Introduction
Malignant fibrous histiocytoma (MFH), which is also
termed undifferentiated pleomorphic sarcoma or pleomorphic spindle
cell sarcoma (PSCS), is a type of malignant sarcoma that occurs
most frequently in patients aged between 50 and 70 years (1). MFH occurs most commonly in the
extremities and the trunk, and it is extremely rare in the
retroperitoneum (1,2). The majority of retroperitoneal MFH cases
are asymptomatic. Compression of nearby organs in the abdomen may
elicit symptoms, including anorexia, abdominal discomfort, nausea
and the sensation of an abdominal mass with abdominal girth
enlargement (3). Chemotherapy is
employed for advanced disease, however, large trials have not
demonstrated a significant benefit (4,5). Recent
insights into the neo-adjuvant chemotherapy of MFH provide exciting
avenues for future research. A review of the clinical literature on
this topic supports the management that was taken in the present
case.
Case report
A 64-year-old male with an unremarkable medical
history presented with a 6-month history of abdominal fullness at
the China Medical University Hospital (Taichung, Taiwan) in April
2012. On abdominal physical examination, a painless mass was
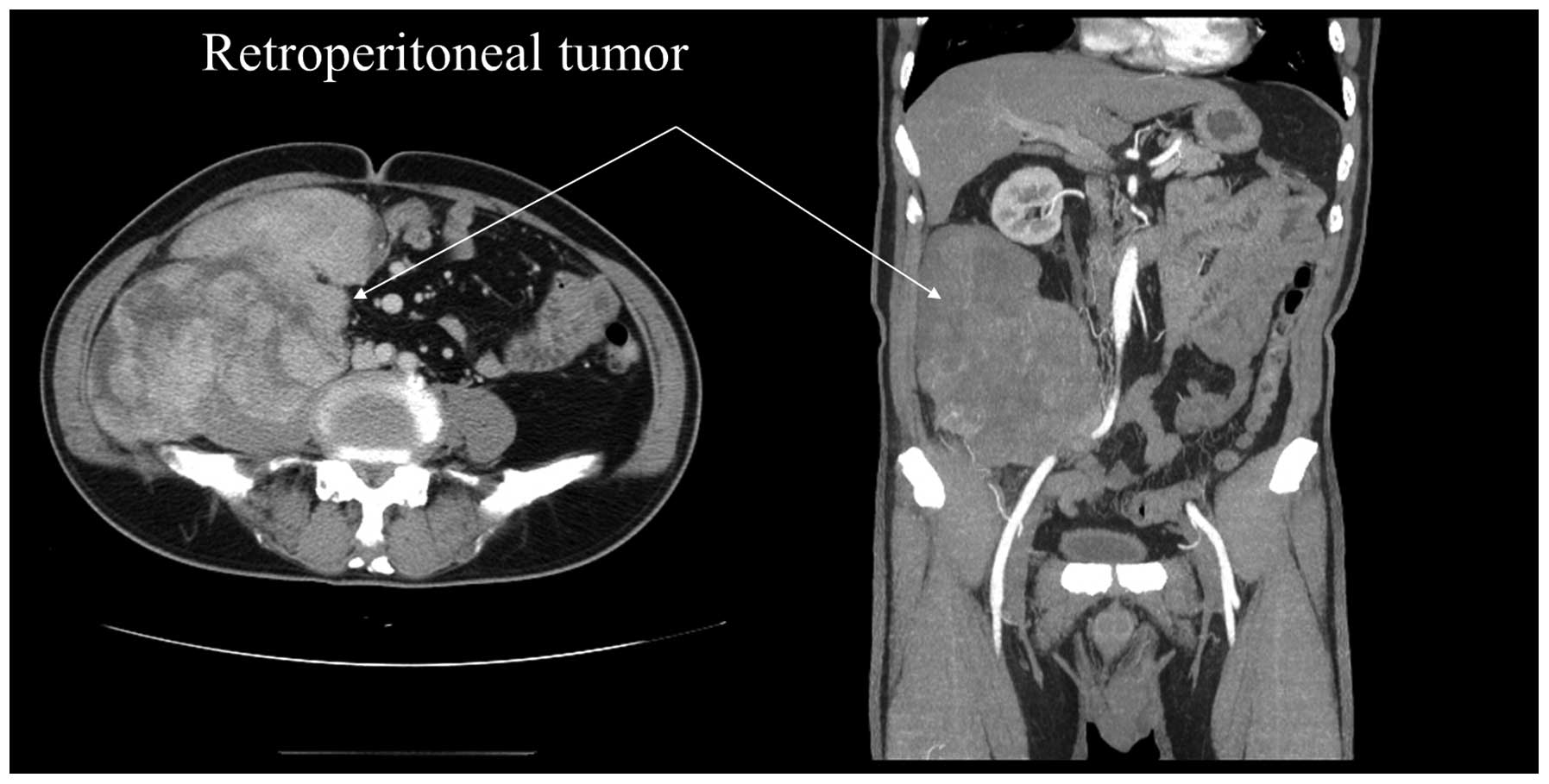
palpated in right abdomen. After thorough investigation, a tumor
measuring ~20×12×8 cm in size was identified in the
retroperitoneum. The right internal and external iliac vessels were
adhered to the tumor and medial deviation. Right mild
hydronephrosis and hydroureter was observed in computed tomography
(CT) scan due to external compression (Fig. 1).
