Introduction
Human lung cancer is one of the most commonly
observed aggressive malignancies, with symptoms including coughing,
weight loss, shortness of breath and chest pains (1). In 2012, 1.8 million cases of lung cancer
occurred worldwide and the disease was responsible for ~1,590,000
mortalities (2), making lung cancer.
A large number of patients with lung cancer are diagnosed at an
advanced stage (2). Despite great
improvement in traditional treatments, including surgery,
supplemented with radiotherapy and chemotherapy, the prognosis of
these patients remains poor, with a low rate of 5-year survival
(10–15%) (3). Therefore, the
identification of potential molecular markers of lung cancer is
required for the prediction of survival and the development of
novel therapeutic targets.
Basic helix-loop-helix (bHLH) transcription factors
are a family of transcriptional regulators that have significant
roles in cell proliferation and differentiation, expression of
intracellular genetic information and cell lineage determination,
as well as other essential processes (4–7).
Activating enhancer-binding protein (AP)-4 is a member of the bHLH
leucine-zipper subgroup of bHLH proteins (8) and is involved in tumor biology (9–11). AP-4 is
upregulated in gastric and hepatocellular carcinoma, and predicts
poor prognosis (9,10). Activation of AP-4 induces
epithelial-mesenchymal transition and enhances migration and
invasion of colorectal cancer cells (11). AP-4 small interfering RNA
(siRNA)-mediated gene silencing has an anticancer role in gastric
carcinoma (12). However, the role of
AP-4 in human lung cancer remains to be elucidated. Increased AP-4
in non-small cell lung cancer tissues compared with their normal
counterparts (13) indicates that
AP-4 may be a potential therapeutic target for the treatment of
human lung cancer.
RNA interference is a process of sequence-specific
post-transcriptional gene silencing mediated by double-stranded
RNA, which is able to silence specific genes and provide a powerful
approach for the analysis of gene functions in vitro and
in vivo (14). At present, the
most commonly utilized nucleic-acid-based sequence-specific gene
silencing molecules are siRNAs, which consist of symmetrical
duplexes of 19–21 base pairs (15).
The siRNA method is able to inhibit target gene expression with
specificity, potency and endurance, and has broad therapeutic
potential for the treatment of various human diseases, including
infections and cancer (16).
In the present study, to investigate the role of
AP-4 in human lung cancer, the expression of AP-4 was measured in
human lung cancer tissues and cells, and the function of AP-4 was
investigated in human lung cancer cells by transfection with
siRNAs. It was observed that the level of AP-4 was increased in
tumor tissues and cells compared with their normal counterparts,
and the silencing of AP-4 mediated by siRNA inhibited cell
proliferation, induced cell cycle arrest and promoted apoptosis by
modulating the expression of p21 and cyclin D1 in lung cancer
cells. The results of the present study imply that AP-4 may be a
potential therapeutic target for the treatment of human lung
cancer.
Materials and methods
Patients and tissue samples
Human lung cancer tissue samples and matched
adjacent noncancerous lung tissue samples were obtained from 52
patients who underwent resection at the First Affiliated Hospital
and Cancer Hospital Affiliated to Zhengzhou University (Zhengzhou,
China) between March 2012 and September 2013. All patients had
received a definite diagnosis by a clinical pathologist prior to
surgery. No patients had received chemotherapy or radiotherapy
prior to surgery. Each patient gave signed informed consent for the
use of their tissues for research purposes. The study protocol was
approved by the Institutional Ethics Committee of Zhengzhou
University. The clinicopathological characteristics of the 52
patients are summarized in Table I.
All lung cancer tissue specimens and adjacent noncancerous lung
tissues were immediately frozen in liquid nitrogen and stored at
−80°C until required.
 | Table I.Expression of activating
enhancer-binding protein-4 messenger RNA in lung cancer cases. |
Table I.
