Introduction
Gliomas are tumors that arise from glial cells, and
malignant gliomas are the most common primary intrinsic brain
tumors of adulthood. Glioblastomas account for 15.6% of all primary
brain tumors and 45.2% of primary malignant brain tumors. The
estimated relative survival rates for glioblastoma are fairly low,
with <5% of patients predicted to survive for five years
post-diagnosis (1).
B-cell-specific Moloney murine leukemia virus
integration site 1 (BMI-1) protein is a member of the polycomb
group of proteins, initially isolated as an oncogene involved in
leukemia (2). The gene is located on
chromosome 10p12.22 and has 10 exons. BMI-1 has been demonstrated
to be a key regulatory factor for the determination of a cellular
phenotype in a variety of therapy-resistant cancers, including
glioma (3–9). A previous study showed that BMI-1 levels
increased in 79% of head and neck carcinoma patients, and a
positive correlation was found between these BMI-1 levels and a
lack of response to radiotherapy or chemotherapy (10). Abdouh et al (2009) reported
that BMI-1 is expressed in glioblastoma tumors, is highly enriched
in cluster of differentiation (CD)133+ tumor-initiating
cells and is necessary for tumor cell growth (11).
There are only a few studies that have analyzed the
role of BMI-1 gene alterations in glioma in clinical samples
(12,13). A DNA-RNA-protein analysis on the same
sample could provide a complete picture in order to establish the
mechanism of BMI-1 regulation. The present study analyzed the copy
number variations and expression of BMI-1 at the RNA and protein
levels, and its correlation with clinical characteristics.
Materials and methods
Sample
Glioma samples were collected from the 50 patients,
enrolled between September 2011 and September 2012, who underwent
surgery in the Department of Neurosurgery at the National Institute
of Mental Health and Neurosciences (NIMHANS; Bangalore, India).
Informed consent was obtained from all patients included in the
study. Tissues were bisected and one half was placed in RNAlater
(Sigma-Aldrich, St. Louis, Missouri, USA) and snap frozen in liquid
nitrogen, then kept at −80° until the isolation of the DNA. The
other half was sent for histopathological analysis.
Histopathological diagnosis was made using the World Health
Organization (2007) grading system (14) in the Department of Neuropathology.
Control brain tissues were collected from 5 patients undergoing
anterior temporal lobectomy for mesial temporal sclerosis. The
study was approved by the NIMHANS Human Ethics Committee.
DNA and RNA isolation and
quantification
Tissues were analyzed histologically and those
containing >95% tumor cells were used for further analysis. DNA
and RNA isolation was performed using an All Prep DNA/RNA Mini
isolation kit (Qiagen GmbH, Hilden, Germany) and quantified by
Nanodrop ND 2000c (Thermo Fisher Scientific, Waltham, MA, USA). DNA
Samples with a purity of 1.75–1.85 and RNA samples with a purity of
1.95–2.05 (A260/280) were used in this study. RNA
stability was checked by formaldehyde agarose gel
electrophoresis.
Copy number variation of BMI-1 using
quantitative polymerase chain reaction
Quantitative PCR amplification was performed on the
Applied Biosystems 7500 (Applied Biosystems, Foster City, CA, USA),
using SYBR® Select master mix (Invitrogen, Life Technologies,
Carlsbad, CA, USA) with a total reaction volume of 20 µl. The PCR
mixtures were then subjected to 50°C for UDG activation for 2 min
and activation at 95°C for 2 min, followed by 40 cycles of
denaturation at 95°C for 15 sec, annealing at 53°C for 15 sec and
extension at 72°C for 60 sec. Each template-primer pair was tested
in three replicates to estimate the mean Ct. The comparative Ct
method was used to check the copy number variation in the BMI-1
gene. The primer sequence was as follows: β-actin forward,
5′-GCATTTAGGTAAGGGGTGGA-3′ and reverse, 5′-GGGTACACAGACGAAGCAGA-3′;
and BMI-1 forward, 5′-TGTGTGCTTTGTGGAGGGTA-3′ and reverse,
5′-CATTTCCACAGATTGCAGGA-3′.
