Introduction
Human papilloma viruses (HPVs) are small
non-enveloped DNA viruses that belong to the papilloma virus
family, and infect cutaneous and mucosal epithelia in humans
(1). HPVs are extremely common
worldwide, and consist of >170 types (2). A persistent infection with certain
mucosa-tropic types of HPV is recognized as the major factor in the
etiology of cervical cancer (3–6). According
to the World Health Organization (WHO), cervical cancer is the
second most common type of cancer in women, with ~530,000 newly
diagnosed cases every year (7). The
incidence of cervical cancer in Germany is one of the highest among
Western countries, and it is currently the second cause of
mortality among women >50 years of age (8).
HPV is usually acquired via sexual transmission, and
may induce the development of cervical cancer within several years
following a persistent infection (9).
The progress of cervical cancer is slow, commencing from cervical
intraepithelial neoplasia (CIN) (3)
and ending with invasive cancer. Precancerous lesions may be
effectively detected by cervical screening using the Papanicolaou
test (Pap smear). HPVs have been classified into high-risk types
(HR-HPVs), which are oncogenic or have oncogenic potential, and
low-risk types (LR-HPVs), which do not exhibit a causal association
with cancer (9). A recent study
provided biological evidence of carcinogenicity for HPV types 26,
53, 66, 67, 68, 70, 73 and 82, which were previously classified as
possibly carcinogenic (HPV26, 53, 66, 68, 73 and 82) and
non-carcinogenic (HPV67 and 70) (10). Whereas LR-HPV types account for
non-fatal conditions, including warts and condylomas, numerous
studies have demonstrated the causal association between HR-HPV and
malignant lesions (4,6,11). In a
retrospective cross-sectional worldwide study concerning the
association between HPV genotype and cervical cancer using data
from >10,000 patients, HPV DNA was identified in 85% of patients
with cervical cancer (12). The
highest carcinogenic risk is attributed to the following HR-HPV
types: 16, 18, 31, 33, 35, 52 and 58 (13). HPV16 and 18 are associated with
>2/3 of all cervical cancer cases worldwide (14–16), which
has led to the development of prophylactic vaccines against these
two HPV types (17).
Currently, there are two approved HPV vaccines in
Germany (18). These are the
quadrivalent vaccine Gardasil® (Merck & Co., Inc.,
Whitehouse Station, NJ, USA), which targets HPV6, 11, 16 and 18,
and the bivalent vaccine Cervarix® (GlaxoSmithKline,
Brentford, UK), which targets HPV16 and 18. Since 2006, numerous
countries have implemented vaccination programs using these two
vaccines (18). In Germany, HPV
vaccination is usually performed during a routine adolescent health
check-up termed J1, which is available for 12–14 year-old girls,
and the recommendation of the Standing Committee on Vaccination at
the Robert Koch Institute is to vaccinate between the ages of 9 and
14 years (19). The results from
clinical trials indicate that vaccinated girls and women have an
increased protection against the development of CIN (20–23). In
addition to the proven efficacy against HPV16 and 18 (10,15), the
above bivalent and quadrivalent HPV vaccines are known to confer
cross-protection against certain non-vaccine HPV types. The
quadrivalent vaccine has been described to cross-protect mainly
against HPV31 (24), while the
bivalent vaccine has been demonstrated to exhibit a protective
effect against HPV31, 33, 45 (25)
and 51 (26).
With the increasing impact of vaccination against
certain HPV types in young women (27), it is important to monitor shifts in
the prevalence of HPV types. Therefore, the aim of the present
study is to determine and evaluate the prevalence of different HPV
types in Southern Bavaria, and to analyze the local HPV type
distribution in women with abnormal cytological diagnostic
findings. Cervical co-infection with multiple HPV types is
widespread (28), and may contribute
to a higher risk of developing cervical lesions with precancerous
potential. Consequently, evaluating the prevalence of infections
with >1 HPV type is an additional aim of the present study.
Materials and methods
Patient cohort
The study cohort consisted of 615 Caucasian women
aged between 16 and 93 years. The participants had undergone
routine cytological evaluation in three Southern Bavaria pathology
centers (Institutes of Pathology at Kaufbeuren, Ravensburg and
Rosenheim, Germany) using the Second Munich Cytological
Classification (29). The cytology
was re-evaluated in the present study using the Bethesda
classification system (30). Only
data from participants with a conspicuous cervical cytological
indication and available HPV analysis were included in the present
study. Complete data on the individual vaccination status of every
participant was not available. Written informed consent was
obtained from all patients for the use of their data. The present
study was approved under the ethical regulations that exist at the
Medical Faculty of the University of Regensburg (Bavaria,
Germany).
Cytology
Cytological specimens were collected between
December 2010 and September 2014 by Pap smear. The specimens were
dehydrated, and subsequently stained with Harris' hematoxylin (Carl
Roth GmbH, Karlsruhe, Germany), bleached with hydrated hydrochloric
acid (Hernicht GmbH, Sulzberg, Germany), and stained orange-red
with Papanicolaou's staining solution Orange G (EA 50; VWR
International GmbH, Darmstadt, Germany), according to the
manufacturer's protocol. The slides were reviewed by qualified
pathologists at the Institutes of Pathology at Kaufbeuren,
Ravensburg and Rosenheim using the Bethesda classification system
(31). Smears with atypical squamous
cells of undetermined significance (ASC-US), low-grade squamous
intraepithelial lesion (LSIL) and high-grade squamous
intraepithelial lesion (HSIL) were subjected to further
analysis.