Expression of activating
enhancer-binding protein-4 messenger RNA in lung cancer cases.
|
|
| Tumor | Non-tumor |
|---|
|
|
|
|
|
|---|
| Parameter | n | Expression level | P-value | Expression level | P-value |
|---|
| Gender |
|
| 0.343 |
| 0.499 |
|
Male | 31 | 1.147±0.3486 |
| 0.250±0.0913 |
|
|
Female | 21 | 1.248±0.4115 |
| 0.265±0.0634 |
|
| Age, years |
|
| 0.615 |
| 0.204 |
|
<60 | 18 | 1.224±0.3393 |
| 0.276±0.0661 |
|
|
>60 | 34 | 1.168±0.3957 |
| 0.245±0.0867 |
|
| Tumor size, cm |
|
| 0.002a |
| 0.850 |
|
<3 | 27 | 1.340±0.4117 |
| 0.258±0.0760 |
|
| ≥3 | 25 | 1.023±0.2460 |
| 0.254±0.0873 |
|
|
Differentiation |
|
| 0.828 |
| 0.747 |
|
Well | 22 | 1.178±0.3472 |
| 0.246±0.0791 |
|
|
Moderate | 22 | 1.220±0.4231 |
| 0.263±0.0860 |
|
|
Poor | 8 | 1.126±0.3413 |
| 0.263±0.0779 |
|
| Histology |
|
| 0.240 |
| 0.289 |
|
Squamous carcinoma | 21 | 1.123±0.3381 |
| 0.239±0.0581 |
|
|
Adenocarcinoma | 28 | 1.261±0.3960 |
| 0.262±0.0909 |
|
|
Other | 3 | 0.954±0.3434 |
| 0.312±0.1142 |
|
|
Tumor-Node-Metastasis stage |
|
|
<0.001a |
| 0.807 |
| I | 19 | 1.421±0.4413 |
| 0.260±0.0835 |
|
| II
& III | 33 | 1.053±0.2515 |
| 0.254±0.0805 |
|
| Lymph node
status |
|
|
<0.001a |
| 0.833 |
|
Negative | 24 | 1.417±0.3857 |
| 0.253±0.0817 |
|
|
Positive | 28 | 0.991±0.2275 |
| 0.258±0.0815 |
|
Cell culture and transfection
The lung cancer cell lines, A549 and H1299 (American
Type Culture Collection, Manassas, VA, USA), were cultured in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
medium supplemented with 10% fetal bovine serum (FBS) (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml streptomycin and
penicillin (Ameresco, Inc., Framingham, MA, USA) at 37°C in a
humidified incubator with an atmosphere containing 5%
CO2. The human bronchial epithelial (HBE) cell line was
obtained from Xiangfu Bio (Shanghai, China), and originated from
the American Type Culture Collection. HBE cells were grown in
Dulbecco's modified Eagle's medium supplemented with 10% FBS;
Gibco; Thermo Fisher Scientific, Inc.), 2 µM glutamine (Beijing
Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China), 100
IU/ml penicillin, and 100 µg/ml streptomycin (Ameresco, Inc.) at
37°C in a humidified incubator with an atmosphere containing 5%
CO2. The siRNA sequences used for silencing of human
AP-4 (NM_003223) were designed by GenScript siRNA Target Finder
online software 2015 (GenScript, Piscataway, NJ, USA) and
synthesized by GenScript. The nucleotide sequences were as follows:
Forward, 5′-CGGGAUUCCAGUCCCUCAATT-3′ and reverse,
5′-UUGAGGGACUGGAAUCCCGCG-3′. The negative control siRNA (scrambled
siRNAs) was supplied by GenScript and the sequences were as
follows: Forward, 5′-CAGUCGUGCGCACUCACUATT-3′ and reverse,
5′-UAGUGAGUGCGCACGACUGCG-3′. The siRNAs were transfected into the
cultured cells using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from lung cancer tissues and
matched adjacent noncancerous lung tissues using RNAiso Reagent
(Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocol. RNA (500 ng) was reverse-transcribed to complementary DNA
using a PrimeScript RT Reagent kit (Takara Bio, Inc.) according to
the manufacturer's protocol. The RT reaction mixture was prepared
on ice, and contained 2 µl 5X PrimeScript Buffer, 0.5 µl 1X
PrimeScript RT Enzyme Mix I, 0.5 µl Oligo dT Primer (50 µM) and 0.5
µl Random 6 mers (100 µM; Takara Bio, Inc.). After dispensing
aliquots of the mix into microtubes (Corning Incorporated, Corning,
NY, USA), 500 ng RNA sample and RNase Free dH2O was
added to total 10 µl. The reaction mixture was subsequently
incubated at 37°C for 5 min, followed by 85°C for 5 sec and 4°C for
a minimum of 10 min. No reverse transcriptase was used as a
negative control. Expression of AP-4 messenger RNA (mRNA) was