The patient had previously sought medical opinion
and palliative treatment was suggested by the majority of those
consulted. Instead, the patient was admitted to our a tertiary
referral hospital (China Medical University Hospital, Taichung,
Taiwan) in April 2010. The patient underwent an exploratory
laparotomy for removal of the tumor in May, 2012. The tumor was not
completely removed due to a severe hemorrhage during the surgery
when the tumor surface was approached. However, excisional biopsy
of the tumor was performed and a double J stent for the right
hydronephrosis was inserted.
A diagnosis of MFH (grade 3/3 based on the
Fédération Nationale des Centres de Lutte Contre le Cancer soft
tissue tumor grading system) was confirmed by a pathologist.
Immunohistochemical analysis was performed by incubation with the
following primary antibodies: Monoclonal mouse anti-human
cytokeratin (CK; low molecular weight; AE1; dilution, 1:800;
catalog no., Z2269; Zeta Corp., Sierra Madre, CA, USA), monoclonal
mouse anti-human CK (high molecular weight; AE3; dilution, 1:800;
catalog no., Z2267; Zeta Corp.) monoclonal mouse anti-human smooth
muscle actin (dilution, 1:100; catalog no., NCL-SMA; Leica
Biosystems, Wetzlar, Germany), monoclonal mouse anti-human desmin
(dilution, 1:200; catalog no., DES-DERII-CE; Leica Biosystems),
monoclonal mouse anti-human vimentin (dilution, 1:400; catalog no.,
61–0066; Genemed Biotechnologies, Inc., San Francisco, CA, USA),
monoclonal mouse anti-human melan-A (dilution, 1:50; catalog no.,
NCL-MelanA; Leica Biosystems), monoclonal rabbit anti-human S100
(1:400; catalog no, NCL-S100p; Leica Biosystems), monoclonal mouse
anti-human cluster of differentiation (CD)68 (dilution, 1:800;
catalog no., CD68-L-CE; Leica Biosystems) and monoclonal mouse
anti-human HMB-45 (dilution, 1:100; catalog no., HMB45-L-CE; Leica
Biosystems). Diaminobenzidine (Leica Biosystems) was used as a
chromogen and Meyer's hematoxylin (Leica Biosystems) as a
counterstain. An Olympus BX-50 microscope (Olympus Corp., Tokyo,
Japan) with DP-20 digital camera (Olympus Corp.) was used to
capture images at a magnification of x100 and magnification, x400
(vimentin immunostaining). Immunohistochemistry revealed positivity
for vimentin; and negativity for CK, desmin, S-100 and CD117
