollingsworth JM, Miller DC, Daignault S
and Hollenbeck BK: Rising incidence of small renal masses: A need
to reassess treatment effect. J Natl Cancer Inst. 98:1331–1334.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chow WH, Devesa SS, Warren JL and Fraumeni
JF Jr: Rising incidence of renal cell cancer in the United States.
JAMA. 281:1628–1631. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lopez-Beltran A, Scarpelli M, Montironi R
and Kirkali Z: 2004 WHO classification of the renal tumors of the
adults. Eur Urol. 49:798–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang W, Ding J, Li Y, Wang C, Zhou L, Zhu
H and Peng W: Magnetic resonance imaging and computed tomography
characteristics of renal cell carcinoma associatedwith Xp11.2
translocation/TFE3 gene fusion. PLoS One. 9:e999902014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Winarti NW, Argani P, De Marzo AM, Hicks J
and Mulyadi K: Pediatric renal cell carcinoma associated with
Xp11.2 translocation/TFE3 gene fusion. Int J Surg Pathol. 6:66–72.
2008. View Article : Google Scholar
|
|
10
|
Camparo P, Vasiliu V, Molinie V, Couturier
J, Dykema KJ, Petillo D, Furge KA, Comperat EM, Lae M, Bouvier R,
et al: Renal translocation carcinomas: Clinicopathologic,
immunohistochemical and gene expression profiling analysis of 31
cases with a review of the literature. Am J Surg Pathol.
32:656–670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dang TT, Ziv E, Weinstein S, Meng MV, Wang
Z and Coakley FV: Computed tomography and magnetic resonance
imaging of adult renal cell carcinoma associated with Xp11.2
translocation. J Comput Assist Tomogr. 36:669–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koo HJ, Choi HJ, Kim MH and Cho KS:
Radiologic-pathologic correlation of renal cell carcinoma
associated with Xp11.2 translocation. Acta Radiol. 54:827–834.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu K, Xie P, Peng W and Zhou Z: Renal
carcinomas associated with Xp11.2 translocations/TFE3 gene fusions:
Findings on MRI and computed tomography imaging. J Magn Reson
Imaging. 40:440–447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He J, Huan Y, Qiao Q, Zhang J and Zhang
JS: Renal carcinomas associated with Xp11.2 translocations: Are CT
findings suggestive of the diagnosis? Clin Radiol. 169:45–51. 2014.
View Article : Google Scholar
|
|
15
|
Kmetec A and Jeruc J: Xp 11.2
translocation renal carcinoma in young adults; recently classified
distinct subtype. Radiol Oncol. 48:197–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lienert AR and Nicol D: Renal
angiomyolipoma. BJU Int. 110:25–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Halpenny D, Snow A, McNeill G and
Torreggiani WC: The radiological diagnosis and treatment of renal
angiomyolipoma-current status. Clin Radiol. 165:99–108. 2010.
View Article : Google Scholar
|
|
18
|
Daniel WW Jr, Hartman GW, Witten DM,
Farrow GM and Kelalis PP: Calcified renal masses. A review of ten
years experience at the Mayo Clinic. Radiology. 103:503–508. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dyer RB, Chen MY and Zagoria RJ: Abnormal
calcifications in the urinary tract. Radiographics. 18:1405–1424.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JY, Kim JK, Kim N and Cho KS: CT
histogram analysis: Differentiation of angiomyolipoma without
visible fat from renal cell carcinoma at CT imaging. Radiology.
246:472–479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sasiwimonphan K, Takahashi N, Leibovich
BC, Carter RE, Atwell TD and Kawashima A: Small (<4 cm) renal
mass: Differentiation of angiomyolipoma without visible fat from
renal cell carcinoma utilizing MR imaging. Radiology. 263:160–168.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chung MS, Choi HJ, Kim MH and Cho KS:
Comparison of T2-weighted MRI with and without fat suppression for
differentiating renal angiomyolipomas without visible fat from
other renal tumors. AJR Am J Roentgenol. 202:765–771. 2014.
View Article : Google Scholar : PubMed/NCBI
|