Histopathology
In addition to the Pap smear, cervical conization
specimens were obtained from a subgroup of 86 out of 615
participants. The cervical conization specimens were reviewed and
classified as grades CIN1, CIN2 and CIN3, according to the WHO
criteria (32). The specimens were
immediately fixed following surgery in buffered 4% formaldehyde
(Hernicht GmbH), and subsequently analyzed with hematoxylin and
eosin staining (VWR International GmbH). Tissue sections (2-µm
thick; RM2235 Microtome; Leica Microsystems GmbH, Wetzlar, Germany)
were obtained from paraffin-embedded tissue (Hasuwax Paraffin;
Sussmann & Steinhauser GmbH, Kaufbeuren, Germany). In certain
tissues, p16 immunohistochemistry was performed using the
CINtec® Histology kit (catalog no., 9517; mouse
monoclonal anti-p16 antibody; dilution, 1:50; Roche Diagnostics
GmbH, Mannheim, Germany), according to the manufacturer's protocol,
to confirm cervical intraepithelial dysplasia.
HPV DNA testing
DNA was isolated from the formalin-fixed,
paraffin-embedded (FFPE) tissues using the QIAamp DNA FFPE Tissue
kit (Qiagen GmbH, Hilden, Germany) and the QIAcube (Qiagen GmbH),
according to the manufacturer's protocol. HPV testing was performed
using a DNA-based liquid-crystal display (LCD)-Array kit (LCD Array
HPV 3.5; Chipron GmbH, Berlin, Germany), which contained 32
specific capture probes for the identification of 32 types of HPV.
In total, 20 µl polymerase chain reaction (PCR) AmpliTaq
Gold® 360 Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used, which contained 1.2
µl pre-labeled primer mix, 0.2 µM dNTPs, 2 mM magnesium, 0.2 U
Taq-Gold polymerase and 2 µl template DNA. Amplification was
performed in a DNA Engine® Thermal Cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) with the following PCR
program: Initial denaturation of 10 min at 95°C followed by 42
cycles of 1 min at 94°C, 1.5 min at 45°C and 1.5 min at 72°C, with
a final elongation of 3 min at 72°C. The present study adapted the
classification of HR- and LR-HPV types according to previous
studies concerning the carcinogenic properties of particular HPV
types (10,33). Therefore, the panel of identifiable
HPV types consisted of 20 HR-HPV types (HPV16, 18, 31, 33, 35, 39,
45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 70, 73, 82 and 83) and 12
LR-HPV types (HPV6, 11, 42, 44, 54, 61, 62, 72, 81, 84, 87, 90 and
91). The positive control contained DNA from a HPV-positive (HPV
16) sample while the negative control contained no DNA. Using the
LCD-Array kit (LCD Array HPV 3.5; Chipron GmbH), the labeled PCR
fragments were combined with hybridization buffer (Chipron GmbH)
and hybridized at 35°C for 30 min to the individual array fields of
one chip with specific capture probes (Chipron GmbH). Following a
washing procedure according to the manufacturer's protocol, each
field was incubated with a secondary label solution for 5 min at
room temperature (enzyme-conjugate, Chipron GmbH). Subsequent to a
second washing step, the bound PCR fragments were visualized by a
blue precipitate formed by the ‘BLUE stain’ enzyme substrate
provided (Chipron GmbH).
PCR results were evaluated using SlideReader
Software v2.0 (Chipron GmbH). PCR, hybridization, labeling and
staining were performed according the manufacturer's protocol
(Chipron GmbH). HPV types that were detected by PCR but did not
generate signals on the LCD array were sequenced and subjected to
Basic Local Alignment Search Tool analysis (National Center for
Biotechnology Information, Bethesda, MD, USA). One tissue sample
with HPV87 was detected by sequencing.
Results
Patient characteristics
The current study consisted of 615 female
participants with abnormal cervical cytological pathology (ASC-US,
LSIL or HSIL). In total, 35.61% (219/615) of the participants were
<30 years of age, 32.36% (199/615) were 30–44 years old, 28.62%
(176/615) were 45–59 years old and 3.41% (21/615) were >60 years
of age. In 470 (76.42%) of these participants, HPV infection was
detected, and 419 (89.15%) of them were infected with ≥1 HR-HPV
type. As shown in Table I, a total of
204 out of 615 participants (33.17%) were infected with a single
type (ST) of HPV, while 266 participants (43.25%) were infected
with multiple types (MTs), providing a total of 775 HPV results
(mean of participants with MT infection, 2.9 types). The
distribution of the detected HPV types divided by age group (<30
years; 30–44 years; 45–59 years; and ≥60 years) is revealed in
Table I. The incidence rates in
Table I are presented as the total
number of participants and the number of HPV+
participants. The prevalence of infected participants declined by
age (<30 years, 86.30%; 30–44 years, 77.89%; 45–59 years,
63.31%). In participants ≥60 years old, there was an unanticipated
high rate (71.43%) of infection. However, this is not significant,
due to the small group size (n=21).
 | Table I.Type-specific HPV incidence in 615
women, grouped according to their age and risk of developing risk
cancer. |
Table I.
