Introduction
Rectal cancer is one of the commonest causes of
cancer-related mortality worldwide, and its incidence is increasing
year-on-year. Surgery remains the mainstay of treatment for rectal
cancer. The aims of surgery for rectal cancer are to achieve cure
and to avoid locoregional recurrence (1). The principle of total mesorectal
excision (TME) has been widely applied, which has significantly
reduced the local recurrence rate for rectal cancer (1,2). However,
the success of TME for the treatment of rectal cancer is influenced
by the surgeon's experience, in addition to patient anatomical and
clinical factors (3–5). The patient's pelvic anatomical factors
are important, as TME is performed in the narrow and funnel-shaped
pelvic cavity, which makes access and visualization in the deep
pelvis, difficult. It is challenging to maintain a clear surgical
field, to recognize precise anatomy, and to accurately perform
rectal mobilization and excision (6).
Surgeons are aware that the female pelvis is, in
general, more accessible than the male pelvis when conducting open
rectal surgery for mid-low rectal cancer. In general, female
pelvises are wider and shallower than male pelvises. However, there
is considerable variation and overlap between the sexes (7). At present, there is no consensus on how
pelvic diameter and angle influence the technical difficulty of
performing open rectal surgery for mid-low rectal cancer. In the
present study, operative time and intraoperative blood loss were
selected as indicators of operative difficulty, as they have been
objectively validated as such in the literature (6,8,9).
The aim of the present study is to evaluate the
predictive value of clinicopathological and pelvic anatomical
parameters in estimating the operative time and intraoperative
blood loss when performing open rectal surgery for mid-low rectal
cancer, in order to assist colorectal surgeons in the
identification of potentially difficult rectal resections and in
the design of appropriate preoperative plans.
Patients and methods
Patients and surgical procedures
Sixty consecutive patients, who underwent low
anterior resection (LAR) with double-stapling technique (DST)
anastomosis or abdominoperineal resection (APR) for mid-low rectal
cancer located within 10 cm of the anal verge, were recruited
between June 2009 and April 2014. The distance from the anal verge
to the lower margin of the tumors (tumor height) was measured by
digital rectal examination and/or colonoscopy. All cases were
confirmed as adenocarcinoma by biopsy prior to surgery. Operations
were performed by the same surgeon and surgical team, who were
experienced in TME techniques, at the Department of Surgery of
Wenzhou Central Hospital (Wenzhou, China).
Patients who had undergone previous abdominal
surgery through a laparotomy, who had a history of pelvic fracture,
who had received neoadjuvant chemoradiotherapy and those with
locally recurrent disease were excluded from the present study.
Patients were also excluded if tumors had infiltrated to the
adjacent organs, or had metastasized to the lateral pelvic wall
lymph nodes or distant sites. The preoperative clinical stage of
rectal cancer was assessed by contrast-enhanced computerized
tomography (CT).
Data on age, gender, body mass index (BMI), the
maximum diameter of the tumor, tumor height, tumor invasive depth,
lymph node metastasis, tumor staging, operative time and
intraoperative blood loss were collected retrospectively. Tumors
were staged according to the 7th tumor-node-metastasis
(TNM) classification of the International Union against Cancer
(UICC) (10), on the basis of the
histological findings of the surgical specimens. Written informed
consent for participation was obtained from participants, or from
their parent or guardian. This study was approved by the
Institutional Review Board of Wenzhou Central Hospital.
Pelvimetry
All patients underwent contrast-enhanced
abdominopelvic CT. Three-dimensional reconstruction of the pelvis
was performed on a workstation, using the syngo CT imaging program
(version VA44A; Siemens AG, Muenchen, Germany) with a scanning
slice thickness of 1.0 mm and interslice interval of 1.0 mm. Pelvic
dimensions and angles were obtained using mid-sagittal and axial
sections of the pelvis.
All measurements were made by a single observer who
was blinded to all clinical information. The following fourteen
pelvic parameters, including twelve dimensions and two angles, were
measured:
1. Anteroposterior
diameter of the pelvic inlet (AB): A line from the superior median
aspect of the pubic symphysis to the sacral promontory.