evaluated by RT-qPCR on a 7500 Fast Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with Premix Ex
Taq (Probe qPCR) Master Mix and a ROX reference DyeII (50x)
(Takara Bio, Inc.). The level of expression was calculated based on
the PCR cycle number, and the relative gene expression level was
determined using the 2−ΔΔCq method as previously
described (17). The ratios of AP-4,
p21 and cyclin D1 to glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) were used to normalize the relative levels of AP-4
expression. The primers and probes used were as follows: AP-4
forward primer, 5′-GCCGATCGCTATGGAGTATT-3′, AP-4 reverse primer,
5′-TTAGTGGAATGTTGGCAAGG-3′ and AP-4 probe, 5′-6-carboxyfluorescein
(FAM)-TGCCCACTCAGAAGGTGCCC-tetramethylrhodamine (TAMRA) 3′; GAPDH
forward primer, 5′-CATCAATGGAAATCCCATCA-3′, GAPDH reverse primer,
5′-TTCTCCATGGTGGTGAAGAC-3′ and GAPDH probe,
5′-FAM-TACTCAGCGCCAGCATCGCC-TAMRA 3′.
Western blot analysis
Cells were lysed with a Nuclear and Cytoplasmic
Protein Extraction kit (Beyotime Institute of Biotechnology,
Haimen, China). Protein (100 µg) was subjected to 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
onto polyvinylidene difluoride membranes (Merck Millipore,
Darmstadt, Germany). The membranes were blocked for 1 h in Tris
buffered saline with Tween-20 (TBST; Beyotime Institute of
Biotechnology) containing 5% non-fat dried milk (Nestlé, Beijing,
China) at room temperature, and subsequently incubated with
polyclonal rabbit primary antibodies against AP-4 (dilution,
1:1,000; #sc-292568; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), p21 (dilution, 1:1,000; #sc-756; Santa Cruz Biotechnology,
Inc.), cyclin D1 (dilution, 1:1,000; #sc-718; Santa Cruz
Biotechnology, Inc.) and GAPDH (dilution, 1:1,000; #sc-25778; Santa
Cruz Biotechnology, Inc.) overnight at 4°C. Following washing three
times with TBST, the membranes were incubated with
horseradish-peroxidase-conjugated goat secondary antibody
(dilution, 1:3,000; #sc-2004; Santa Cruz Biotechnology, Inc.) for 2
h at room temperature. Protein bands were detected using enhanced
chemiluminescence reagents (GE Healthcare Life Science, Chalfont,
UK), and quantified using Adobe Photoshop CS4 software (Adobe
Systems, Inc., San Jose, CA, USA).
Cell proliferation assay
Cell proliferation following transfection with siRNA
was assessed by Cell Counting Kit-8 (CCK-8) (Beyotime Institute of
Biotechnology). A549 and H1299 cells were seeded into 96-well
plates (Corning Incorporated) at 3×103 cells/well. The
cells were cultured at 37°C for 0, 24, 48 and 72 h. CCK-8 reagent
(10 µl) was added to each well. Following incubation for 4 h at
37°C, the absorbance was measured at 450 nm with a multi-detection
microplate reader (Synergy HT; Bio-Tek Instruments, Inc., Winooski,
VT, USA).
Apoptosis assay
Cells were collected in tubes 48 h subsequent to
transfection with siRNA, washed once using cold phosphate-buffered
saline (PBS; Beijing Dingguo Changsheng Biotechnology Co., Ltd.),
suspended in binding buffer (eBioscience, Inc., San Diego, CA, USA)
and stained with Annexin V-fluorescein isothiocyanate (eBioscience,
Inc.) in the dark at room temperature for 15 min. In total, 10 µl
propidium iodide (PI; 25 µg/ml; eBioscience, Inc.) was added to
each tube and cells were incubated at room temperature for 10 min.
Within 1 h of staining, the cells underwent flow cytometry, which
was performed a FACSCantoII flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA), and cells were analyzed using BD FACSDiva
Software 6.0 (BD Biosciences). Viable cells were Annexin
V−/PI−, early apoptotic cells were
Annexin+/PI− and late apoptotic/dead cells
were Annexin+/PI+. Nonviable cells that had
undergone necrosis were Annexin−/PI+.