Type-specific HPV incidence in 615
women, grouped according to their age and risk of developing risk
cancer.
|
|
|
|
|
| Age groups
(HPV+ participants only) |
|---|
|
|
|
|
|
|
|
|---|
|
|
| HPV+
participants | Group I (<30
years) | Group II (30–44
years) | Group III (45–59
years) | Group IV (≥60
years) |
|---|
|
|
|
|
|
|
|
|
|---|
| Variable | Total n (%) | n (%) | ST | MT | n (%) | MT | n (%) | MT | n (%) | MT | n (%) | MT |
|---|
| Abnormal
cytology | 615 (NA) | 470
(76.42) | 204 | 266 | 189 (NA) | 121 | 155 (NA) | 80 | 111 (NA) | 58 | 15 (NA) | 7 |
| HPV findings | 979 (NA) | 979 (NA) | 204 | 775 | 440 (NA) | 372 | 294 (NA) | 219 | 219 (NA) | 166 | 26 (NA) | 18 |
| Patients with ≥1
HR-HPV type | 419
(68.13) | 419
(89.15) | – | – | 173
(91.53) | – | 143
(92.26) | – | 92
(82.88) | – | 11
(73.33) | – |
| HPV type |
|
|
|
|
|
|
|
|
|
|
|
|
| 16 | 155
(25.20) | 155
(32.98) | 51 | 104 | 78
(41.27) | 52 | 48
(30.97) | 30 | 25
(22.52) | 19 | 4
(26.67) | 3 |
| 18 | 30
(4.88) | 30
(6.38) | 10 | 20 | 12
(6.35) | 10 | 8
(5.16) | 4 | 9
(8.11) | 5 | 1
(6.67) | 1 |
| 31 | 83
(13.50) | 83
(17.66) | 21 | 62 | 35
(18.52) | 32 | 29
(18.71) | 18 | 19
(17.12) | 12 | 0
(0.00) | 0 |
| 33 | 18
(2.93) | 18
(3.83) | 3 | 15 | 8
(4.23) | 8 | 5
(3.23) | 3 | 4
(3.60) | 3 | 1
(6.67) | 1 |
| 35 | 14
(2.28) | 14
(2.98) | 1 | 13 | 8
(4.23) | 7 | 4
(2.58) | 4 | 2
(1.80) | 2 | 0
(0.00) | 0 |
| 39 | 29
(4.72) | 29
(6.17) | 8 | 21 | 12
(6.35) | 10 | 11
(7.10) | 6 | 5
(4.50) | 4 | 1
(6.67) | 1 |
| 45 | 24
(3.90) | 24
(5.11) | 4 | 20 | 8
(4.23) | 8 | 7
(4.52) | 6 | 7
(6.31) | 4 | 2
(13.33) | 2 |
| 51 | 65
(10.57) | 65
(13.83) | 11 | 54 | 36
(19.05) | 31 | 16
(10.32) | 13 | 12
(10.81) | 9 | 1
(6.67) | 1 |
| 52 | 36
(5.85) | 36
(7.66) | 6 | 30 | 15
(7.94) | 14 | 12
(7.74) | 8 | 9
(8.11) | 8 | 0
(0.00) | 0 |
| 53 | 41
(6.67) | 41
(8.72) | 4 | 37 | 16
(8.47) | 13 | 12
(7.74) | 12 | 11
(9.91) | 11 | 2
(13.33) | 1 |
| 56 | 47
(7.64) | 47
(10.00) | 14 | 33 | 18
(9.52) | 15 | 18
(11.61) | 12 | 11
(9.91) | 6 | 0
(0.00) | 0 |
| 58 | 15
(2.44) | 15
(3.19) | 5 | 10 | 6
(3.17) | 4 | 4
(2.58) | 3 | 4
(3.60) | 3 | 1
(6.67) | 0 |
| 59 | 29
(4.72) | 29
(6.17) | 2 | 27 | 16
(8.47) | 16 | 10
(6.45) | 8 | 3
(2.70) | 3 | 0
(0.00) | 0 |
| 66 | 41
(6.67) | 41
(8.72) | 8 | 33 | 20
(10.58) | 18 | 9
(5.81) | 8 | 8
(7.21) | 4 | 4
(26.67) | 3 |
| 67 | 28
(4.55) | 28
(5.96) | 1 | 27 | 16
(8.47) | 15 | 8
(5.16) | 8 | 3
(2.70) | 3 | 1
(6.67) | 1 |
| 68 | 6
(0.98) | 6
(1.28) | 2 | 4 | 2
(1.06) | 2 | 2
(1.29) | 1 | 2
(1.80) | 1 | 0
(0.00) | 0 |
| 70 | 22
(3.58) | 22
(4.68) | 6 | 16 | 8
(4.23) | 7 | 8
(5.16) | 5 | 6
(5.41) | 4 | 0
(0.00) | 0 |
| 73 | 13
(2.11) | 13
(2.77) | 2 | 11 | 7
(3.70) | 6 | 2
(1.29) | 1 | 4
(3.60) | 4 | 0
(0.00) | 0 |
| 82 | 5
(0.81) | 5
(1.06) | 0 | 5 | 2
(1.06) | 2 | 3
(1.94) | 3 | 0
(0.00) | 0 | 0
(0.00) | 0 |
| 83 | 9
(1.46) | 9
(1.91) | 1 | 8 | 5
(2.65) | 5 | 2
(1.29) | 1 | 2
(1.80) | 2 | 0
(0.00) | 0 |
| Patients with ≥1
LR-HPV type | 212 (34.47) | 212 (45.11) | – | – | 90