2. Anteroposterior
diameter of the mid-pelvis (CD): A line from the inferior median
aspect of the pubic symphysis to the sacrococcygeal junction.
3. Anteroposterior
diameter of the pelvic outlet (CE): A line from the inferior median
aspect of the pubic symphysis to the tip of the coccyx.
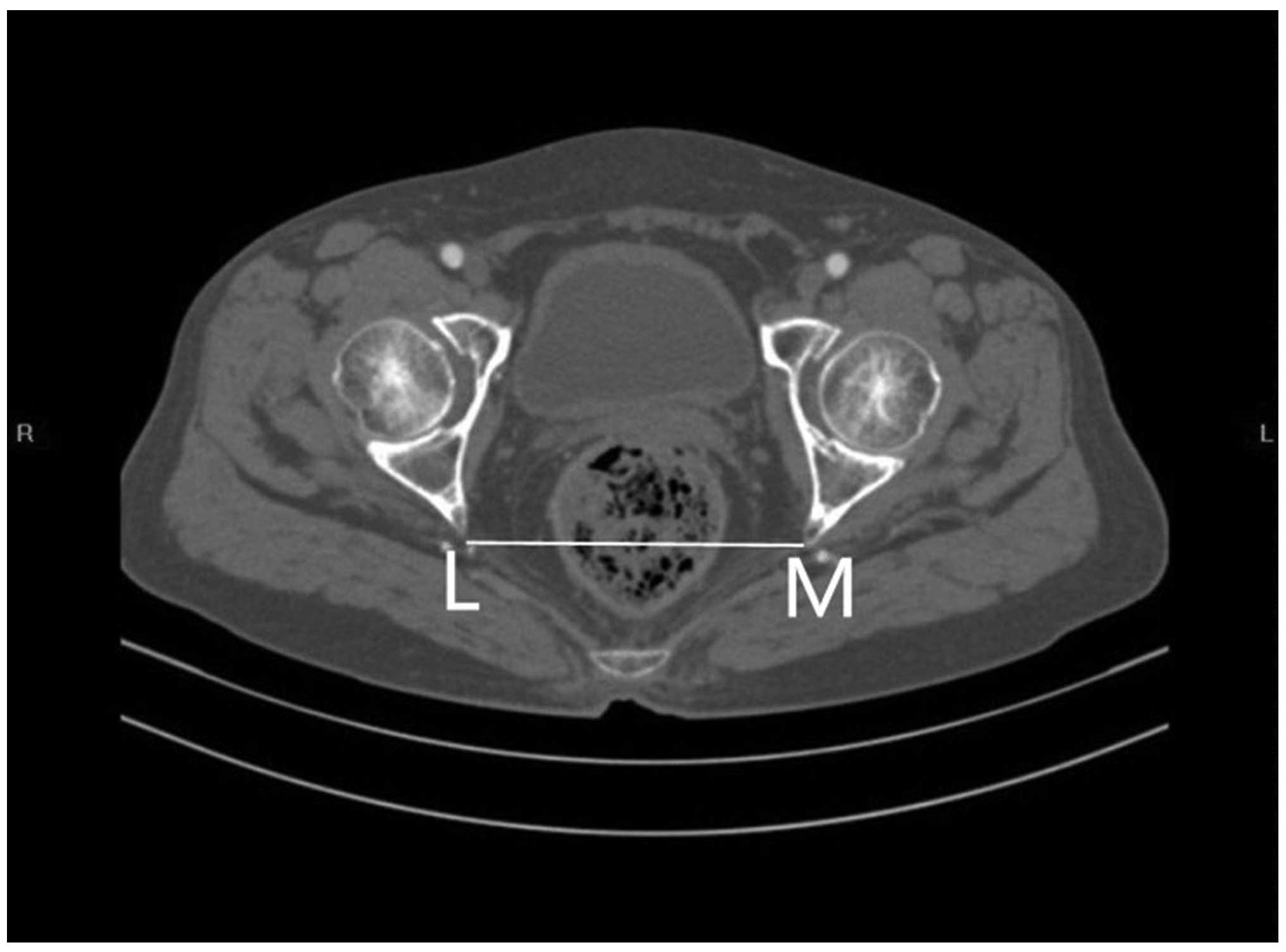
4. The interspinous
diameter (LM).