Cell cycle assay
Cells were harvested 48 h subsequent to transfection
with siRNA, fixed in 70% ice-cold ethanol (Beijing Dingguo
Changsheng Biotechnology Co., Ltd.) overnight, washed with 1X PBS
and stained by PI (50 µg/ml) with RNase (50 µg/ml) in the dark at
room temperature for 30 min. Cell cycle stage analysis was
performed by flow cytometry (FACSCantoII) using ModFit software
version 3.0 (Verity Software House, Topsham, ME, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Means were compared two groups by Student's t-test,
while means of three or more groups were compared with one-way
analysis of variance using SPSS for Windows version 17.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
AP-4 expression is significantly
increased in lung cancer tissue
Expression of AP-4 in 52 human lung cancer tissue
samples and matched adjacent noncancerous lung tissue samples was
assessed using RT-qPCR. The level of AP-4 mRNA in lung cancer
tissues was three times higher compared with that in the
noncancerous tissue samples (P=0.00005; Fig. 1A). The association between AP-4 mRNA
expression and clinicopathological characteristics of the lung
cancer patients was additionally analyzed (Table I). The levels of AP-4 mRNA were
significantly associated with tumor size (P=0.002), lymph node
status (P<0.001) and TNM stage (P=0.0006), but not with sex
(P=0.343), age (P=0.615), differentiation degree (P=0.828) or
histology (P=0.240) in tumor tissue samples. In addition, western
blotting indicated that the levels of AP-4 protein in A549 and
H1299 human lung cancer cells were significantly increased compared
with those in human bronchial epithelial (HBE) cells (P=0.006;
Fig. 1B).
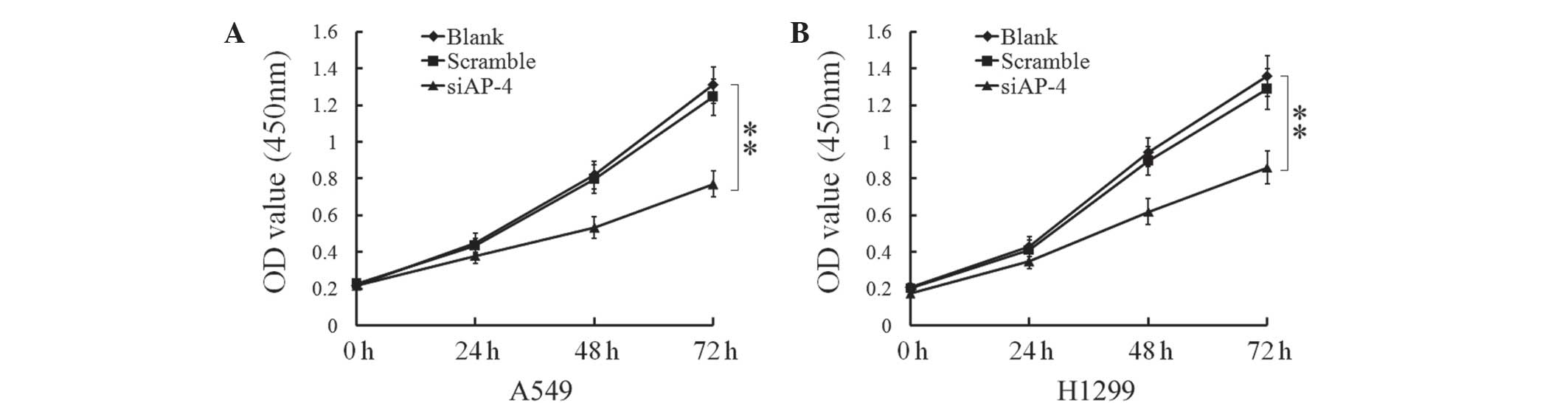
Silencing of AP-4 inhibits the
proliferation of human lung cancer cells
In order to investigate the involvement of AP-4 in
the proliferation of human lung cancer cells, A549 and H1299 cells
were transfected with scrambled siRNA or AP-4-specific siRNA, and
cell proliferation was analyzed using CCK-8. The proliferation of
A549 and H1299 cells was decreased following transfection with
AP-4-specific transfection for 24 h (P=0.0276, P=0.0322,
respectively). Following 72 h of transfection, the proliferation of
the two cell lines was significantly decreased (A549, P=0.0017;
H1299, P=0.0035) (Fig. 2). These
results demonstrated that silencing of AP-4 expression was able to
inhibit the proliferation of human lung cancer cells.