(47.62) | – | 59
(38.06) | – | 55 (49.55) | – | 8
(53.33) | – |
| HPV type |
|
|
|
|
|
|
|
|
|
|
|
|
| 6 | 21 (3.41) | 21 (4.47) | 6 | 15 | 9
(4.76) | 7 | 7
(4.52) | 5 | 5 (4.50) | 3 | 0 (0.00) | 0 |
| 11 | 7
(1.14) | 7
(1.49) | 1 | 6 | 1
(0.53) | 1 | 3
(1.94) | 3 | 1 (0.90) | 1 | 2
(13.33) | 1 |
| 42 | 85
(13.82) | 85
(18.09) | 18 | 67 | 38
(20.11) | 33 | 18
(11.61) | 14 | 28 (25.23) | 20 | 1 (6.67) | 0 |
| 44 | 17 (2.76) | 17 (3.62) | 2 | 15 | 7
(3.70) | 6 | 6
(3.87) | 6 | 4 (3.60) | 3 | 0 (0.00) | 0 |
| 54 | 32 (5.20) | 32 (6.81) | 2 | 30 | 16 (8.47) | 14 | 7
(4.52) | 7 | 9 (8.11) | 9 | 0 (0.00) | 0 |
| 61 | 13 (2.11) | 13 (2.77) | 0 | 13 | 8
(4.23) | 8 | 2
(1.29) | 2 | 3 (2.70) | 3 | 0 (0.00) | 0 |
| 62 | 24 (3.90) | 24 (5.11) | 3 | 21 | 5
(2.65) | 4 | 12 (7.74) | 12 | 6 (5.41) | 4 | 1 (6.67) | 1 |
| 72 | 1
(0.16) | 1
(0.21) | 1 | 0 | 0
(0.00) | 0 | 1
(0.65) | 0 | 0 (0.00) | 0 | 0 (0.00) | 0 |
| 81 | 8
(1.30) | 8
(1.70) | 1 | 7 | 3
(1.59) | 3 | 2
(1.29) | 2 | 3 (2.70) | 2 | 0 (0.00) | 0 |
| 84 | 19 (3.09) | 19 (4.04) | 0 | 19 | 9
(4.76) | 9 | 6
(3.87) | 6 | 3 (2.70) | 3 | 1 (6.67) | 1 |
| 87 | 1
(0.16) | 1
(0.21) | 1 | 0 | 1
(0.53) | 0 | 0
(0.00) | 0 | 0 (0.00) | 0 | 0 (0.00) | 0 |
| 90 | 26 (4.23) | 26 (5.53) | 5 | 21 | 9
(4.76) | 8 | 7
(4.52) | 4 | 8 (7.21) | 8 | 2
(13.33) | 1 |
| 91 | 15 (2.44) | 15 (3.19) | 4 | 11 | 6
(3.17) | 4 | 5
(3.23) | 4 | 3 (2.70) | 3 | 1 (6.67) | 0 |
| Single
infection | 204 (33.17) | 204 (43.40) | – | – | 68
(35.98) | – | 75
(48.39) | – | 53 (47.75) | – | 8
(53.33) | – |
| Double
infection | 129 (20.98) | 129 (27.45) | – | – | 57
(30.16) | – | 41
(26.45) | – | 27 (24.32) | – | 4
(26.67) | – |
| Multiple (>1)
infections | 266 (43.25) | 266 (56.60) | – | – | 121 (64.02) | – | 80
(51.61) | – | 58 (52.25) | – | 7
(46.67) | – |
The present study additionally analyzed participants
with ST or MT infection. The highest rate of MT infection (64.02%
of all infected patients) was observed in participants <30 years
old. In participants >30 years old, the percentage of patients
with MT infection was lower (30–44 years, 51.61%; 45–59 years,
52.25%; ≥60 years, 46.67%). The highest prevalence of HPV type was
HPV16 (HR), which was present in 32.98% of participants that tested
HPV+, followed by HPV42 (LR; 18.09%), HPV31 (HR;
17.66%), HPV51 (HR; 13.83%), HPV56 (HR; 10.00%), HPV66 (HR; 8.72%)
and HPV53 (HR; 8.72%). HR-HPV type infections that may be directly
prevented by vaccination or via cross-protection with available
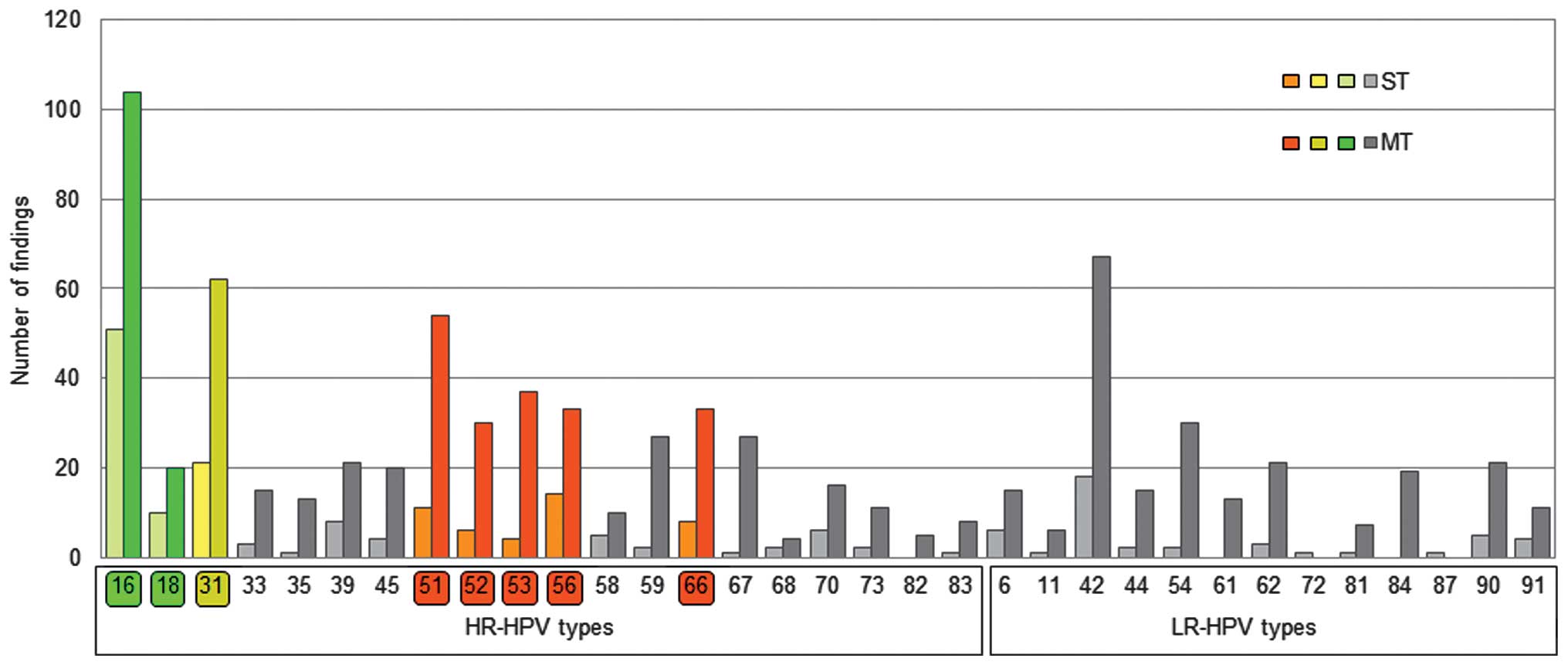
vaccines are marked in green (HPV16 and 18) and yellow (HPV31) in
Figs. 1 and 2, while HR-HPV types with the highest
prevalence (HPV51, 53, 56 and 66), with no protection from
vaccinations, are marked in red. HPV18 infection occurred in 6.38%
of participants that tested HPV+. The prevalence of
HPV16 was highest in participants <30 years (41.27%), and
declined with older age (30–44 years, 30.97%; 45–59 years, 22.52%;
≥60 years, 26.67%).