RNA extraction and reverse
transcription-quantitative PCR
Total RNA (1 µg) was reverse transcribed into cDNA
using MMLV-reverse transcriptase, oligo-dT, dNTPs and buffer
following the manufacturer's instructions (Life Technologies). The
sequences of the BMI-1 and GAPDH detection probes and primers were
as follows: BMI-1 sense, 5′-CTGGTTGCCCATTGACAGC-3′; BMI-1
antisense, 5′-CAGAAAATGAATGCGAGCCA-3′; probe for BMI-1,
5′-CAGCTCGCTTCAAGATGGCCGC-3′, labeled with FAM and TAMRA as the
reporter dye; GAPDH sense, 5′-GAAGGTGAAGGTCGGAGTCAAC-3′; GAPDH
antisense, 5′-CAGAGTTAAAAGCAGCCCTGG T-3′; GAPDH probe,
5′-TTTGGTCGTATTGGGCGCCT-3′, labeled with VIC dye. The quantitative
assay amplified 1 µl of cDNA in three replicates using the
aforementioned primers and probes, and the Taqman Universal master
mix (Applied Biosystems). Comparative analysis by the
2−ΔΔCt method was used to compare the expression in each
gene.
Western blotting
Whole-cell protein extracts were obtained from all
50 tissues. Cell lysates were prepared in cold lysis buffer [50 mM
Tris, 150 mM NaCl, 1 mol/l EDTA, 0.1% sodium dodecyl sulfate, 1%
Triton X-100, 1 mol/l phenylmethylsulfonyl fluoride (pH 8.0); SRL
Ltd., Bangalore, India]. The lysate was collected and stored at
−80°C. The protein content in the lysates was measured by Bradford
assay. For western blot analysis, 50 µg protein was resolved on 12%
SDS-PAGE gels, transferred onto polyvinyldifluoride membranes
(Bio-Rad Laboratories Inc., Hercules, CA, USA) and subsequently
incubated in blocking buffer (5% bovine serum albumin, 1% Tween 20
in 20 mmol/l Tris-buffered saline (pH 7.6); SRL Ltd.] for 1 h. The
blots were incubated with mouse anti-human monoclonal
IgG1 antibody against BMI-1 (#sc-390443; 1:1,000
dilution), followed by rabbit anti-mouse IgG horseradish
peroxidase-conjugated antibody (#sc-358914; 1:5,000 dilution) (both
Santa Cruz Biotechnology Inc., Dallas, TX, USA), and detected by
enhanced chemiluminescence kit (Bio-Rad Laboratories Inc.)
according to the manufacturer's instructions. Equal loading of
protein was confirmed by stripping the blots and re-probing with
monoclonal mouse anti-human β-tubulin antibody (#sc-58882; 1:2,000
dilution; Santa Cruz Biotechnology Inc.). Bands were detected by
chemiluminescence and analyzed using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
R-2.3.1 (R Foundation for Statistical Computing,
Vienna, Austria) was used for the statistical analysis. Due to the
non-normal distribution of the samples, the statistical evaluation
was performed using non-parametric tests. Comparison between mRNA
and protein expression levels in the different grades of glioma was
performed using the Kruskal-Wallis test. Correlation between mRNA
and protein was analyzed using Spearman's correlation test.
P<0.05 was used to indicate a statistically significant
difference.
Results
Patient details
A total of 50 patients with varying grades of
glioma, enrolled between 2011 and 2012, were included in this
study. All tumors were pathologically confirmed as glioma, and the
patients underwent radiological and histopathological examinations
to establish the clinical profile. The age of the patients ranged
from 8–60 years (mean, 35.6±14.86).
BMI-1 gene copy number variation in
glioma
BMI-1 gene amplification analysis was performed
using SYBR Green quantitative PCR in all tumor samples and
non-glioma brain tissues. The copy number variation was analyzed in
comparison with the β-actin gene. The comparative Ct value analysis
did not show any difference between the BMI-1 and β-actin genes in
the glioma samples or the non-glioma brain tissues.
BMI-1 mRNA expression and correlation
of clinical data
The BMI-1 mRNA levels were analyzed in all the
glioma samples. BMI-1 mRNA expression was found to be overexpressed
in the glioma tissues compared with the non-glioma samples. In
total, 36 out of 50 samples demonstrated overexpression (72%.0),
which was statistically significant when compared with the
non-glioma control tissues (P=0.01). The median fold-change of
expression was 2.055. The mRNA expression ranged from 1.41-fold to
60.97-fold. Grade-wise gene expression analysis was performed and
BMI-1 was found to be expressed more in high-grade glioma than in
low-grade glioma (Kruskal-Wallis rank sum test; P=0.025), as shown
in Fig. 1. The clinical details are
provided in Table I.
 | Table I.BMI-1 mRNA and protein expression data
with clinical characteristics. |
Table I.