5. The intertuberous
diameter (NO).
6. The height of the
pubic symphysis (AC).
7. The sacrococcygeal
distance (BE): Distance from the sacral promontory to the tip of
the coccyx.
8. The sacral distance
(BD): Distance from the sacral promontory to the sacrococcygeal
junction.
9. Sacrococcygeal-pubic
angle (α): The angle between an extension of the line forming the
anteroposterior diameter of the pelvic inlet and that of the
anteroposterior diameter of the pelvic outlet.
10. Sacropubic angle (β):
The angle between an extension of the line forming the
anteroposterior diameter of the pelvic inlet and that of the
anteroposterior diameter of the mid-pelvis.
11. The depth of the
sacrococcygeal curvature (FI): A perpendicular line from the
deepest portion of the sacrococcygeal hollow to the sacrococcygeal
distance.
12. The depth of the
sacral curvature (FH): A perpendicular line from the deepest
portion of the sacral hollow to the sacral distance line.
13. Diameter of the upper
pubis to the coccyx (AE): A line from the superior median aspect of
the pubic symphysis to the tip of the coccyx.
14. Sacropubic distance
(FG): A perpendicular line from the deepest portion of the
sacrococcygeal hollow to the height of the pubic symphysis or an
extension of this line.
Figs. 1 and 2 outline the mid-sagittal view of the pelvis
in a female patient. Fig. 3 outlines
the axial section, showing the interspinous diameter of the
mid-pelvis. Fig. 4 outlines the axial
section, showing the intertuberous diameter of the pelvic outlet.
The relevant measurements are indicated in Figs. 1–4.
Assessment of intraobserver error was conducted as detailed in the
statistics section.
Statistical analysis
Statistical analyses were performed using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are shown as the
mean ± standard deviation or medians (range), as appropriate.
Operative time and intraoperative blood loss were used as
indicators of operative difficulty. Operative time and
intraoperative blood loss were defined as dependent variables, and
pelvic anatomical and clinicopathological parameters were defined
as independent variables. Determination of statistically
significant factors was made by univariate and multivariate
analysis.
Where appropriate, an independent samples t-test was
applied to analyze differences in pelvic anatomical parameters
between males and females. Pearson's product-moment correlation
coefficient or Spearman's rank correlation coefficient were applied
to analyze associations between patients' pelvic anatomical and
clinicopathological parameters, and operative difficulty.
Multivariate analysis was performed using a multiple linear
regression model with a backward method. P≤0.05 was considered to
indicate a statistically significant difference.
In order to assess intraobserver variation,
measurements of the pelvic anatomical parameters of 20 patients
were repeated following an interval of 4 weeks, with the observer
blinded to the initial results. A paired-samples t-test was then
applied to the data. Intraobserver variation was calculated using
Pearson's product-moment correlation coefficient. The lowest value
obtained was 0.991. The two sets of measurements were highly
correlated (P<0.001), indicating that measurements were
reproducible and accurate.
Results
Patients' clinicopathological data and pelvic
anatomical parameters are summarized in Tables I and II, respectively. The nine pelvic
parameters, in which there were significant differences between the
sexes, were anteroposterior diameter of the pelvic inlet,
anteroposterior diameter of the mid-pelvis, anteroposterior
diameter of the pelvic outlet, the interspinous diameter, the
intertuberous diameter, sacrococcygeal-pubic angle, the depth of
the sacrococcygeal curvature, diameter of the upper pubis to the
coccyx and sacropubic distance (P<0.05). No significant
differences were detected between the sexes in the remaining five
pelvic parameters; namely, the height of the pubic symphysis, the
sacrococcygeal distance, the sacral distance, sacropubic angle and
the depth of the sacral curvature (P>0.05).
 | Table I.Clinicopathological data (n=60). |
Table I.