Incidence of ST and MT infection
Fig. 1 represents the
prevalence of ST and MT infections for all the HPV types analyzed.
HPV16 and 18, known to be cervical carcinogens, were observed in
39.36% of HPV+ participants (ST or MT infection) and in
44.05% of HR-HPV infected participants. In 67.10% (104/155) of
participants, the presence of HPV16 was associated with MT
infection, while this percentage was 66.67% (20/30) for HPV18. The
most frequent HPV types that exhibited co-infection with HPV16 were
HPV42 (n=22), HPV51 (n=21), HPV31 (n=17), HPV66 (n=16), HPV56
(n=12), HPV67 (n=12), HPV18 (n=11), HPV39 (n=11) and HPV90 (n=11).
In total, 55.95% of HR-HPV-infected participants were not infected
with HPV16 or 18. The less prevalent HPV18 was identified in 20 out
of 30 participants with MT-HPV infection, most frequently with
HPV16 (n=11), and rarely with HPV51 (n=5), HPV31 (n=3), HPV44
(n=3), HPV59 (n=3) and HPV66 (n=3).
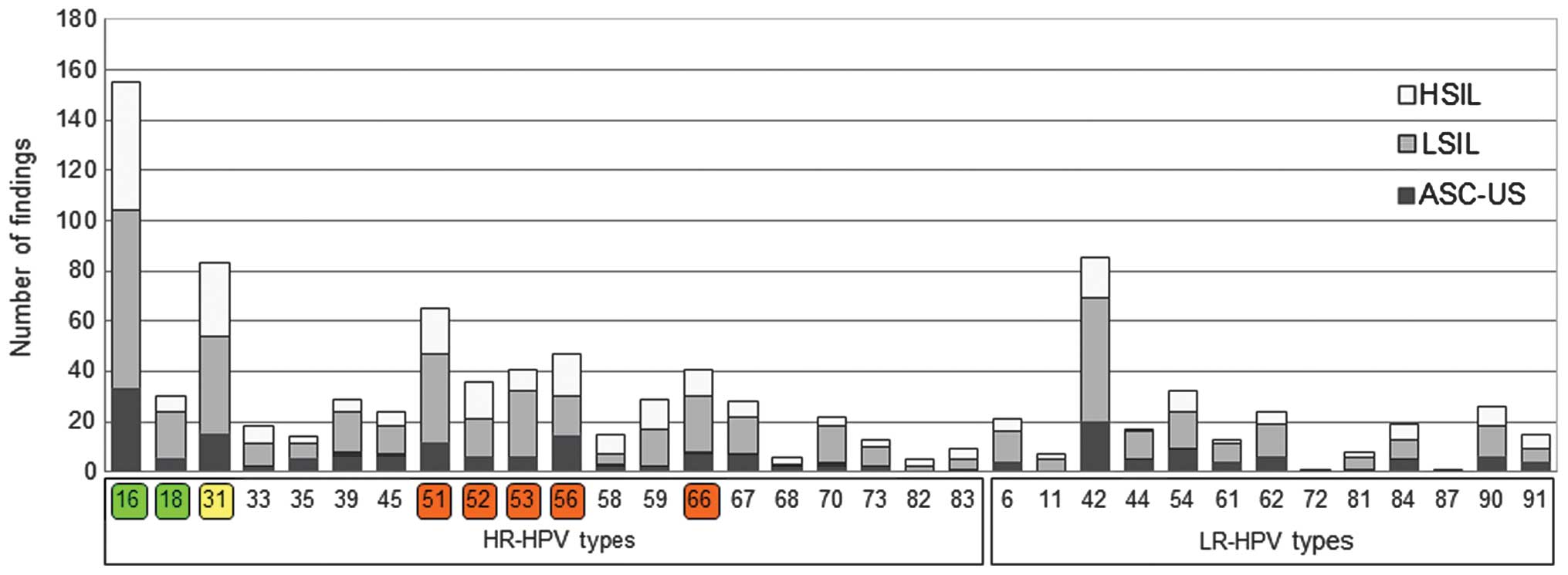
HPV type and association with cervical
cancer
Fig. 2 represents the
number of findings of each HPV type, and the corresponding cervical
cytological finding, based on the Bethesda classification system
(30). The relative distribution of
cytological diagnosis in the group of HPV+ participants
was clearly different, compared with the cytological diagnosis of
HPV− participants (Fig.