Silencing of AP-4-induced cell cycle
arrest at G0/G1 phase
AP-4 silencing inhibited the proliferation of lung
cancer cells; therefore, the present study aimed to establish
whether this effect was caused by cell cycle arrest. Following
transfection with scrambled siRNA or AP-4-specific siRNA for 48 h,
the cell cycles of A549 and H1299 cells were analyzed using flow
cytometry. There was an increased proportion of AP-4
siRNA-transfected cells in the G0/G1 phase (A549, P=0.0083; H1299,
P=0.012) and a reduced proportion of these cells in the S phase
compared with the G0/G1 and S phase proportions observed in
untransfected and scrambled siRNA-transfected cells (Fig. 3A and B). Therefore, silencing of AP-4
may be capable of inducing cell cycle arrest at the G0/G1
phase.
Silencing of AP-4 promotes apoptosis
in human lung cancer cells
The present study measured the effects of AP-4
silencing on the apoptosis of A549 and H1299 cells. As demonstrated
in Fig. 4, the percentages of
apoptotic cells in non-transfected or scramble siRNA-transfected
A549 and H1299 cells were 4.5% and 2.8%, respectively. Following
transfection with AP-4-specific siRNA, the percentages of apoptotic
A549 and H1299 cells significantly increased to 15% (P=0.005) and
10% (P=0.006), respectively. These findings indicated that
silencing of AP-4 may promote the apoptosis of lung cancer
cells.
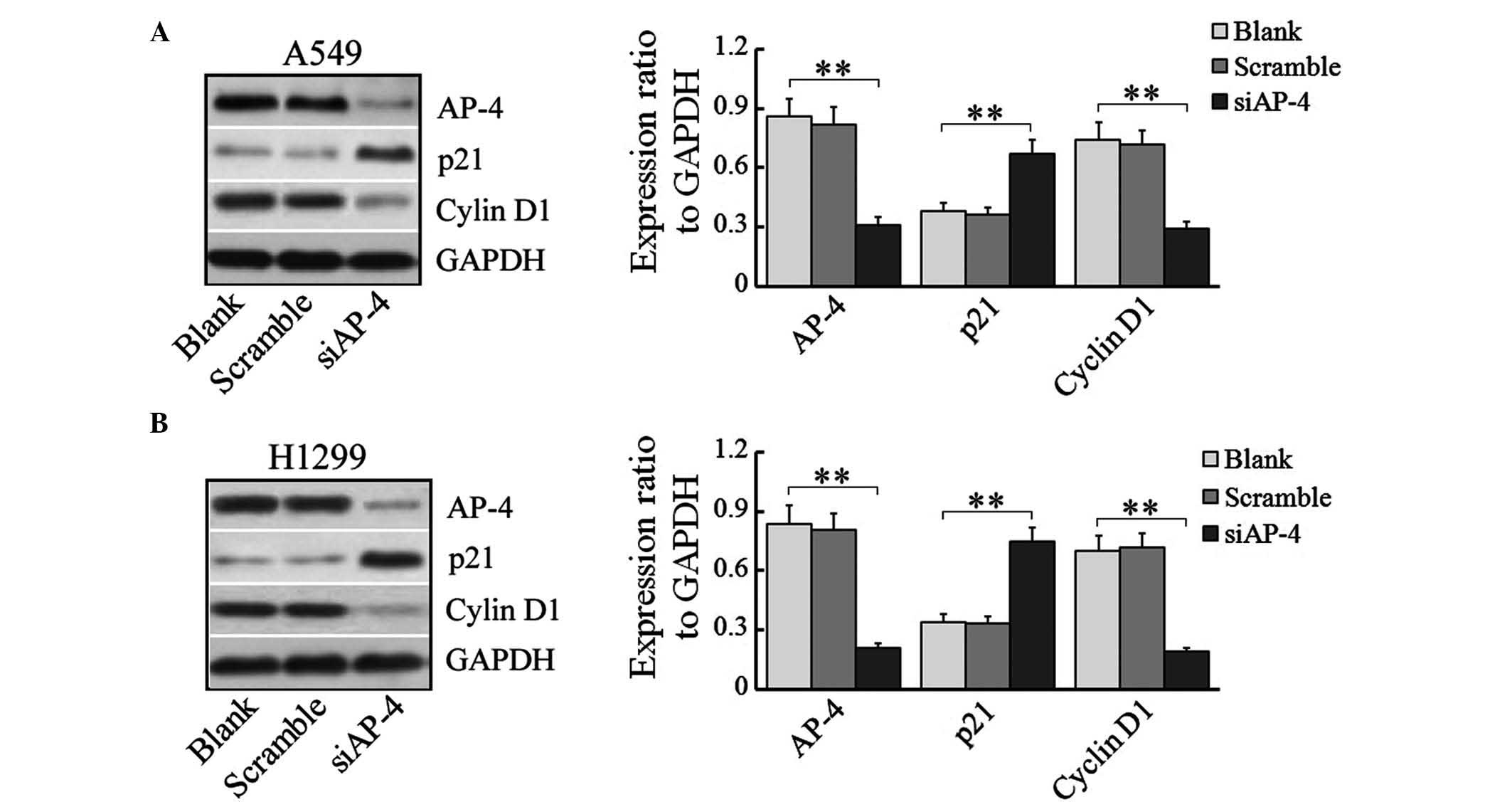
AP-4 silencing alters the expression
of p21 and cyclin D1
In order to investigate the mechanisms underlying
the effect of AP-4 silencing on the cell cycle of human lung cancer
cells, the expression of certain cell-cycle-associated regulators,
cyclin-dependent kinase inhibitor p21 and cyclin D1, were examined
using western blotting in siRNA-transfected A549 and H1299 cells.
Transfection with AP-4-specific siRNA in A459 and H1299 cells
reduced the expression of AP-4 (P=0.0009 and P=0.0002,
respectively) and cyclin D1 protein (P=0.0023 and P=0.0007,
respectively), but increased the levels of p21 expression (P=0.0062
and P=0.0039, respectively) (Fig. 5A and
B). This suggested that AP-4 silencing may be capable of
suppressing the proliferation of human lung cancer cells and
inducing cell cycle arrest at the G0/G1 phase by modulating the
expression of certain cell cycle regulators.
Discussion
Despite numerous advances in diagnosis and therapy
for lung cancer, it still possesses one of the highest mortality
rates worldwide, and has a poor patient prognosis and quality of
life (1). Given the frequent failure
