Introduction
Due to changes in diet structure and lifestyle, as
well as the increase in the aging population, colorectal cancer
(CRC) has become one of the most commonly observed malignancies
worldwide, accounting for >1.2 million new cases and >600,000
mortalities each year (1,2). Although surgical resection provides the
most positive prognosis for long-term survival, the majority of CRC
patients are not suitable for surgery, as at the time of diagnosis
they already present with metastatic disease (3,4).
Therefore, chemotherapy remains a major therapeutic approach for
the treatment of patients exhibiting advanced CRC. Despite the
progress that has been achieved in the field of chemotherapy, the
long-term prognosis of CRC remains poor due to the development of
drug resistance, severe adverse effects, metastasis and recurrence
(4–6).
It has been proposed that cancer may arise from a small population
of cells known as cancer stem cells (CSCs) (7). CSCs demonstrate stem cell properties,
including continuous self-renewal and multi-directional
differentiation, as well as natural resistance to chemo- and
radiotherapy, leading to the initiation, progression and relapse of
cancer (8). Thus, targeting CSCs may
be a promising strategy for anticancer treatment (9).
Currently, natural products have received great
interest due to their therapeutic efficacy and reduced adverse
effects compared with modern chemotherapeutics (10–12).
Hedyotis diffusa Willd. (HDW), a member of the Rubiaceae
family, is a well-known traditional Chinese medicinal herb that is
widely distributed throughout northeast Asia. As a critical
ingredient of several traditional Chinese medicine formulas, HDW
has long been utilized in China to clinically treat various
malignancies, including CRC. Previously, the authors of the present
study demonstrated that HDW demonstrates a wide range of antitumor
activities, by affecting multiple intracellular targets (13–16). To
additionally elucidate the underlying mechanism of the tumoricidal
activity of HDW, the present study isolated a stem-like side
population (SP) from CRC HT-29 cells to investigate the effect of
HDW on CSCs.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM), DMEM/F12,
fetal bovine serum (FBS), penicillin, streptomycin, 0.25%
trypsin-ethylenediaminetetraacetic acid (EDTA), 50X B27 supplement,
Pierce radioimmunoprecipitation assay Buffer, Pierce bicinchoninic
acid (BCA) Protein assay kit, SuperSignal™ West Pico
Chemiluminescent Substrate and DreamTaq Green PCR Master mix (2X)
were all purchased from Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). Epidermal growth factor (EGF) and basic fibroblast growth
factor (bFGF) were obtained from PeproTech (Rocky Hill, NJ, USA).
Hoechst 33342 and verapamil were purchased from Sigma Chemicals
(St. Louis, MO, USA). Leucine-rich repeat-containing G-protein
coupled receptor 5 (Lgr5; catalog. no. ab75732) and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH; catalog. no.
ab181602) rabbit polyclonal antibodies were purchased from Abcam
(Cambridge, UK). Horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibody (catalog. no. E030120-01) was
purchased from Earthox (Millbrae, CA, USA). RNAiso Plus reagent and
PrimeScript™ Reverse Transcription (RT) Reagent kit were purchased
from Takara Bio, Inc. (Dalian, Liaoning, China). Water-soluble
tetrazolium salts (WST)-1 assay kit and Blocking buffer were
purchased for Beyotime Institute of Biotechnology (Shanghai,
China).
Preparation of ethanol extract of HDW
(EEHDW)
EEHDW was prepared as previously described (16). Stock solutions of EEHDW were prepared
by dissolving the EEHDW powder in a concentration of 40% dimethyl
sulfoxide (DMSO; catalog. no. 67-68-5; Amresco, Solon, OH, USA) to
achieve a final concentration of 400 mg/ml. Working concentrations
of EEHDW were created by diluting the stock solution in culture
medium (DMEM for HT-29 cells or DMEM/F12 for SP cells). The final
concentration of DMSO in the culture medium was <0.5%.
HT-29 cell culture
Human CRC HT-29 cells were purchased from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China). The
cells were grown in DMEM containing 10% (v/v) FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin in a 37°C humidified
incubator with an atmosphere of 5% CO2.