Clinicopathological data (n=60).
| Variable | Value |
|---|
| Gender, n
(male:female) | 38:22 |
| Age, years
(range) |
65.3±13.0a
(29–85) |
| Body mass index,
kg/m2 (range) |
21.31±3.07a (16.2–30.8) |
| Tumor height, cm
(range) | 5.9±2.0a (2.0–10.0) |
| Maximum diameter of
the tumor, cm (range) | 4.0b (3.0–8.0) |
| Operative time, min
(range) |
161.3±41.9a (75–275) |
| Intraoperative blood
loss, ml (range) | 50b (50–600) |
| Surgical procedure,
n |
|
|
Abdominoperineal
resection | 40 |
| Low
anterior resection | 20 |
| Tumor invasive depth,
n |
|
| T1 | 3 |
| T2 | 16 |
| T3 | 28 |
| T4 | 13 |
| Lymph node
metastasis, n |
|
| N0 | 25 |
| N1 | 23 |
| N2 | 12 |
| Tumor stage, n |
|
| I | 14 |
| II | 11 |
| III | 35 |
 | Table II.Pelvic anatomical parameters
(n=60). |
Table II.
Pelvic anatomical parameters
(n=60).
|
| Total (n=60) | Male (n=38) | Female (n=22) | P-value |
|---|
| Anteroposterior
diameter of the pelvic inlet, mm |
110.31±12.10 |
105.22±8.78 |
119.10±12.13 | <0.001 |
| Anteroposterior
diameter of the mid-pelvis, mm |
111.40±8.44 |
107.96±6.74 |
117.34±7.85 | <0.001 |
| Anteroposterior
diameter of the pelvic outlet, mm |
88.92±8.62 |
85.54±6.37 |
94.76±9.00 | <0.001 |
| Interspinous
diameter, mm |
98.58±10.23 |
93.24±7.32 |
107.79±7.68 | <0.001 |
| Intertuberous
diameter, mm |
98.46±14.25 |
91.28±9.52 |
110.87±12.50 | <0.001 |
| Height of pubic
symphysis, mm |
36.73±3.50 |
37.36±3.38 |
35.65±3.53 |
0.068 |
| Sacrococcygeal
distance, mm |
121.49±13.80 |
122.22±14.17 |
120.24±13.36 |
0.595 |
| Sacral distance,
mm |
107.09±10.52 |
107.37±10.37 |
106.62±11.00 |
0.794 |
|
Sacrococcygeal-pubic angle, ° |
51.21±8.76 |
53.61±8.81 |
47.04±7.07 |
0.003 |
| Sacropubic angle,
° |
36.98±6.41 |
38.00±6.75 |
35.21±5.46 |
0.104 |
| Depth of the
sacrococcygeal curvature, mm |
37.98±5.38 |
39.20±4.81 |
35.87±5.76 |
0.020 |
| Depth of the sacral
curvature, mm |
20.78±5.05 |
21.43±5.28 |
19.66±4.52 |
0.177 |
| Diameter of the
upper pubis to the coccyx, mm |
113.17±8.54 |
110.91±6.68 |
117.08±10.06 |
0.015 |
| Sacropubic
distance, mm |
123.13±10.15 |
120.17±6.82 |
128.24±12.81 |
0.011 |
Univariate analyses of the association between
patients' clinicopathological and pelvic anatomical parameters, and
operative difficulty are summarized in Table III. Multivariate analyses of the
associations between patients' clinicopathological and pelvic
anatomical parameters, and operative difficulty are summarized in
Tables IV–VIII. Univariate analysis showed that BMI
(P=0.02), tumor height (P=0.02), lymph node metastasis (P=0.04) and
tumor staging (P=0.03) were significantly associated with operating
time. However, no significant associations were detected between
gender, age, the maximum diameter of the tumor, tumor invasive
depth or pelvic anatomical parameters, and operating time
(P>0.05). Univariate analysis showed that the maximum diameter
of the tumor (P=0.05) was the only clinicopathological parameter
that was significantly associated with intraoperative blood loss,
while no association was detected between the remaining
clinicopathological parameters or the pelvic anatomical parameters,
and intraoperative blood loss (P>0.05).
 | Table III.Correlations between
clinicopathological and pelvic anatomical parameters, and operative
difficulty. |
Table III.