of conventional treatment strategies, a number of cancer-associated
molecules have been investigated with the aim of developing novel
anticancer therapies (18).
A number of studies have demonstrated that
transcription factor AP-4 is upregulated in several human cancers,
including gastric, hepatocellular, colorectal and breast cancer,
and that it may be associated with prognosis (9,10,19,20). These
observations suggest that AP-4 may have a significant role in the
tumorigenesis or progression of a number of types of cancer
(11,12,21,22).
In the present study, AP-4 expression was
significantly increased in lung cancer tissue compared with normal
tissue, as described previously (13). However, AP-4 mRNA levels were
negatively correlated with tumor size (P=0.002), lymph node status
(P<0.001) and Tumor-Node-Metastasis stage (23) (P<0.001), which contrasts with the
results of a number of previous studies (9,10,13). These differences may have been due to
the use of RT-qPCR in the present study, compared with the use of
immunohistochemical staining in these previous studies (9,10,13).
Transcription factors form a transcription
initiation complex with RNA polymerase II, which participates in
transcription initiation in order to regulate gene expression
(24). As an ubiquitously-expressed
transcription factor, AP-4 was initially identified to activate
viral late gene transcription by binding the simian virus 40
enhancer (25). AP-4 is a direct
target gene of c-Myc, which has crucial involvement in cell
proliferation, cell cycle progression, apoptosis and
differentiation (26). In addition,
AP-4 binds the recognition motifs located in the vicinity of the
p21 promoter and is able to mediate the transcriptional repression
of p21, which is a key cell cycle inhibitor (27). Ge et al (22) reported that c-Myc activated by the Wnt
signaling pathway may promote hepatocellular carcinoma progression,
through a direct inhibitory effect of AP-4 on p21. The present
study identified that silencing of AP-4 was able to inhibit the
proliferation of human lung cancer cells and induce cell cycle
arrest at the G0/G1 phase. In order to investigate the potential
mechanisms underlying the effect of AP-4 silencing on human lung
cancer cells, several cell-cycle-associated regulators, cyclin D1
and p21, were examined. Cyclin D1 promotes progression through the
G1/S phase of the cell cycle (28),
while p21 has been hypothesized to be a negative regulator of the
cell cycle and proliferation (29).