BMI-1 mRNA and protein expression data
with clinical characteristics.
|
|
| BMI-1 mRNA
expression |
| BMI-1 protein
expression |
|
|---|
| Characteristics | No. of patients | (mean
fold-change) | P-value | (mean
fold-change) | P-value |
|---|
| Age, years |
|
| 0.467 |
| 0.907 |
| ≤35 | 24 | 7.10 |
| 1.97 |
|
|
>35 | 26 | 7.89 |
| 2.77 |
|
| Gender |
|
| 0.066 |
| 0.276 |
| Male | 31 | 9.56 |
| 1.95 |
|
|
Female | 19 | 4.17 |
| 2.54 |
|
| Grade |
|
| 0.025 |
| 0.024 |
| Low | 8 | 1.79 |
| 1.43 |
| High | 42 | 8.60 |
| 2.67 |
| Tumor type |
|
| 0.448 |
| 0.8253 |
|
Astrocytoma | 12 | 4.21 |
| 1.76 |
|
|
Oligodendroglioma | 22 | 8.96 |
| 2.49 |
|
|
Glioblastoma multiforme | 16 | 7.99 |
| 2.35 |
|
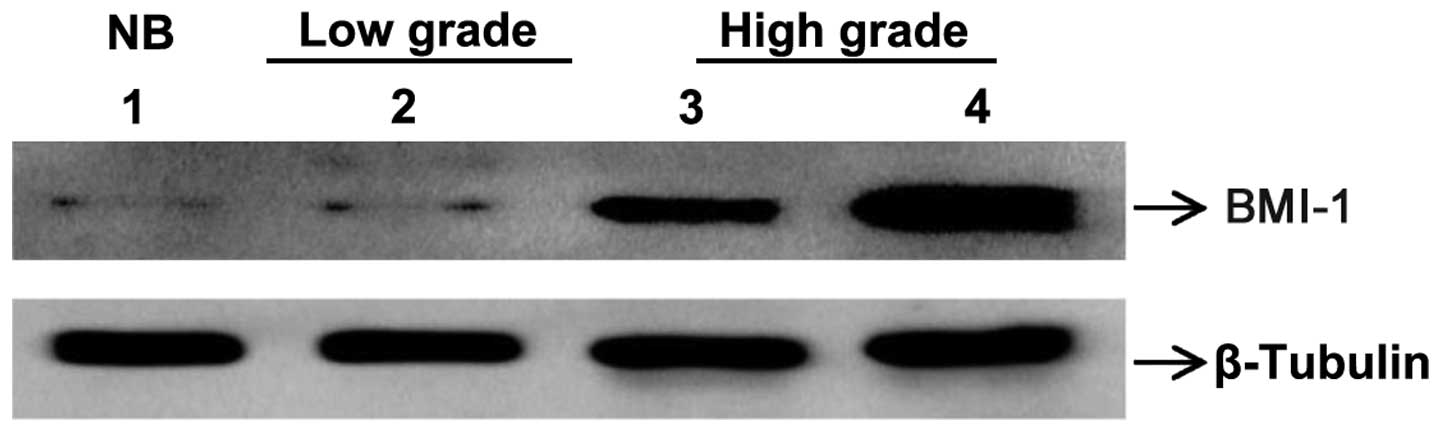
BMI-1 protein expression in glioma and
correlation of clinical data
BMI-1 protein levels were analyzed in all glioma
tissue samples. BMI-1 protein expression was found to be
overexpressed in the glioma tissues compared with the non-glioma
samples upon western blot analysis (Fig.
2). In total, 37 out of 50 samples demonstrated overexpression
(74.0%), which was statistically significant compared with the
non-glioma control tissues (P=0.037). The median fold-change of
expression was 2.0408. The protein expression ranged from 1.47-fold
to 16.36-fold. Grade-wise gene expression analysis was performed
and BMI-1 was found to be expressed more in high-grade glioma than
in low-grade glioma (Kruskal-Wallis rank sum test; P=0.024), as
shown in Fig. 1. There were no other
clinical correlations with protein expression (Table I).
Correlation of BMI-1 mRNA and protein
expression
The correlation between BMI-1 mRNA and protein
levels was assessed by Spearman's correlation test. A positive
correlation was found with a coefficient ρ-value of 0.474 (Fig. 3); this was statistically significant
(P=0.0005). This indicates that the protein expression was
concordant with the mRNA expression, and suggests that there may
not be much post-translational regulation in this protein in
glioma.