Correlations between
clinicopathological and pelvic anatomical parameters, and operative
difficulty.
|
| Correlation with
operative time | Correlation with
intraoperative blood loss |
|---|
|
|
|
|
|---|
| Variable | Correlation | P-value | Correlation | P-value |
|---|
| Gender | 0.115 | 0.38 | 0.055 | 0.67 |
| Age | −0.142a | 0.28 | 0.124 | 0.35 |
| Body mass
index | 0.310a | 0.02 | −0.149 | 0.25 |
| Tumor height | −0.300a | 0.02 | 0.059 | 0.65 |
| Maximum diameter of
the tumor | −0.117 | 0.37 | 0.253 | 0.05 |
| Tumor invasive
depth | −0.031 | 0.81 | −0.005 | 0.97 |
| Lymph node
metastasis | −0.262 | 0.04 | −0.012 | 0.93 |
| Tumor stage | −0.284 | 0.03 | 0.073 | 0.58 |
| Anteroposterior
diameter of the pelvic inlet | 0.014a | 0.92 | 0.043 | 0.74 |
| Anteroposterior
diameter of the mid-pelvis | −0.129a | 0.32 | −0.027 | 0.84 |
| Anteroposterior
diameter of the pelvic outlet | −0.107a | 0.41 | −0.125 | 0.34 |
| Interspinous
diameter | −0.090a | 0.49 | −0.015 | 0.91 |
| Intertuberous
diameter | −0.060a | 0.65 | 0.037 | 0.78 |
| Height of pubic
symphysis | 0.001a | 0.99 | 0.219 | 0.09 |
| Sacrococcygeal
distance | −0.013a | 0.92 | −0.041 | 0.76 |
| Sacral
distance | −0.103a | 0.44 | −0.047 | 0.72 |
|
Sacrococcygeal-pubic angle |
0.039a | 0.77 | −0.019 | 0.89 |
| Sacropubic
angle | −0.003a | 0.98 | −0.152 | 0.25 |
| Depth of the
sacrococcygeal curvature | 0.048a | 0.72 | 0.188 | 0.15 |
| Depth of the sacral
curvature | 0.104a | 0.43 | 0.024 | 0.85 |
| Diameter of the
upper pubis to the coccyx | −0.106a | 0.42 | −0.179 | 0.17 |
| Sacropubic
distance | −0.033a | 0.80 | 0.093 | 0.48 |
 | Table IV.Multivariate analysis in the final
backward linear regression model of clinicopathological parameters
and operative time. |
Table IV.
Multivariate analysis in the final
backward linear regression model of clinicopathological parameters
and operative time.
|
| Unstandardized
coefficient | Standardized
coefficient |
|
|
|---|
|
|
|
|
|
|
|---|
| Model | B | SE | β | t | P-value |
|---|
| (Constant) | 132.295 | 38.672 |
|
3.421 | 0.001 |
| Body mass
index |
3.456 | 1.603 |
0.253 |
2.156 | 0.035 |
| Tumor height |
−5.613 | 2.383 | −0.273 | −2.355 | 0.022 |
| Lymph node
metastasis | −15.069 | 6.466 | −0.274 | −2.330 | 0.023 |
 | Table VIII.Multivariate analysis of the
association between pelvic anatomical parameters and intraoperative
blood loss in the abdominoperineal resection group. |
Table VIII.
Multivariate analysis of the
association between pelvic anatomical parameters and intraoperative
blood loss in the abdominoperineal resection group.