In the present study, the expression of cyclin D1 and AP-4 was
reduced following transfection with siRNA, but levels of p21 were
upregulated. These results implied that AP-4 silencing suppressed
the proliferation of human lung cancer cells and induced cell cycle
arrest at the G0/G1 phase by modulating the expression of certain
cellular regulators. Furthermore, the present study identified that
silencing of AP-4 expression promoted apoptosis. AP-4 silencing is
able to increase the levels of caspase-9 and downregulates
expression of B-cell lymphoma (Bcl)-2 and Bcl-extra large
expression in human gastric cancer cells (12), suggesting that knockdown of AP-4 may
be able to activate mitochondrial (intrinsic) and death receptor
(extrinsic) signaling pathways of apoptosis in cancer cells
(30,31).
In conclusion, the present study demonstrated that
expression of transcription factor AP-4 was significantly increased
in lung cancer tissues and cells, and siRNA-mediated silencing of
AP-4 inhibited cell proliferation, induced cell cycle arrest and
promoted apoptosis of human lung cancer cells, as well as causing
decreased expression of cyclin D1 and increased expression of p21.
The results of the present study suggest that AP-4 may be an
oncoprotein that has a significant role in lung cancer. Although
additional investigation is required in order to investigate the
precise mechanisms underlying this role, AP-4-specific-siRNAs may
be worth investigating as a novel therapeutic agent for the
treatment of human lung cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China, Beijing, China (grant no.
81272188).
References
|
1
|
Chen YT, Feng B and Chen LB: Update of
research on drug resistance in small cell lung cancer chemotherapy.
Asian Pac J Cancer Prev. 13:3577–3581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Sánchez Cos J, Sojo González MA,
Montero MV, Pérez Calvo MC, Vicente MJ and Valle MH: Non-small cell
lung cancer and silent brain metastasis. Survival and prognostic
factors. Lung Cancer. 63:140–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jones S: An overview of the basic
helix-loop-helix proteins. Genome Biol. 5:2262004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grau R, Punzón C, Fresno M and Iñiguez MA:
Peroxisome-proliferator-activated receptor alpha agonists inhibit
cyclo-oxygenase 2 and vascular endothelial growth factor
transcriptional activation in human colorectal carcinoma cells via
inhibition of activator protein-1. Biochem J. 395:81–88. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakar Y, Duca FA, Langelier B, Devime F,
Blottiere H, Delorme C, Renault P and Covasa M: Impact of high-fat
feeding on basic helix-loop-helix transcription factors controlling
enteroendocrine cell differentiation. Int J Obes (Lond).
38:1440–1448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wushou A, Hou J, Zhao YJ and Shao ZM:
Twist-1 up-regulation in carcinoma correlates to poor survival. Int
J Mol Sci. 15:21621–21630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SU, Song HO, Lee W, Singaravelu G, Yu
JR and Park WY: Identification and characterization of a putative
basic helix-loop-helix (bHLH) transcription factor interacting with
calcineurin in C. elegans. Mol Cells. 28:455–461. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xinghua L, Bo Z, Yan G, Lei W, Changyao W,
Qi L, Lin Y, Kaixiong T, Guobin W and Jianying C: The
overexpression of AP-4 as a prognostic indicator for gastric
carcinoma. Med Oncol. 29:871–877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu BS, Zhao G, Yu HF, Chen K, Dong JH and
Tan JW: High expression of AP-4 predicts poor prognosis for
hepatocellular carcinoma after curative hepatectomy. Tumour Biol.
34:271–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jackstadt R, Röh S, Neumann J, Jung P,
Hoffmann R, Horst D, Berens C, Bornkamm GW, Kirchner T, Menssen A
and Hermeking H: AP4 is a mediator of epithelial-mesenchymal
transition and metastasis in colorectal cancer. J Exp Med.