(Fig. 2). Neo-adjuvant chemotherapy
was suggested for palliative treatment. The patient received
neo-adjuvant chemotherapy with 4 cycles of MAID [mesna 2,500
mg/m2/6 h (days 1–3), tetrahydropyranyl adriamycin 20
mg/m2/0. 5 h (days 1–3), ifosfamide 2,500
mg/m2/6 h (days 1–3) and dacarbazine 300
mg/m2/1 h (days 1–3)] (6)
from June to October 2012. CT scan was arranged after undergoing 2
cycles of chemotherapy. The mass was reduced in size (minor
response) with central necrosis components in comparison to the
last CT scan (Fig. 3). Six months
later, the patient underwent whole tumor resection in November
2012. The tumor was carefully removed from the right gonadal vein,
right common iliac, internal and external iliac arteries. (Fig. 4) The tumor had also invaded the right
psoas muscle and anterior longitudinal ligament of the lumbar
spine. Part of the muscle and ligament were also therefore
resected. En bloc resection was undergone successfully
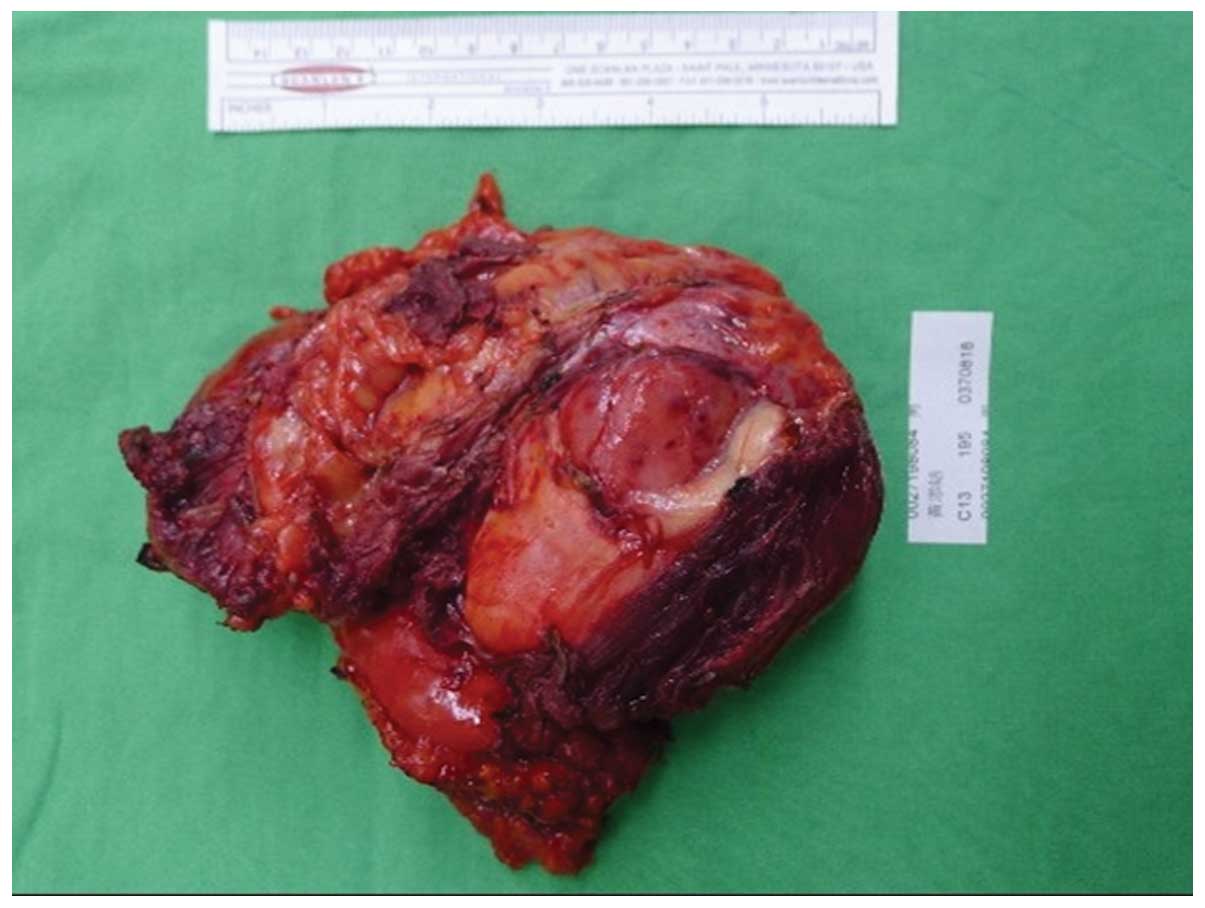
without any remaining tumor. The surgical specimen measured
13.3×8.8×5.7 cm in size and weighed 4.3 kg. (Fig. 5) The specimen had heterogeneous
yellowish content and necrotic components inside. Extensive tumor
necrosis and fibrosis were noted microscopically (Fig. 6). Complete response of chemotherapy
was observed following pathological review, where a pathological
complete response was defined as 99–100% necrosis (7–9). Pathology
composed of a mixture of spindle cells in a storiform pattern and
polygonal or rounded cells. Nuclear pleomorphism and necrosis were
present in the resected tumor. Degenerative foamy tumor cells with
intracellular brown pigment deposition were observed inside the
tumor. Tumor cells responded well to chemotherapy with extensive
tumor necrosis and fibrosis. Immunohistochemistry demonstrated that
the tumor cells were negative for cytokeratin, smooth muscle actin,
desmin, S-100, and melan-A expression, but positive with diffuse
cytoplasmic staining for vimentin, CD68, and HMB-45. The
Fontana-Masson and iron stains were negative for intracellular
pigments. The negative margin was also proven under microscopy
(Fig. 7). The patient did not receive
any further treatment and remained alive without tumor recurrence
at the final follow-up appointment in February 2015.