Discussion
Our understanding of the pathophysiology of glioma
has progressed in recent years. New studies are focused on
targeting the cancer stem cells that are responsible for treatment
resistance. A few cancer stem cell factors, such as CD133, Nestin
and CD44, are being considered as potent biomarkers for therapeutic
targeting. The BMI-1 gene is one of the stem cell factors that has
a promising role. BMI-1 has been found to be overexpressed in
gliomas and to interact with other oncogenes to make them resistant
to anticancer drugs (15–19).
BMI-1 is a component of polycomb repressive complex
1 (PRC1) involved in epigenetic regulation of gene activity
(20). Gli1 protein can directly bind
to the promoter region of BMI-1 and regulate its expression, and
this has been found to be the major external stimuli of BMI-1
(21). BMI-1 is an upstream regulator
of the CDKN2A pathway, ultimately downregulating TP53 and RB1
genes, which are involved in cell cycle processes (22). Other downstream pathways include the
DNA damage response pathway (23),
the phosphatase and tensin homolog-Akt pathway (24), the nuclear factor κB pathway (25) and the glutathione-reactive oxygen
species pathway (22). BMI-1 has been
found to be overexpressed in various human cancers, including
mammary epithelial cancer, prostate cancer, medulloblastoma,
melanoma, neuroblastoma, endometrial carcinoma and non-small cell
lung cancer (3–9).
It is important to identify the pattern of
expression of BMI-1 and to identify therapeutic targets that will
downregulate its expression and reduce the aggressiveness of the
tumor. In order to understand the BMI-1 gene alterations and
expression, the present study analyzed 50 glioma samples and 5
non-glioma samples, and correlated the results with clinical
status. Each sample was analyzed for alterations of BMI-1
expression at all levels (DNA-RNA-protein).
BMI-1 gene alterations are uncommon in human
neoplasms and are reported only in Mantle cell lymphomas (MCL) and
gliomas. High-level DNA amplification of the 10p23 region, where
BMI-1 is located, has been observed in head and neck carcinoma and
other solid tumors (26). Beà et
al (2001) could not find any gene amplification in the BMI-1
gene in any hematological malignancies except for MCL. The study
observed tumors expressing high levels of mRNA and protein without
any gene amplification, and concluded that this may be due to
unknown mechanisms (26).
Previous studies showed BMI-1 gene alterations such
as copy number variations (amplifications and deletions), and
overexpression of mRNA and protein in different grades of gliomas
(12,13,27–29). Häyry
et al (2008) showed that BMI-1 protein was overexpressed in
the majority of grade 2–4 gliomas (12). In another study, roughly two-thirds of
the total tumors exhibited copy number alterations of BMI-1. It was
also shown that there was no correlation between BMI-1 copy number
variation and protein expression in glioma (13). Abdouh et al (2009) performed
quantitative PCR for the gene amplification analysis and they found
that there was no gene amplification, but that there was
overexpression of BMI-1 in the glioblastoma samples (11). All the glioma samples in the present
study failed to show any copy number variations using the
quantitative PCR technique. There is a constant debate over the
amplification or deletion status in BMI-1 gene in gliomas and other
cancers.
Cenci et al (2012) studied high-grade gliomas
and revealed the overexpression of BMI-1 protein in 72.9% of
samples (27). Farivar et al
(2013) showed BMI-1 gene expression in various types of pediatric
brain tumors and found that gliomas exhibited 5.54-fold more BMI-1
expression when compared with normal brain tissues. The BMI-1
expression and other patho-clinical parameters were also found to
be significantly correlated in the study (28). Wu et al (2013) observed that
the BMI-1 protein expression level in glioma was significantly
higher than that in corresponding non-neoplastic brain tissue
(29).
The present study showed that BMI-1 mRNA was
overexpressed in 72% of the samples. Low-grade glioma exhibited
comparatively less expression. There was no statistical correlation
between gender and BMI-1 mRNA expression. BMI-1 protein expression
(quantitative) was analyzed by western blotting and BMI-1 protein
was found to be overexpressed in 74% of glioma samples, similar to
the previously reported range of 67–99% (12,27,29). In
the present study, it was observed that high-grade gliomas
exhibited higher levels of protein expression compared with
low-grade gliomas. No difference was found between BMI-1 mRNA and
protein expression in the glioma subtypes. High transcriptional
activity may be the one true reason for the overexpression of BMI-1
at the RNA and protein levels without copy number variation at the
gene level.