|
| Unstandardized
coefficient | Standardized
coefficient |
|---|
|
|
|
|
|---|
| Model | B | SE | β | t | P-value |
|---|
| (Constant) | 229.465 | 214.829 |
|
1.068 |
0.304 |
| Anteroposterior
diameter of the mid-pelvis |
22.429 |
4.322 | 2.498 |
5.189 | <0.001 |
| Anteroposterior
diameter of the pelvic outlet | −14.673 |
2.908 | −1.239 | −5.045 | <0.001 |
| Interspinous
diameter |
−5.594 |
1.600 | −0.619 | −3.495 |
0.004 |
| Depth of the
sacrococcygeal curvature |
13.276 |
4.510 |
0.747 |
2.944 |
0.011 |
| Sacropubic
distance |
−8.840 |
2.767 | −1.004 | −3.195 |
0.006 |
In the whole group, multivariate analysis
demonstrated that predictive factors for operating time included
BMI, tumor height, lymph node metastasis, anteroposterior diameter
of the pelvic inlet, anteroposterior diameter of the pelvic outlet,
the height of pubic symphysis, the sacrococcygeal distance,
sacrococcygeal-pubic angle and the diameter of the upper pubis to
the coccyx (all P<0.05), while the only predictive factor for
intraoperative blood loss was the maximum diameter of the tumor
(P<0.05).
Subgroup analyses may provide more specific
information, and multivariate analysis of the association between
clinicopathological parameters and operative time in the LAR group,
showed that predictive factors for operative time included tumor
height and tumor stage (adjusted coefficient of determination of
the regression equation, RC2=0.312;
P<0.001). Multivariate analysis of the association between
pelvic anatomical parameters and intraoperative blood loss in the
APR group demonstrated that predictive factors for intraoperative
blood loss included anteroposterior diameter of the mid-pelvis,
anteroposterior diameter of the pelvic outlet, the interspinous
diameter, the depth of the sacral curvature and the sacropubic
distance (RC2=0.608; P=0.002).
Discussion
Patients with rectal cancer usually require spiral
CT enhanced scanning for preoperative staging assessment. Spiral CT
has high sensitivity and specificity, and a low radiation dose, and
is also relatively inexpensive compared with magnetic resonance
imaging. Therefore CT pelvimetry may be used conveniently in
patients with rectal cancer. CT pelvimetry is also an accurate and
reliable technique for obtaining pelvimetric measurements, which
has been utilized in patients with rectal cancer for a number of
years (6,11–13).
Colorectal surgeons recognize that the female pelvis
is generally more accessible than the male pelvis, in terms of
conducting open rectal surgery for mid-low rectal cancer. In the
present study, nine pelvic parameters exhibited significant
differences between the sexes; namely, anteroposterior diameter of
the pelvic inlet, anteroposterior diameter of the mid-pelvis,
anteroposterior diameter of the pelvic outlet, the interspinous
diameter, the intertuberous diameter, sacrococcygeal-pubic angle,
the depth of the sacrococcygeal curvature, diameter of the upper
pubis to the coccyx and sacropubic distance (P<0.05). These
pelvic parameters, with the exception of the sacrococcygeal-pubic
angle and the depth of the sacrococcygeal curvature, represent the
pelvic width, and were greater in females. By contrast, the depth
of the sacrococcygeal curvature represents the sacrococcygeal
bending degree, and was greater in the male pelvis. The
sacrococcygeal-pubic angle indicates sacrococcygeal arc length and
bending degree; in general, the larger the sacrococcygeal-pubic
angle, the longer the sacrococcygeal arc length and the smaller the
sacrococcygeal bending degree, in individuals of a similiar height.
Usually, the sacrococcygeal-pubic angle is greater in the male
pelvis than the female pelvis. The remaining five pelvic parameters
measured in the present study, exhibited no significant differences
between the sexes; namely, the height of the pubic symphysis, the
sacrococcygeal distance, the sacral distance, sacropubic angle and
the depth of the sacral curvature (P>0.05). The height of the
pubic symphysis, the sacrococcygeal distance and the sacral
distance, represent the pelvic depth; the depth of the sacral
curvature represents the sacral bending degree; and the sacropubic
angle indicates sacral arc length and sacral bending degree, as
well as the distance between pubis and the sacrum, which exhibited
a greater overlap between the sexes, suggesting that for these
factors, the measurements themselves may be a more useful predictor
of difficulty than gender alone, as previously mentioned by Killeen
et al (8).