Discussion
MFH was first described as soft-tissue sarcomas
arising from fibroblasts and histiocytes in 1964 (5). MFH is generally divided into 5
histological types: Storiform-pleomorphic, myxoid, giant cell,
angiomatoid and inflammatory subtypes (10). MFH presents with varied histology
morphology, but the classic form is composed of spindle-shaped and
round histiocytes arranged in storiform pattern as in the present
case.
The primary treatment of retroperitoneal MFH is
surgical resection (11). The main
structures in the retroperitoneum are the aorta, vena cava,
superior mesenteric vessels, celiac trunk, kidney, ureter and
duodenum (12). Damage to these
structures may cause severe post-operative complications such as
hemorrhaging and fatalities (12). If
the tumor is encased or adheres to these structures, the mortality
and morbidity rate will be high during and following tumor excision
surgery.
Although the 5-year survival rate of all MFH
patients that receive surgical resection is 67.2% (13), the 5-year survival rate of patients
with those unresectable MFH is <10% (14). To the best of our knowledge, no
studies regarding the management of a marginally resectable or
unresectable retroperitoneal MFH have been published.
Retroperitoneal MFH may be shrunk following an initial course of
chemotherapy. Multimodal therapy for extremity sarcoma has provided
an inference for the role of chemotherapy in retroperitoneal
lesions (2). There are certain
survival benefits if the tumor can be removed after chemotherapy
(15). Overall, the arguments for
neo-adjuvant therapy are reasonable.
A study reported 70% of 63 patients underwent
complete surgery after neoadjuvant chemotherapy of ifosfamide,
cisplatin, adriamycin and mitomycin. But 65% of patients had
disease relapsed and 34% of patients died of metastasis within 30
months following up. Median survival of patients was 30 months and
median relapse-free survival was 13 months (16).
A meta-analysis study reported a slight advantage of
4% overall survival for the use of adjuvant chemotherapy to
decrease the risk of death and recurrence in patients with
high-grade lesions (17,18).
In the present study, a combination regimen of MAID
was used for neoadjuvant chemotherapy. The regimen has been
successful in neo-adjuvant programs for sarcomas of the extremities
(19,20) compared with historical controls, yet
less data are available with regard to other soft tissue sites.
Bui-Nguyen et al (21)
designed a randomized control trial that patients who had sarcoma
of extremities received 4 cycles of standard dose and high dose of
MAID. Their 3-year overall survival rate was 49.4 and 32.7%; the
progression-free survival rate was 32.4 and 14.0%, respectively.
Other studies have also indicated the benefit of MAID regimen;
pre-operative MAID is effective in advanced adult soft tissue
sarcomas (22,23). Elias et al (24) demonstrated that MAID treatment is
effective for retroperitoneal sarcoma: The overall response rate
was 47% (10% complete response CR). The majority of responses
(~70%) were observed within 2 cycles, the median times to
progression was 9 months; and the median survival was 16 months
(24). Variations in chemosensitivity
between different histological types have been observed (25). For example, response rates of >50%
have been reported for synovial sarcoma (26) Similarly, myxoid liposarcomas are
considered to be significantly more responsive than the majority of
soft tissue sarcoma, although the evidence remains controversial
(27,28) Limited data was reported for MFH type
in retroperitoneal tumors. Therefore, further prospective
randomized trials on special types of soft tissue sarcomas and on
subtypes of MFH are needed in order to make a more conclusive
evaluation.
In conclusion, palliative treatment is not the only
option for a borderline resectable or unresectable MFH in
retroperitoneum. The age and any comorbidity of the patient
together with the histology of the tumor need to be taken into
account. If the tumor is chemosensitive and adjacent to critical
organs, chemotherapy may render the tumor suitable for radical
surgery. However, patients with MFH exceeding 5 cm are at a
significant risk of developing metastases. The present study
provides evidence that neo-adjuvant chemotherapy for those high
grading or unresectable retroperitoneal tumor may lead to the
possibility to perform complete resection or an improvement in
prognostic outcome.