Isolation and culture of SP
The SP assay is based on the efflux of Hoechst dye
from cells via the ATP-binding cassette (ABC) family of transporter
proteins expressed within the cell membrane. In the two-dimensional
flow analysis chart, the cells are located on the side of a main
cell population in a comet-like distribution; these cells are
termed the ‘side population’. The verapamil control is an ABC
transporter inhibitor. The SP from HT-29 cells was isolated and
analyzed using MoFloTM XDP cell sorter flow cytometry (Beckman
Coulter, Inc., Brea, CA, USA) as described previously (17). Briefly, the HT-29 cells were digested
with 0.25% trypsin-EDTA and re-suspended in DMEM (supplemented with
2% FBS) at a concentration of 2.5×106 cells/ml. Fresh Hoechst 33342
(10 µg/ml final concentration) was added for 30 min at 37°C in a
rotary shaker. As a control, certain cells were incubated with
Hoechst 33342 in the presence of 50 µM verapamil. At the end of
incubation, the cells were washed and re-suspended in cold
phosphate-buffered saline (PBS), then 1 mg/ml propidium iodide was
added, and the cells were kept at 4°C in the dark. Excitation of
Hoechst dye was performed with an ultraviolet laser (catalog. no.
CY-355-100; JDSU, Milpitas, CA, USA) at 355 nm, and the
fluorescence was measured using a 450±25 nm filter (Hoechst blue)
and a 620±15-nm filter (Hoechst red). Sorted SP cells were cultured
in serum-free stem cell DMEM/F12 culture medium, containing B27
supplement (1X), 20 ng/ml EGF and 20 ng/ml bFGF.
Sphere formation assay
Isolated HT-29 SP cells were seeded at a density of
2×105 cells/well into 6-well plates (NEST Biotechnology
Co., Ltd., Wuxi, Jiangsu, China) in 2 ml DMEM culture medium.
Following treatment with various concentrations of EEHDW (0, 0.5, 1
and 2 mg/ml) for 24 h at 37°C, cells were digested with 0.25%
trypsin-EDTA and seeded at a density of 1.0×103
cells/well into Costar® 6-well Ultra-Low attachment
plates (Corning, Inc., Corning, NY, USA), and cultured in DMEM/F12
serum-free stem cell culture medium. The medium was replaced every
2 days. Following 15 days of incubation, cells were collected and
transferred into a new well in a 96-well plate (catalog. no.
205512; BD Diagnostics, Sparks Glencoe, MD, USA); and images were
captured using a BD Pathway™ 855 at magnification, ×100 (BD
Biosciences, Franklin Lakes, NJ, USA). A spheroid with >50 cells
inside was considered to be a full sphere.
Cell viability analysis
Cell viability was analyzed by WST-1 assay. Sorted
SP cells were seeded at a density of 2.0×104 cells/well
into 96-well plates, and incubated with serum-free stem cell
culture medium for a total of 48 h at 37°C. Subsequently, cells
were treated with various concentrations of EEHDW (0, 0.5, 1 and 2
mg/ml) for 24 h at 37°C. At the conclusion of the treatment period,
10 µl WST-1 were added to each well, and the samples were incubated
for an additional 2 h at 37°C. The absorbance was measured at 450
nm using a microplate reader (ELx800 Absorbance Reader; BioTek
Instruments, Inc., Winooski, VT, USA).
Observation of morphological
changes
The sorted SP cells were seeded into 96-well plates
at a density of 2.0×104 cells/well in 0.1 ml serum-free
stem cell culture medium. The cells were treated with various
concentrations of EEHDW (0, 0.5, 1 and 2 mg/ml) for 24 h at 37°C.
Cell morphology was observed using a phase-contrast microscope
(DMIL/DFC295; Leica Microsystems GmbH, Wetzlar, Germany). Images
were captured at ×200 magnification.