In the present study, multivariate analyses
demonstrated that higher BMI, lower tumor height, fewer lymph node
metastasis, shorter anteroposterior diameter of the pelvic inlet,
shorter anteroposterior diameter of the pelvic outlet, shorter
height of the pubic symphysis, longer sacrococcygeal distance,
smaller sacrococcygeal-pubic angle and longer diameter of the upper
pubis to the coccyx, were significantly associated with a longer
operative time (all P<0.05), while a larger maximum diameter of
the tumor was significantly associated with increased
intraoperative blood loss (P<0.05). Between the two procedures,
the clinicopathological parameters appeared to have greater
predictive value in the LAR group, in which lower tumor height and
lower tumor staging were significantly associated with longer
operative time (RC2=0.312; P<0.001). By
contrast, the pelvic anatomical parameters appeared to be more
valuable predictors in the APR group, in which longer
anteroposterior diameter of the mid-pelvis, shorter anteroposterior
diameter of the pelvic outlet, shorter interspinous diameter,
longer depth of the sacral curvature and shorter sacropubic
distance were significantly associated with increased
intraoperative blood loss (RC2=0.608;
P=0.002).
The present findings suggest that BMI, tumor height
and the maximum diameter of the tumor may be valuable for
predicting operative difficulty encountered during open rectal
surgery for mid-low rectal cancer, which is in accordance with
previous studies (6,12,14). A
higher BMI is associated with greater mesorectal volume, which
restricts the pelvic working space during surgical procedures.
Furthermore, a larger maximum diameter of the tumor reflects larger
tumor volume, which also restricts the pelvic working space
(6). The pelvic working space will
also be affected by tumor height from the anal verge. Particularly
in certain patients with lower rectal cancer, it is difficult to
expose the deep pelvic structures to obtain an adequate working
space. In addition, the present findings indicated that fewer lymph
node metastases and lower tumor stage may be significantly
associated with longer operative time. A possible explanation for
this unusual finding, is that thin patients with rectal cancer are
more prone to lymph node metastasis and intestinal wall invasion,
compared with obese patients. Since the findings also showed a
correlation between higher BMI and longer operative time, this
group may therefore include those with fewer metastases. It has
been reported that the mesorectum presents a considerable obstacle
to the growth of cancer (15). The
volume of perirectal fatty tissue is likely to be smaller in lean
patients than in obese patients. A small volume of perirectal fatty
tissue may therefore contribute to early tumor infiltration of the
pelvic wall and/or adjacent organs (16), meaning that this parameter may be
negatively correlated with operative time for a similar reason to
that of lymph node metastasis.
The present findings concerning the predictive value
of pelvic anatomical features, may, in part, be explained by the
fact that open rectal surgery for mid-low rectal cancer is easier
to complete in wider, shallower and less curved pelvises, although
the influence of the height of the pubic symphysis, diameter of the
upper pubis to the coccyx and anteroposterior diameter of the
mid-pelvis, is more difficult to be rationalize. A possible reason
for the effect of these factors, is that the sample size is
relatively small. Therefore, studies with larger sample sizes need
to be conducted for more definite results in future. In view of the
results of the multivariate analyses in the APR subgroup,
demonstrating the predictive value of pelvic anatomical parameters,
it is more difficult for surgeons to gain entry into the presacral
space by sharp dissection in narrower and more curved concave
pelvises. This is due to the fact that any inaccuracy may lead to
deviation from the correct anatomical level (‘holy plane’), and the
avascular area, resulting in severe presacral hemorrhage.
Therefore, such cases should be performed by highly experienced
colorectal surgeons in order to reduce the risk of serious
complications and recurrence in patients with positive margins.
The present findings indicate that BMI, tumor height
and the maximum diameter of the tumor may be used to predict
operative difficulty in performing open rectal surgery for mid-low
rectal cancer. Furthermore, the associated clinicopathological
parameters, and wider, shallower and less curved pelvises may make
the greatest contribution to reducing operating times and
intraoperative blood loss. Operative difficulty is likely to be
increased in deeper and narrower pelvises, or in those with larger
sacrococcygeal curvature.
Acknowledgements
This study was supported by a grant from the
Technology Planning Project of Wenzhou Science & Technology
Bureau (grant no. Y20130383).