References
|
1
|
Weiss SW and Goldblum JR: Malignant
fibrous histiocytoma (pleomorphic undifferentiated sarcoma).
Enzinger and Weiss's Soft Tissue Tumors (5th). (Mosby, London).
1161–1182. 2008.
|
|
2
|
Marchese R, Bufo P, Carrieri G and Bove G:
Malignant fibrous histiocytoma of the kidney treated with
nephrectomy and adjuvant radiotherapy: A case report. Case Rep Med
2010. 802026:pii. 2010.
|
|
3
|
Bhavsar T, Saeed-Vafa D, Harbison S and
Inniss S: Retroperitoneal cystic lymphangioma in an adult: A case
report and review of the literature. World J Gastrointest
Pathophysiol. 1:171–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tarján M, Cserni G and Szabó Z: Malignant
fibrous histiocytoma of the kidney. Scand J Urol Nephrol.
35:518–520. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Brien JE and Stout AP: Malignant fibrous
xanthomas Cancer. 17:1445–1455. 1964.PubMed/NCBI
|
|
6
|
Anagnostopoulos G, Sakorafas GH,
Grigoriadis K and Kostopoulos P: Malignant fibrous histiocytoma of
the liver: A case report and review of the literature. Mt Sinai J
Med. 72:50–52. 2005.PubMed/NCBI
|
|
7
|
Eilber FC, Rosen G, Eckardt J, Forscher C,
Nelson SD, Selch M, Dorey F and Eilber FR: Treatment-induced
pathologic necrosis: A predictor of local recurrence and survival
in patients receiving neoadjuvant therapy for high-grade extremity
soft tissue sarcomas. J Clin Oncol. 19:3203–3209. 2001.PubMed/NCBI
|
|
8
|
Canter RJ, Martinez SR, Tamurian RM,
Wilton M, Li CS, Ryu J, Mak W, Monsky WL and Borys D: Radiographic
and histologic response to neoadjuvant radiotherapy in patients
with soft tissue sarcoma. Ann Surg Oncol. 17:2578–2584. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shah D, Borys D, Martinez SR, Li CS,
Tamurian RM, Bold RJ, Monjazeb A and Canter R: Complete pathologic
response to neoadjuvant radiotherapy is predictive of oncological
outcome in patients with soft tissue sarcoma. Anticancer Res.
32:3911–3915. 2012.PubMed/NCBI
|
|
10
|
Hassan I, Park SZ, Donohue JH, Nagorney
DM, Kay PA, Nasciemento AG, Schleck CD and Ilstrup DM: Operative
management of primary retroperitoneal sarcomas: A reappraisal of an
institutional experience. Ann Surg. 239:244–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tseng WW, Wang SC, Eichler CM, Warren RS
and Nakakura EK: Complete and safe resection of challenging
retroperitoneal tumors: Anticipation of multi-organ and major
vascular resection and use of adjunct procedures. World J Surg
Oncol. 9:1432011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pezzi CM, Rawlings MS Jr, Esgro JJ,
Pollock RE and Romsdahl MM: Prognostic factors in 277 patients with
malignant fibrous histiocytoma. Cancer. 69:2098–2103. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogura K, Goto T, Imanishi J, Shinoda Y,
Okuma T, Tsuda Y, Kobayashi H, Akiyama T, Hirata M, Yamamoto A and
Kawano H: Neoadjuvant and adjuvant chemotherapy with modified
mesna, adriamycin, ifosfamide, and dacarbazine (MAID) regimen for
adult high-grade non-small round cell soft tissue sarcomas. Int J
Clin Oncol. 18:170–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sherman KL, Wayne JD, Chung J, Agulnik M,
Attar S, Hayes JP, Laskin WB, Peabody TD, Bentrem DJ, Pollock RE
and Bilimoria KY: Assessment of multimodality therapy use for
extremity sarcoma in the United States. J Surg Oncol. 109:395–404.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wendtner CM, Abdel-Rahman S, Krych M,
Baumert J, Lindner LH, Baur A, Hiddeman W and Issels RD: Response
to neoadjuvant chemotherapy combined with regional hyperthermia
predicts long-term survival for adult patients with retroperitoneal
and visceral high-risk soft tissue sarcomas. J Clinical Oncol.