Western blotting
HT-29 cells were seeded at a density of
2.5×105 cells/well into 6-well plates in 2 ml DMEM
medium, and were treated with various concentrations of EEHDW (0,
0.5, 1 and 2 mg/ml) for a total of 24 h at 37°C. The treated cells
were washed with PBS and scraped off into a tube, then lysed using
lysis buffer containing protease and phosphatase inhibitor
cocktails on ice for 15 min. Following high-speed centrifugation
(12,000 × g) for 20 min at 4°C, supernatant containing the sample
proteins was collected. The concentration of proteins was
determined using the BCA Protein Assay Reagent kit. A total of 50
µg of protein was resolved using 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis and blotted onto nitrocellulose
membranes. The membranes were blocked with blocking buffer and
probed with primary antibodies against Lgr5 or GAPDH (1:1,000)
overnight at 4°C, and subsequently with the appropriate
HRP-conjugated goat anti-rabbit secondary antibody (1:5,000) for 1
h at room temperature. The chemiluminescence signals were
visualized using the SuperSignal™ West Pico Chemiluminescent
Substrate. Image Lab™ Software, version 3.0, was used for
densitometric analysis/quantification of the western blotting
(Bio-Rad Laboratories Inc., Hercules, CA, USA).
RT-polymerase chain reaction (RT-PCR)
analysis
Total RNA from the HT-29 cells was isolated with
RNAiso Plus reagent. Oligo (dT)-primed RNA (1 µg) was reverse
transcribed using the PrimeScript RT Reagent kit according to the
manufacturer's protocol. Briefly, the gDNA Eraser (1 µl) contained
in the kit was used to remove the genomic DNA following incubation
with the total RNA for 2 min at 42°C, then the PrimeScript RT
Enzyme mix and RT Primer mix were added to perform RT using
incubation for 15 min at 37°C. The obtained complementary DNA was
used to determine the messenger (m)RNA quantity of c-Myc,
β-catenin, PCNA, survivin and ABCB1 by PCR, using the DreamTaq
Green PCR Master mix (2X). PCR was performed by the 3-step method,
with a denaturation stage at 95°C for 30 sec, an annealing stage at
an appropriate temperature (55°C for c-Myc, β-catenin and survivin,
and 58°C for PCNA, ABCB1 and GAPDH) for 30 sec and an extension
stage at 60°C for 30 sec for 30 cycles. GAPDH was used as an
internal control. The primer were synthesized by Invitrogen, Thermo
Fisher Scientific Inc., (Waltham, MA, USA) and the sequences used
in the RT-PCR are listed in Table I.
A negative control with no DNA and an RT control with no reverse
transcription were used as the experimental controls. The PCR was
repeated in three independent experiements. A Bio-Rad S1000 Thermal
Cycler was used to perform the experiment, and Image Lab™ Software,
version 3.0, was used for quantification/densitometric analysis
(both Bio-Rad Laboratories Inc.).
 | Table I.Primer sequences for reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-polymerase chain reaction.