3). The prevalence of LSIL and HSIL was increased in the group
of HPV+ participants, which suggests that HPV may cause
the development of a precancerous lesion. The distribution of
cytological findings among participants with ≥1 HR-HPV type,
partially with an additional LR-HPV appearance, differed from the
distribution of cytological findings among participants with ≤1
LR-HPV type and without any HR-HPV appearance. Therefore, this
suggests that there were proportionally more LSIL and HSIL cases,
compared with ASC-US cases. Three HR-HPV types, namely HPV16, 18
and 31 were present singularly in 65.25% of HSIL cases, compared
with 37.79 and 26.37% in LSIL and ASC-US cases, respectively. The
general distribution of cytological diagnosis according to HPV
type, HR- or LR-HPV prevalence and ST or MT infection status are
presented in Fig. 3 (ASC-US, n=182;
LSIL, n=307; HSIL, n=126). There were proportionally more
participants with MT-HPV infection classified as LSIL and HSIL,
compared with participants with a single infection, who were
classified as ASC-US. Notably, 7 out of 266 participants (2.63%)
with MT possessed LR-HPV types and, in contrast, 259 women (97.37%)
possessed HR-HPV types or a mixture of HR- and LR-HPV types.
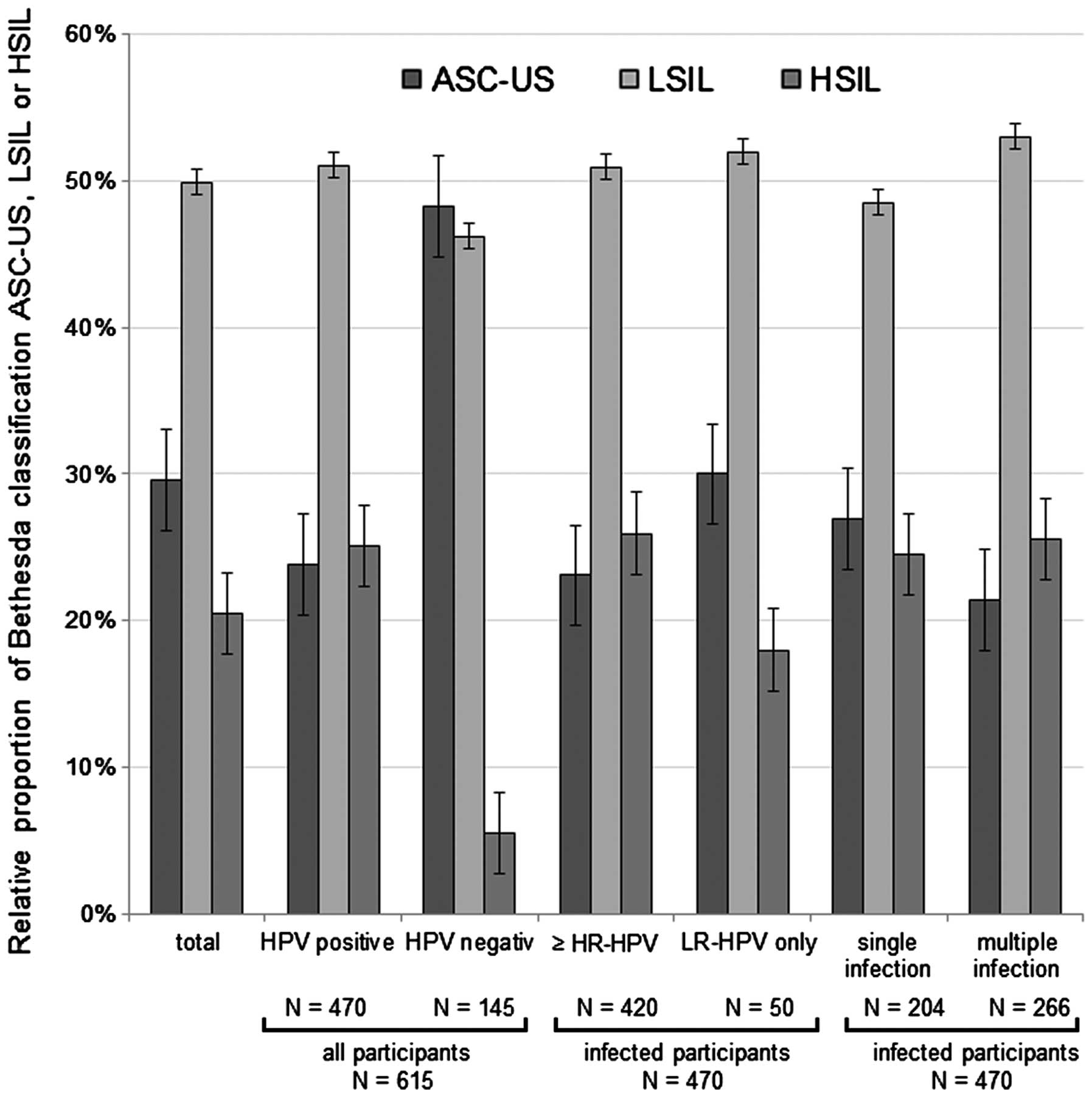 | Figure 3.Relative distribution of
HPV+ and HPV− participants according to the
Bethesda cervical cytological classification (ASC-US, LSIL or
HSIL). The distribution of cervical cytological classification
among participants in the present study with ≥1 HR-HPV or 1
probably HR-HPV type with additional LR-HPV appearance, and the
distribution of cervical cytological classification among
participants with ≥1 LR-HPV type without any HR-HPV appearance, are
depicted. Furthermore, the relative distribution of participants
according to single or multiple infection is represented in the
graph. HPV, human papilloma virus; ASC-US, atypical squamous cell
of undetermined significance; LSIL, low-grade squamous
intraepithelial lesion; HSIL, high-grade squamous intraepithelial
lesion; HR, high risk; LR, low risk. |
Cervical conization specimens
In a subgroup of 86 participants, cervical
conization specimens were obtained, and 80 of these participants
exhibited cervical intraepithelial neoplasia, which were classified
as CIN1 (22/80), CIN2 (27/80) or CIN3 (31/80). The prevalence of
HR-HPVs was associated with a more severe cervical intraepithelial
neoplasia (HR-HPV appearances: CIN1, 77.27%; CIN2, 81.48%; and
CIN3, 90.32%). The total prevalence of HR-HPV types 16, 18 and 31
was highest in participants whose neoplasms were classified as CIN3
(64.52%; 20/31), followed by 55.56% (15/27) of neoplasms classified
as CIN2 and 50.00% (11/22) of neoplasms classified as CIN1. By
contrast, there was no evidence that MT infections were more
prevalent in neoplasms classified as CIN3 (48.39%), compared with
those classified as CIN2 (50.85%). In addition, the present study
demonstrated that in participants with CIN3 lesions, a cytological
diagnosis of ASC-US was present in 19.35% of participants, LSIL in
32.26% participants and HSIL in 48.39% of participants.