Glossary
Abbreviations
Abbreviations:
|
DST
|
double-stapling technique
|
|
CT
|
computerized tomography
|
|
BMI
|
body mass index
|
|
TME
|
total mesorectal excision
|
|
APR
|
abdominoperineal resection
|
|
LAR
|
low anterior resection
|
|
TNM
|
tumor-node-metastasis
|
References
|
1
|
Dorudi S, Steele RJ and McArdle CS:
Surgery for colorectal cancer. Br Med Bull. 64:101–118. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heald RJ, Husband EM and Ryall RD: The
mesorectum in rectal cancer surgery - the clue to pelvic
recurrence? Br J Surg. 69:613–616. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baik SH, Kim NK, Lee KY, Sohn SK, Cho CH,
Kim MJ, Kim H and Shinn RK: Factors influencing pathologic results
after total mesorectal excision for rectal cancer: Analysis of
consecutive 100 cases. Ann Surg Oncol. 15:721–728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salerno G, Daniels IR, Brown G, Norman AR,
Moran BJ and Heald RJ: Variations in pelvic dimensions do not
predict the risk of circumferential resection margin (CRM)
involvement in rectal cancer. World J Surg. 31:1313–1320. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boyle KM, Petty D, Chalmers AG, Quirke P,
Cairns A, Finan PJ, Sagar PM and Burke D: MRI assessment of the
bony pelvis may help predict resectability of rectal cancer.
Colorectal Dis. 7:232–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogiso S, Yamaguchi T, Hata H, Fukuda M,
Ikai I, Yamato T and Sakai Y: Evaluation of factors affecting the
difficulty of laparoscopic anterior resection for rectal cancer:
Narrow pelvis is not a contraindication. Surg Endosc. 25:1907–1912.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salerno G, Daniels IR, Brown G, Heald RJ
and Moran BJ: Magnetic resonance imaging pelvimetry in 186 patients
with rectal cancer confirms an overlap in pelvic size between males
and females. Colorectal Dis. 8:772–776. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Killeen T, Banerjee S, Vijay V, Al-Dabbagh
Z, Francis D and Warren S: Magnetic resonance (MR) pelvimetry as a
predictor of difficulty in laparoscopic operations for rectal
cancer. Surg Endosc. 24:2974–2979. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Veenhof AA, Engel AF, van der Peet DL,
Sietses C, Meijerink WJ, de Lange-de Klerk ES and Cuesta MA:
Technical difficulty grade score for the laparoscopic approach of
rectal cancer: A single institution pilot study. Int J Colorectal
Dis. 23:469–475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobin LH and Brierly J: Colon and rectum.
International Union Against Cancer (UICC) TNM Classification of
Malignant Tumors. Sobin LH, Gospodarowicz MK and Wittekind C:
(7th). (Oxford). Wiley-Blackwell. 100–105. 2009.
|
|
11
|
Targarona EM, Balague C, Pernas JC,
Martinez C, Berindoague R, Gich I and Trias M: Can we predict
immediate outcome after laparoscopic rectal surgery? Multivariate
analysis of clinical, anatomic and pathologic features after
3-dimensional reconstruction of the pelvic anatomy. Ann Surg.
247:642–649. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akiyoshi T, Kuroyanagi H, Oya M, Konishi
T, Fukuda M, Fujimoto Y, Ueno M, Miyata S and Yamaguchi T: Factors
affecting the difficulty of laparoscopic total mesorectal excision
with double stapling technique anastomosis for low rectal cancer.
Surgery. 146:483–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu J, Bo XF, Xiong CY, Wu AW, Zhang XP, Li
M, An Q, Fang J, Li J, Zhang X, et al: Defining pelvic factors in
sphincter-preservation of low rectal cancer with a
three-dimensional digital model of pelvis. Dis Colon Rectum.
49:1517–1526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Law WL and Chu KW: Anterior resection for
rectal cancer with mesorectal excision: A prospective evaluation of
622 patients. Ann Surg. 240:260–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heald RJ and Karanjia ND: Results of
radical surgery for rectal cancer. World J Surg. 16:848–857. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Görög D, Tóth A, Péter A and Perner F: Is
obesity a favorable factor for resectability of rectal cancer?
Hepatogastroenterology. 51:630–633. 2004.PubMed/NCBI
|