20:3156–3164. 2002. View Article : Google Scholar
|
|
16
|
Mohagheghi MA, Sadighi S, Raafat J,
Mosavi-Jarrahi AR, Seddigh Z and Ghaemi A: Neoadjuvant chemotherapy
with ifosfamide, cisplatin, adriamycin and mitomycin (IMAP) for
high risk adult soft tissue sarcomas. Acta Med Iran. 47:133–138.
2009.
|
|
17
|
Gilbeau L, Kantor G, Stoeckle E, Lagarde
P, Thomas L, Kind M, Richaud P, Coindre JM, Bonichon F and Bui BN:
Surgical resection and radiotherapy for primary retroperitoneal
soft tissue sarcoma. Radiother Oncol. 65:137–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pervaiz N, Colterjohn N, Farrokhyar F,
Tozer R, Figueredo A and Ghert M: A systematic meta-analysis of
randomized controlled trials of adjuvant chemotherapy for localized
resectable soft-tissue sarcoma. Cancer. 113:573–581. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mullen JT, Kobayashi W, Wang JJ, Harmon
DC, Choy E, Hornicek FJ, Rosenberg AE, Chen YL, Spiro IJ and
DeLaney TF: Long-term follow-up of patients treated with
neoadjuvant chemotherapy and radiotherapy for large, extremity soft
tissue sarcomas. Cancer. 118:3758–3765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Glabbeke M, van Oosterom AT,
Oosterhuis JW, Mouridsen H, Crowther D, Somers R, Verweij J,
Santoro A, Buesa J and Tursz T: Prognostic factors for the outcome
of chemotherapy in advanced soft tissue sarcoma: An analysis of
2,185 patients treated with anthracycline-containing first-line
regimens-a european organization for research and treatment of
cancer soft tissue and bone sarcoma group study. J Clin Oncol.
17:150–157. 1999.PubMed/NCBI
|
|
21
|
Bui-Nguyen B, Ray-Coquard I, Chevreau C,
Penel N, Bay JO, Coindre JM, Cupissol D, Italiano A, Bonichon F,
Lotz JP, et al: High-dose chemotherapy consolidation for
chemosensitive advanced soft tissue sarcoma patients: An
open-label, randomized controlled trial. Ann Oncol. 23:777–784.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fayette J, Penel N, Chevreau C, Blay JY,
Cupissol D, Thyss A, Guillemet C, Rios M, Rolland F, Fargeot P, et
al: Phase III trial of standard versus dose-intensified
doxorubicin, ifosfamide and dacarbazine (MAID) in the first-line
treatment of metastatic and locally advanced soft tissue sarcoma.
Invest New Drugs. 27:482–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kasper B, Ouali M, van Glabbeke M, Blay
JY, Bramwell VH, Woll PJ, Hohenberger P and Schöffski P: Prognostic
factors in adolescents and young adults (AYA) with high risk soft
tissue sarcoma (STS) treated by adjuvant chemotherapy: A study
based on pooled European organisation for research and treatment of
cancer (EORTC) clinical trials 62771 and 62931. Eur J Cancer.
49:449–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elias A, Ryan L, Sulkes A, Collins J,
Aisner J and Antman KH: Response to mesna, doxorubicin, ifosfamide,
and dacarbazine in 108 patients with metastatic or unresectable
sarcoma and no prior chemotherapy. J Clin Oncol. 7:1208–1216.
1989.PubMed/NCBI
|
|
25
|
Salgado Rand and vanMarck E: Soft tissue
tumours: The surgical pathologist's perspective. Imaging of Soft
Tissue Tumors. De Schepper AM, Vanhoemacker F, Gielen J and Parizel
PM: (3rd). Springer. (Berlin, Germany). 107–116. 2006. View Article : Google Scholar
|
|
26
|
Spurrell EL, Fisher C, Thomas JM and
Judson IR: Prognostic factors in advanced synovial sarcoma: An
analysis of 104 patients treated at the Royal Marsden Hospital. Ann
Oncol. 16:437–444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karavasilis V, Seddon BM, Ashley S,
Al-Muderis O, Fisher C and Judson I: Significant clinical benefit
of first-line palliative chemotherapy in advanced soft-tissue
sarcoma: Retrospective analysis and identification of prognostic
factors in 488 patients. Cancer. 112:1585–1591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jones RL, Fisher C, Al-Muderis O and
Judson IR: Differential sensitivity of liposarcoma subtypes to
chemotherapy. Eur J Cancer. 41:2853–2860. 2005. View Article : Google Scholar : PubMed/NCBI
|