Participants that possessed CIN1 or CIN2 neoplasms were more likely
to exhibit LSIL (50.00%; 66.67%), compared with HSIL (31.82%;
18.52%). There was no accumulation of specific HPV types among the
non-vaccinated HR-HPV participants that could be assigned to one of
the CIN classes.
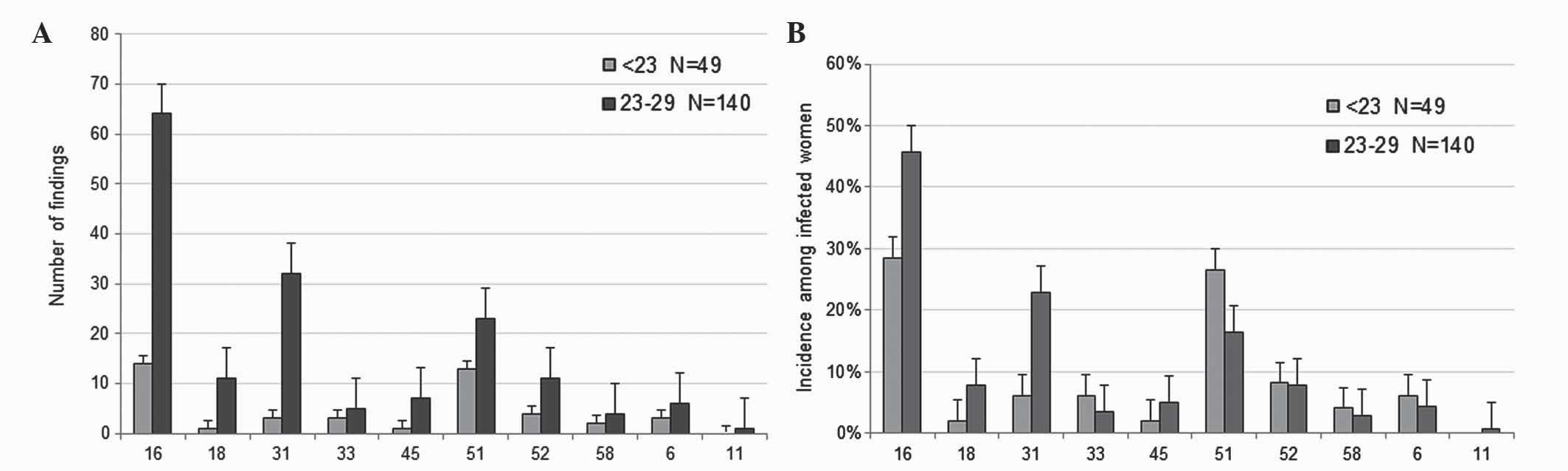
HPV vaccination
In Germany, a routine HPV vaccine has been
recommended for 12–17 year-old girls since 2007 (34). Consequently, in the present study,
participants aged 19–21 years may have been vaccinated, while women
>22 years presumably had not been vaccinated. Therefore, a
disparity was expected among the participants in the
<30-year-old group. As a result, this age group was divided into
two subgroups: Those aged <23 years (n=49), and those aged 23–29
years (n=140). For participants <23 years old, information on
vaccination status was obtained from 24 participants, and the
information was as follows: 37.50% of patients (9/24) were
vaccinated (Gardasil®, 7; unknown, 2), while 62.50%
(15/24) were not vaccinated. Although the present study did not
include data on the individual vaccination status of all
participants, based on the results from the 9 participants in the
<23-year-old group, it was estimated that ~1/3 of women <23
years of age were protected against HPV by vaccination. The number
of HPV types that may be suppressed directly by vaccination (HPV6,
11, 16 and 18) or potentially reduced via cross-protection by ≥1 of
the current vaccines (HPV31, 33, 45, 52 and 58) are presented in
Fig. 4. The prevalence of HPV16, 18
and 31 was considerably decreased in participants aged <23 years
old (HPV16, 28.57%; HPV18, 2.04%; HPV31, 6.12%), compared with
those aged 23–29 years old (HPV16, 45.71%; HPV18, 7.86%; HPV31,
22.86%). These results support that vaccination of young women is
important in preventing infections with HR-HPV. However, the
prevalence of certain HR-HPVs that are not affected by direct
vaccination or cross-protection remains quite high. The most
frequent HR-HPVs identified by the present study were HPV51
(13.83%), HPV56 (10.00%) and HPV66 (8.72%), which are labeled red
in Figs. 1 and 2.