BMI-1-knockdown has effectively suppressed cancer
cell proliferation in several cancer types (22,30,31).
Studies have reported that the blocking of BMI-1 inhibits the
proliferation and accelerates the apoptosis of colorectal cancer
cells (30), breast cancer cells
(31) and ovarian carcinoma (22). The silencing of BMI-1 expression can
be a potential therapeutic strategy to eliminate these cancer stem
cells from the brain.
In conclusion, the BMI-1 gene plays a major role in
glioma pathogenesis. The present study showed that BMI-1 mRNA and
protein levels are concordantly high in glioma. A
post-transcriptional regulation study is required in order to
detect the discrepancy of correlation between DNA alteration and
RNA/protein expression.
Acknowledgements
The authors would like to kindly acknowledge the
financial support provided by the Council of Scientific and
Industrial Research, New Delhi. This study was also financially
supported by Department of Science and Technology-Science and
Engineering Research Board, Government of India (no.
SR/SO/HS-233/2012). The authors would also like to thank the
Department of Neuropathology, NIMHANS, for performing the
histopathological diagnosis.
References
|
1
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2006–2010. Neuro-Oncology.
15(Suppl 2): ii1–ii56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Lohuizen M, Verbeek S, Scheljen B,
Wientjens E, van der Guidon H and Berns A: Identification of
cooperating oncogenes in E mu-myc transgenic mice by provirus
tagging. Cell. 65:737–752. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Datta S, Hoenerhoff MJ, Bommi P, Sainger
R, Guo WJ, Dimri M, Band H, Band V, Green JE and Dimri GP: Bmi-1
cooperates with H-Ras to transform human mammary epithelial cells
via dysregulation of multiple growth-regulatory pathways. Cancer
Res. 67:10286–10295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lukacs RU, Memarzadeh S, Wu H and Witte
ON: Bmi-1 is a crucial regulator of prostate stem cell self-renewal
and malignant transformation. Cell Stem Cell. 7:682–693. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Venugopal C, Manoranjan B,
McFarlane N, O'Farrell E, Nolte S, Gunnarsson T, Hollenberg R,
Kwiecien J, Northcott P, et al: Sonic hedgehog regulates Bmi1 in
human medulloblastoma brain tumor-initiating cells. Oncogene.
31:187–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bachmann IM, Puntervoll HE, Otte AP and
Akslen LA: Loss of BMI-1 expression is associated with clinical
progress of malignant melanoma. Mod Pathol. 21:583–590. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui H, Hu B, Li T, Ma J, Alam G, Gunning
WT and Ding HF: Bmi-1 is essential for the tumorigenicity of
neuroblastoma cells. Am J Pathol. 170:1370–1378. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engelsen IB, Mannelqvist M, Stefansson IM,
Carter SL, Beroukhim R, Øyan AM, Otte AP, Kalland KH, Akslen LA and
Salvesen HB: Low BMI-1 expression is associated with an activated
BMI-1-driven signature, vascular invasion and hormone receptor loss
in endometrial carcinoma. Br J Cancer. 98:1662–1669. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vonlanthen S, Heighway J, Altermatt HJ,
Gugger M, Kappeler A, Borner MM, van Lohuizen M and Betticher DC:
The bmi-1 oncoprotein is differentially expressed in non-small cell
lung cancer and correlates with INK4A-ARF locus expression. Br J
Cancer. 84:13722001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vormittag L, Thurnher D, Geleff S, Pammer
J, Heiduschka G, Brunner M, Grasl MCH and Erovic BM: Co-expression
of Bmi-1 and podoplanin predicts overall survival in patients with
squamous cell carcinoma of the head and neck treated with radio
(chemo) therapy. Int J Radiat Oncol Biol Phys. 73:913–918. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abdouh M, Facchino S, Chatoo W, Balasingam
V, Ferreira J and Bernier G: BMI1 sustains human glioblastoma
multiforme stem cell renewal. J Neurosci. 29:8884–8896. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Häyry V, Tynninen O, Haapasalo HK, Wölfer
J, Paulus W, Hasselblatt M, Sariola H, Paetau A, Sarna S, Niemelä
M, et al: Stem cell protein BMI-1 is an independent marker for poor
prognosis in oligodendroglial tumours. Neuropathol Appl Neurobiol.