210:1331–1350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Zhang B, Guo Y, Liang Q, Wu C, Wu
L, Tao K, Wang G and Chen J: Down-regulation of AP-4 inhibits
proliferation, induces cell cycle arrest and promotes apoptosis in
human gastric cancer cells. PLoS One. 7:e370962012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gong H, Han S, Yao H, Zhao H and Wang Y:
AP-4 predicts poor prognosis in non-small cell lung cancer. Mol Med
Rep. 10:336–340. 2014.PubMed/NCBI
|
|
14
|
Sioud M: RNA interference: Mechanisms,
technical challenges, and therapeutic opportunities. Methods Mol
Biol. 1218:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karagiannis TC and El-Osta A: siRNAs:
Mechanism of RNA interference, in vivo and potential
clinical applications. Cancer Biol Ther. 3:1069–1074. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bora RS, Gupta D, Mukkur TK and Saini KS:
RNA interference therapeutics for cancer: Challenges and
opportunities (review). Mol Med Rep. 6:9–15. 2012.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao J, Tang M, Li WL, Xie J, Du H, Tang
WB, Wang H, Chen XW, Xiao H and Li Y: Upregulation of activator
protein-4 in human colorectal cancer with metastasis. Int J Surg
Pathol. 17:16–21. 2009.PubMed/NCBI
|
|
20
|
Buechler S: Low expression of a few genes
indicates good prognosis in estrogen receptor positive breast
cancer. BMC Cancer. 9:2432009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amin S, Kumar A, Nilchi L, Wright K and
Kozlowski M: Breast cancer cells proliferation is regulated by
tyrosine phosphatase SHP1 through c-jun N-terminal kinase and
cooperative induction of RFX-1 and AP-4 transcription factors. Mol
Cancer Res. 9:1112–1125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ge Z, Zhang B, Bu X, Wang Y, Xiang L and
Tan J: Molecular mechanism of activating protein-4 regulated growth
of hepatocellular carcinoma. Tumor Biol. 35:12441–12447. 2014.
View Article : Google Scholar
|
|
23
|
Chee KG, Nguyen DV, Brown M, Gandara DR,
Wun T and Lara PN: Positron emission tomography and improved
survival in patients with lung cancer: The Will Rogers phenomenon
revisited. Arch Intern Med. 168:1541–1549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nikolov DB and Burley SK: RNA polymerase
II transcription initiation: A structural view. Proc Nat Acad Sci
USA. 94:15–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mermod N, Williams TJ and Tjian R:
Enhancer binding factors AP-4 and AP-1 act in concert to activate
SV40 late transcription in vitro. Nature. 332:557–561. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung P and Hermeking H: The c-MYC-AP4-p21
cascade. Cell Cycle. 8:982–989. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jung P, Menssen A, Mayr D and Hermeking H:
AP4 encodes a c-MYC-inducible repressor of p21. Proc Natl Acad Sci
USA. 105:15046–15051. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tane S, Kubota M, Okayama H, Ikenishi A,
Yoshitome S, Iwamoto N, Satoh Y, Kusakabe A, Ogawa S, Kanai A, et
al: Repression of cyclin D1 expression is necessary for the
maintenance of cell cycle exit in adult mammalian cardiomyocytes. J
Biol Chem. 289:18033–18044. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gartel AL and Radhakrishnan SK: Lost in
transcription: p21 repression, mechanisms, and consequences. Cancer
Res. 65:3980–3985. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gupta SK, Sahoo AP, Rosh N, Gandham RK,
Saxena L, Singh AK, Harish DR and Tiwari AK: Canine parvovirus NS1
induced apoptosis involves mitochondria, accumulation of reactive
oxygen species and activation of caspases. Virus Res. 213:46–61.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Do MT, Na M, Kim HG, Khanal T, Choi JH,
Jin SW, Oh SH, Hwang IH, Chung YC, Kim HS, et al: Ilimaquinone
induces death receptor expression and sensitizes human colon cancer
cells to TRAIL-induced apoptosis through activation of ROS-ERK/p38
MAPK-CHOP signaling pathways. Food Chem Toxicol. 71:51–59. 2014.
View Article : Google Scholar : PubMed/NCBI
|