| Gene | Primers, 5′→3′ |
|---|
| ABCB1 |
|
|
Forward |
TGACATTTATTCAAAGTTAAAAGCA |
|
Reverse |
TAGACACTTTATGCAAACATTTCAA |
| β-catenin |
|
|
Forward |
CCCACTGGCCTCTGATAATGG |
|
Reverse |
ACGCAAAGGTGCATGATTTG |
| c-Myc |
|
|
Forward |
CAGCTGCTTAGACGCTGGATT |
|
Reverse |
GTAGAAATACGGCTGCACCGA |
| PCNA |
|
|
Forward |
CCAAACCAGGAGAAAGT |
|
Reverse |
GTGTCACCGTTGAAGAG |
| Survivin |
|
|
Forward |
CAGATTTGAATCGCGGGACCC |
|
Reverse |
CCAAGTCTGGCTCGTTCTCAG |
| GAPDH |
|
|
Forward |
CGACCACTTTGTCAAGCTCA |
|
Reverse |
AGGGGTCTACATGGCAACTG |
Statistical analysis
Data were analyzed using SPSS for Windows (version
17.0; SPSS, Inc. Chicago, IL, USA). Statistical analysis of the
data was performed with Student's t-test and analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
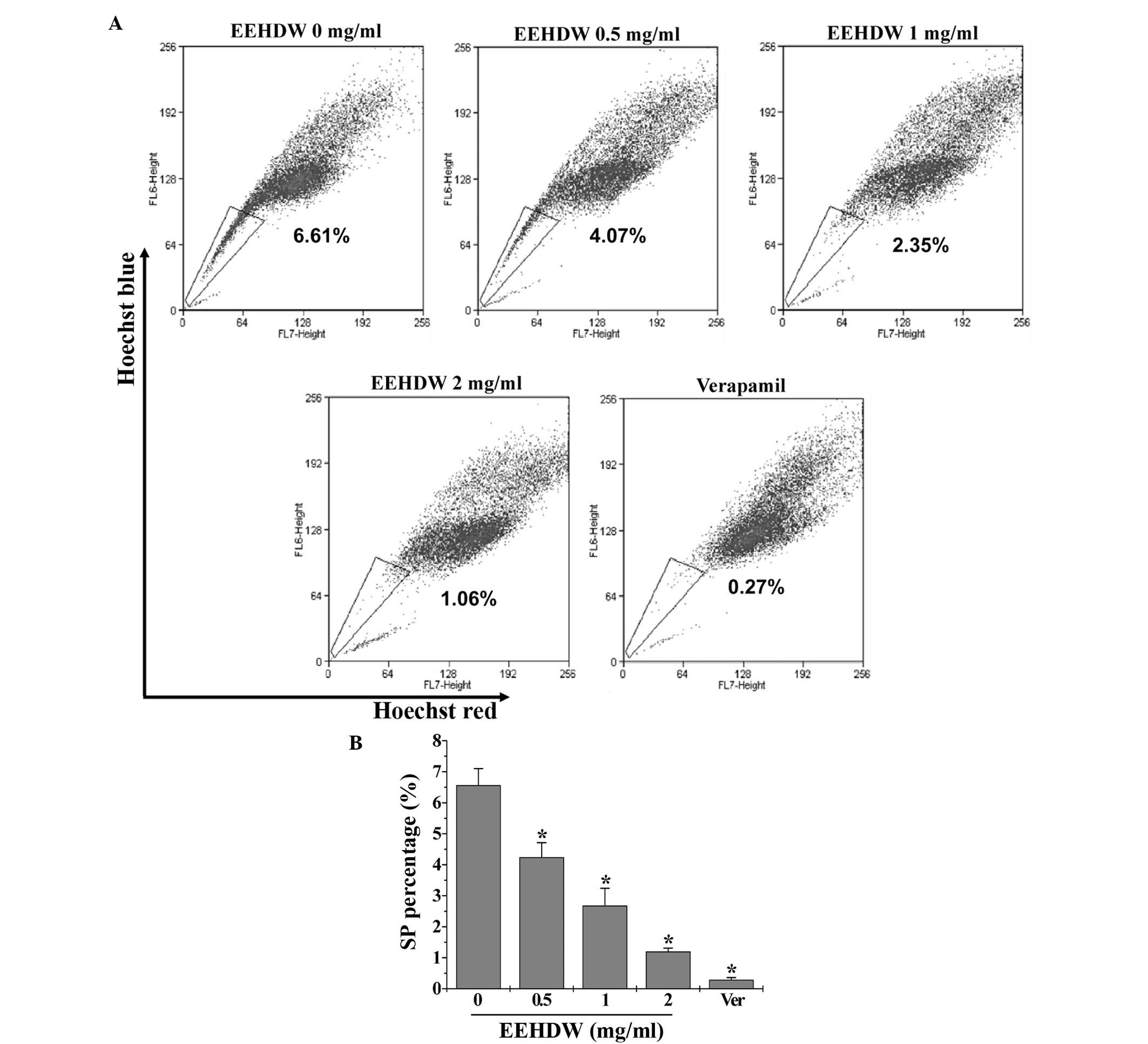
EEHDW reduces the SP proportion in
HT-29 cells
The effect of EEHDW on cancer stem cells was
determined by examining the SP proportion in HT-29 cells. As
demonstrated in Fig. 1, similar to
verapamil, a multi-drug transporter inhibitor that is typically
used as a positive control in SP analysis, EEHDW significantly
reduced the percentage of SP in HT-29 cells in a dose-dependent
manner compared with the control (P<0.05). In order to confirm
these results, the expression of Lgr5, which is considered to be a
bio-marker of CSCs, was detected. As shown in Fig. 2, EEHDW significantly and
dose-dependently downregulated Lgr5 protein expression compared
with the control (P<0.05).