34:555–563. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Häyry V, Tanner M, Blom T, Tynninen O,
Roselli A, Ollikainen M, Sariola H, Wartiovaara K and Nupponen NN:
Copy number alterations of the polycomb gene BMI1 in gliomas. Acta
Neuropathol. 116:97–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hemmati HD, Nakano I, Lazareff JA,
Masterman-Smith M, Geschwind DH, Bronner-Fraser M and Kornblum HI:
Cancerous stem cells can arise from pediatric brain tumors. Proc
Natl Acad Sci USA. 100:15178–15183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tso CL, Shintaku P, Chen J, Liu Q, Liu J,
Chen Z, Yoshimoto K, Mischel PS, Cloughesy TF, Liau LM and Nelson
SF: Primary glioblastomas express mesenchymal stem-like properties.
Mol Cancer Res. 4:607–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen R, Nishimura MC, Bumbaca SM,
Kharbanda S, Forrest WF, Kasman IM, Greve JM, Soriano RH, Gilmour
LL, Rivers CS, et al: A hierarchy of self-renewing tumor-initiating
cell types in glioblastoma. Cancer Cell. 17:362–375. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huber GF, Albinger-Hegyi A, Soltermann A,
Roessle M, Graf N, Haerle SK, Holzmann D, Moch H and Hegyi I:
Expression patterns of Bmi-1 and p16 significantly correlate with
overall, disease-specific and recurrence-free survival in
oropharyngeal squamous cell carcinoma. Cancer. 117:4659–4670. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vaissière T, Sawan C and Herceg Z:
Epigenetic interplay between histone modifications and DNA
methylation in gene silencing. Mutat Res. 659:40–48. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park IK, Morrison SJ and Clarke MF: Bmi1,
stem cells and senescence regulation. J Clin Invest. 113:175–179.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang E, Bhattacharyya S, Szabolcs A,
Rodriguez-Aguayo C, Jennings NB, Lopez-Berestein G, Mukherjee P,
Sood AK and Bhattacharya R: Enhancing chemotherapy response with
Bmi-1 silencing in ovarian cancer. PLoS One. 6:e179182011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu S, Dontu G, Mantle ID, Patel S, Ahn
NS, Jackson KW, Suri P and Wicha MS: Hedgehog signaling and Bmi-1
regulate self-renewal of normal and malignant human mammary stem
cells. Cancer Res. 66:6063–6071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu
LJ, Kong QL, Xu LH, Zhang X, Liu WL, et al: The polycomb group
protein Bmi-1 represses the tumor suppressor PTEN and induces
epithelial-mesenchymal transition in human nasopharyngeal
epithelial cells. J Clin Invest. 119:3626–3636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Gong LY, Song LB, Jiang LL, Liu LP,
Wu J, Yuan J, Cai JC, He M, Wang L, et al: Oncoprotein Bmi-1
renders apoptotic resistance to glioma cells through activation of
the IKK-nuclear factor-κB pathway. Am J Pathol. 176:699–709. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beà S, Tort F, Pinyol M, Puig X, Hernández
L, Hernández S, Fernandez PL, van Lohuizen M, Colomer D and Campo
E: BMI-1 gene amplification and overexpression in hematological
malignancies occur mainly in mantle cell lymphomas. Cancer Res.
61:2409–2412. 2001.PubMed/NCBI
|
|
27
|
Cenci T, Martini M, Montano N,
D'Alessandris QG, Falchetti ML, Annibali D, Savino M, Bianchi F,
Pierconti F, Nasi S, et al: Prognostic relevance of c-Myc and BMI1
expression in patients with glioblastoma. Am J Clin Pathol.
138:390–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Farivar S, Keikha R Zati, Shiari R and
Jadali F: Expression of bmi-1 in pediatric brain tumors as a new
independent prognostic marker of patient survival. Biomed Res Int.
2013:1925482013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu Z, Wang Q, Wang L, Li G, Liu H, Fan F,
Li Z, Li Y and Tu Y: Combined aberrant expression of Bmi1 and EZH2
is predictive of poor prognosis in glioma patients. J Neurol Sci.
335:191–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Molofsky AV, He S, Bydon M, Morrison SJ
and Pardal R: Bmi-1 promotes neural stem cell self-renewal and
neural development but not mouse growth and survival by repressing
the p16Ink4a and p19Arf senescence pathways. Genes Dev.
19:1432–1437. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bommi PV, Dimri M, Sahasrabuddhe AA,
Khandekar J and Dimri GP: The polycomb group protein BMI1 is a
transcriptional target of HDAC inhibitors. Cell Cycle. 9:2663–2673.
2010. View Article : Google Scholar : PubMed/NCBI
|