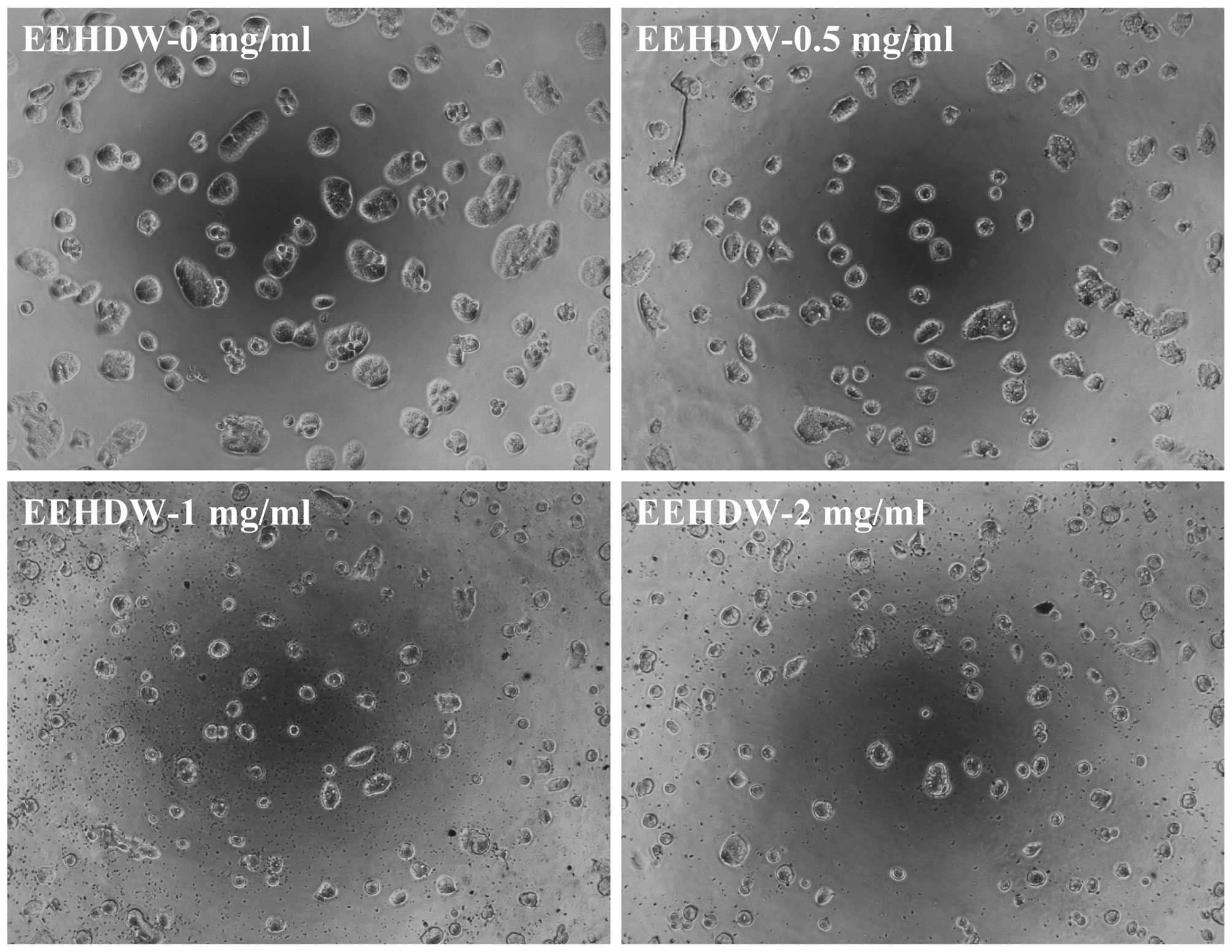
EEHDW inhibits the sphere formation
capacity and viability of isolated HT-29 SP cells
In order to investigate EEHDW's effect on CSC
growth, the present study evaluated the sphere formation of
isolated HT-29 SP cells. As shown in Fig.
3A and B, EEHDW dose-dependently suppressed SP sphere formation
compared with the control (P<0.05). In order to verify the
growth-suppressive activity of EEHDW in CSCs, the viability of
isolated HT-29 SP cells was determined. As demonstrated in Fig. 3C, EEHDW treatment significantly
reduced SP viability in a dose-dependent manner compared with the
control (P<0.05). The growth inhibition ability of EEHDW was
confirmed by its effect on SP cell morphology via phase-contrast
microscopy, as cell morphology in culture is indicative of the
healthy status of the cells. As shown in Fig. 4, following incubation with various
concentrations of EEHDW (0, 0.5, 1 and 2 mg/ml) for 24 h the
majority of the cells became shrunken and less confluent. Taken
together, these data demonstrate that EEHDW inhibits the growth of
isolated HT-29 SP cells.
EEHDW suppresses the expression of
ABCB1, β-catenin c-Myc, PCNA and survivin in isolated HT-29 SP
cells
In order to elucidate the underlying mechanism of
the anti-CSC activity of EEHDW, the present study examined the
expression of ABCB1, β-catenin, c-Myc, PCNA and survivin in
isolated HT-29 SP cells. As demonstrated in Fig. 5, EEHDW treatment markedly reduced the
mRNA levels of the aforementioned genes in the SP cells compared
with the control (P<0.05).
 | Figure 5.EEHDW suppresses the mRNA expression
of ABCB1, β-catenin, c-Myc, PCNA and survivin in isolated HT-29 SP
cells. (A) Following treatment with the indicated concentrations
(0, 0.5, 1 and 2 mg/ml) of EEHDW for 24 h, the mRNA expression of
ABCB1, β-catenin, c-Myc, PCNA and survivin in sorted HT-29 SP cells
was determined by reverse transcription-polymerase chain reaction.
GAPDH was used as the internal control. (B) Densitometric analysis.
The data were normalized to the mean mRNA expression of untreated
controls (100%). Images are representative and data are presented
as the mean ± standard deviation of 3 independent experiments.
*P<0.05 vs. untreated control cells. EEHDW, ethanol extract of
Hedyotis diffusa Willd.; mRNA, messenger RNA; ABCB1,
ATP-binding cassette, sub-family B, member 1; PCNA, proliferating
cell nuclear antigen; SP, side population; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase. |
Discussion
Accumulating evidence has revealed the existence of
cancer stem cells (CSCs) in the majority of leukemias and a number
of solid tumors, including colorectal cancer (18–21). Their
stem cell-like characteristics give CSCs the capacity for
continuous self-renewal, multi-directional differentiation and
natural drug resistance, resulting in cancer relapse and
metastasis, and leading to the eventual failure of clinical
anticancer treatment (8,9). Therefore, discovering novel agents that
target CSCs has the potential to improve the effectiveness of
anticancer treatment. Natural products, including traditional
Chinese medicines, have gained great attention as certain
naturally-occurring compounds have been demonstrated to possess
anti-CSC activity (17,22). HDW is a well-known medicinal herb that
is utilized in traditional Chinese medicine formulas as an
alternative treatment for various types of cancer. Previous studies
have proposed that HDW may possess a wide range of anticancer
activities by affecting multiple intracellular targets (13–16),
suggesting that it may be a novel and potent therapeutic agent for
the treatment of cancer. However, to the best of our knowledge, the
effect of HDW on CSCs has never previously been studied.
Side population (SP) analysis is a commonly utilized
technique for the identification and isolation of CSCs, and is
based on the ability of CSCs to efflux Hoechst dye, due to the
overexpression of ABC transporter proteins (23–26). SP
cells have been identified in various types of cancer and have been
observed to be correlated with tumor grade and patient prognosis
(27–30). In the present study, the stem-like
cells were isolated from the colon cancer cell line HT-29 as SP
using fluorescence-activated cell sorting. It was observed that HDW
was able to reduce the percentage of SP in HT-29 cells, and inhibit
the viability, sphere formation and cell growth of isolated SP
cells, indicating that HDW may be a useful agent for suppressing
the growth of cancer stem cells.
ABC transporter proteins are part of the superfamily
of membrane pumps that remove certain xenobiotics from cells,
including chemotherapeutic drugs and lipophilic fluorescent dyes,
and contribute to the SP phenotype and chemotherapy resistance. ABC
transporters are frequently overexpressed in CSCs (23–26). In
addition, the differentiation and self-renewal of CSCs is regulated
strictly by multiple signal transduction pathways, including Wnt
signaling (31). The activity of this
signaling pathway is typically determined by the amount of
stabilized β-catenin in the cytoplasm. When β-catenin accumulates
in the cytosol, it will translocate into the nucleus where it is
able to interact with Tcf/Lcf transcription factors to regulate the
transcription of target genes that mediate cell apoptosis and/or
proliferation, including c-Myc, PCNA and survivin, which gives CSCs
a survival advantage. It has been demonstrated that β-catenin is
important in the maintenance of the CSC phenotype (32–34). In
order to investigate the underlying mechanism whereby HDW was able
to inhibit the growth of colorectal cancer stem-like cells, the
present study examined the mRNA expression of ABCB1, β-catenin,
c-Myc, PCNA and survivin. It was observed that HDW treatment
markedly reduced the mRNA levels of the above-mentioned genes in
isolated HT-29 SP cells, suggesting that HDW may suppress CSCs in
HT-29 cells, potentially via inhibition of the expression of ABC
transporters and the Wnt signaling pathway.
The present study reported that HDW was able to
markedly downregulate the expression of the CSC marker, Lgr5, and
also significantly decrease the proportion of stem-like SP
colorectal cancer HT-29 cells. In addition, HDW treatment
significantly inhibited the viability and sphere formation, and
induced morphological changes in the isolated HT-29 SP cells.
Furthermore, HDW greatly suppressed the expression of several
critical genes that mediate CSC features. The findings in this
study suggest that HDW may exert inhibitory effects on CSCs.
Acknowledgements
The present study was sponsored by the Research Fund
for the Doctoral Program of Higher Education of China (Beijing,
China; grant no. 20133519110003) and the Developmental Fund of Chen
Keji Integrative Medicine (Fujian, China; grant nos. CKJ2014013 and
CKJ2015007).
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
HDW
|
Hedyotis diffusa Willd.
|
|
CSCs
|
cancer stem cells
|
|
SP
|
side population
|
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Montagnani F, Chiriatti A, Turrisi G,
Francini G and Fiorentini G: A systematic review of FOLFOXIRI
chemotherapy for the first-line treatment of metastatic colorectal
cancer: Improved efficacy at the cost of increased toxicity.
Colorectal Dis. 13:846–852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lippman SM: The dilemma and promise of
cancer chemoprevention. Nat Clin Pract Oncol. 3:5232006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Longley DB, Allen WL and Johnston PG: Drug
resistance, predictive markers and pharmacogenomics in colorectal
cancer. Biochim Biophys Acta. 1766:184–196. 2006.PubMed/NCBI
|
|
7
|
Alison MR, Lin WR, Lim SM and Nicholson
LJ: Cancer stem cells: In the line of fire. Cancer Treat Rev.
38:589–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou BB, Zhang H, Damelin M, Geles KG,
Grindley JC and Dirks PB: Tumour-initiating cells: Challenges and
opportunities for anticancer drug discovery. Nat Rev Drug Discov.
8:806–823. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin JM, Wei LH, Chen YQ, Liu XX, Hong ZF,
Sferra TJ and Peng J: Pien Tze Huang induced apoptosis in human
colon cancer HT-29 cells is associated with regulation of the Bcl-2
family and activation of caspase 3. Chin J Integr Med. 17:685–690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang on angiogenesis
in vivo and in vitro. Chin J Integr Med. 18:431–436.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin J, Chen Y, Wei L, Shen A, Sferra TJ,
Hong Z and Peng J: Ursolic acid promotes colorectal cancer cell
apoptosis and inhibits cell proliferation via modulation of
multiple signaling pathways. Int J Oncol. 43:1235–1243.
2013.PubMed/NCBI
|
|
14
|
Lin J, Wei L, Shen A, Cai Q, Xu W, Li H,
Zhan Y, Hong Z and Peng J: Hedyotis diffusa Willd extract
suppresses Sonic hedgehog signaling leading to the inhibition of
colorectal cancer angiogenesis. Int J Oncol. 42:651–656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai Q, Lin J, Wei L, Zhang L, Wang L, Zhan
Y, Zeng J, Xu W, Shen A, Hong Z and Peng J: Hedyotis diffusa
Willd inhibits colorectal cancer growth in vivo via
inhibition of STAT3 signaling pathway. Int J Mol Sci. 13:6117–6128.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis diffusa Willd extract
induces apoptosis via activation of the mitochondrion-dependent
pathway in human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.PubMed/NCBI
|
|
17
|
Wei L, Chen P, Chen Y, Shen A, Chen H, Lin
W, Hong Z, Sferra TJ and Peng J: Pien Tze Huang suppresses the
stem-like side population in colorectal cancer cells. Mol Med Rep.
9:261–266. 2014.PubMed/NCBI
|
|
18
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Efferth T: Stem cells, cancer stem-like
cells, and natural products. Planta Med. 78:935–942. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Golebiewska A, Brons NH, Bjerkvig R and
Niclou SP: Critical appraisal of the side population assay in stem
cell and cancer stem cell research. Cell Stem Cell. 8:136–147.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou S, Schuetz JD, Bunting KD, Colapietro
AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M,
Nakauchi H and Sorrentino BP: The ABC transporter Bcrp1/ABCG2 is
expressed in a wide variety of stem cells and is a molecular
determinant of the side-population phenotype. Nat Med. 7:1028–1034.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robey RW, To KK, Polgar O, Dohse M, Fetsch
P, Dean M and Bates SE: ABCG2: A perspective. Adv Drug Deliv Rev.
61:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic.
Cancer Res. 65:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bleau AM, Hambardzumyan D, Ozawa T,
Fomchenko EI, Huse JT, Brennan CW and Holland EC: PTEN/PI3K/Akt
pathway regulates the side population phenotype and ABCG2 activity
in glioma tumor stem-like cells. Cell Stem Cell. 4:226–235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiba T, Kita K, Zheng YW, Yokosuka O,
Saisho H, Iwama A, Nakauchi H and Taniguchi H: Side population
purified from hepatocellular carcinoma cells harbors cancer stem
cell-like properties. Hepatology. 44:240–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haraguchi N, Utsunomiya T, Inoue H, Tanaka
F, Mimori K, Barnard GF and Mori M: Characterization of a side
population of cancer cells from human gastrointestinal system. Stem
Cells. 24:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ho MM, Ng AV, Lam S and Hung JY: Side
population in human lung cancer cell lines and tumors is enriched
with stem-like cancer cells. Cancer Res. 67:4827–4833. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klaus A and Birchmeier W: Wnt signaling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Komiya Y and Habas R: Wnt signal
transduction pathways. Organogenesis. 4:68–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rao TP and Kühl M: An updated overview on
Wnt signaling pathways: A prelude for more. Circ Res.
106:1798–1806. 2010. View Article : Google Scholar : PubMed/NCBI
|