Discussion
The present study evaluated the prevalence of
important HPV types in a local cohort of participants from Southern
Bavaria, and analyzed the HPV type distribution in participants
with abnormal cytological diagnostic findings, who were separated
by various age groups. It is well known that the distribution of
HPV types varies around the world (14), and it is hypothesized, based on a
pooled analysis from women with normal cytological findings in
various studies, that the prevalence of HPV16 is more prominent in
Europe and North America, compared with other regions of the world
(12,14,33,34). In
the current study, HPV infection was detected in 76.42% of
participants with an abnormal cytological finding. In total, 89.15%
of participants were infected with ≥1 HR-HPV, which is
significantly higher than the incidence previously reported in a
similar study conducted in Italy (68.90%) (35). However, it taken into consideration
the fact that the present study classified more HPV types as HR-HPV
than the aforementioned previous study did. In the present study,
an outdated HPV classification system with several probably HR-HPV
types identified that the HR-HPV prevalence was 81.50% in
participants <30 years of age. This is slightly lower than the
prevalence previously described for other regions in Germany
(88.40%) (36). However, using a
classification system based on novel biological evidence, which
revealed that more HPV types exhibit carcinogenicity than
previously acknowledged (10,33), the prevalence of HR-HPV types
identified by the present study increased to 91.53%.
In addition, the present study observed that the
prevalence of HPV16 and 18 in participants >30 years old was
33.81%, which is close to the prevalence of 34.50% identified in a
previous study (36). In participants
aged <30 years old, the highest incidence of HPV16 and 18 was
47.62%, which is higher than the prevalence of 37.40% identified in
a previous study on young women <30 years of age in Germany
(37). The decreasing prevalence of
HPV16 in older participants demonstrated by the current study has
previously been described in a cross-national study conducted prior
to the initiation of the HPV vaccination program (11).
It was previously demonstrated that MT HPV are more
common in women with cervical lesions, compared with women with
normal cervical cytology (38).
However, it has been observed that the presence of MT HPV does not
affect the severity of infection, and HPV infections are
independent of one another (39). In
the present study, 43.40% of all HPV+ participants were
infected with one type of HPV (ST HPV), whereas the majority of
participants exhibited MT HPV (56.60%). In patients who were<30
years old, the prevalence of MT HPV differed considerably from
participants aged 30–44, 45–59 and ≥60 years old. Thus, 64.02% of
participants aged <30 years presented MT HPV, compared with
51.61% of participants aged >29 years old. Similar
age-stratified proportions of ST and MT infections have been
previously described in other countries (38,40,41). This
may be explained by differences in the immune system, and
individuals may be capable of eradicating, at least in part, HPV
infections over time.
In addition to the prevalence of specific HPV types,
the present study analyzed the distribution of HPV types in
participants with abnormal cervical cytology. Since HPV may cause
the development of precancerous lesions (3), it was expected that the cytological
findings from HPV+ participants would be different to
findings from HPV− participants. HPV+
participants exhibited higher grades of neoplasms (LSIL and HSIL)
than HPV− participants. Furthermore, participants with
≥1 HR-HPV type were more often diagnosed with HSIL than
participants with LR-HPV. Similar observations concerning the
association of poor cytological diagnosis with carcinogenic HPV
types have been previously reported (36,38). In
all grades of cytological diagnosis, HPV16 was the most common HPV
type (ASC-US, 29.46%; LSIL, 29.58%; HSIL, 43.22%). In a similar
study conducted previously in Italy (35), HPV16 was the most common HPV type
observed in women with HSIL (27.30%). However, in the present
study, HPV51 and HPV56 had the highest incidence in participants
with ASC-US lesions or LSIL (24.82 and 19.17%, respectively).
Future studies on which HPV type may become more important in the
development of higher grade SILs as HPV vaccination coverage
increases may be of great interest.
In the current study, 80 participants with cervical
intraepithelial neoplasia were classified as grade CIN1 (22/80),
CIN2 (27/80) or CIN3 (31/80). As previously reported, the
incidences of particular HR-HPV infections were observed to
increase in association with the severity of the cervical
intraepithelial neoplasia in the present study (5,35,42,43). In
previous studies, each high-grade cervical neoplasia was attributed
to a single HR-HPV infection, and HPV16 and 31 were the predominant
HR-HPV types (44). These results are
similar to the cytological findings of the present study, which
demonstrated that the most frequent HPV types were HPV16 and
31.
Although the present study revealed that the highest
prevalence of HPV16 was in participants <30 years of age, it is
important to emphasize that a subgroup of the youngest women,
namely those aged <23 years old, exhibited a significantly lower
incidence, compared with women aged 23–29 years old (28.57 vs.
45.71%, respectively). The lower relative incidences of HPV16, 18
and 31 observed in the present study may be explained by a higher
rate of vaccinated participants aged <23 years old. The low
vaccination coverage in Germany (45,46) may be
attributed to the fact that women, who at the time of the present
study were in their mid-twenties, were excluded from the
recommendation for HPV vaccination, due to the restriction of the
vaccination program to girls aged 12–17 years (47). In a recent study with a group of
vaccinated women, the prevalence of HPV16 and 18 was significantly
lower in women aged 20–21 years old, compared with non-vaccinated
woman of the same age (48). The
present study observed that the incidence of HPV31 was also lower
in participants aged <23 years old, which may be due to the
cross-protective effect of the two vaccines (25,26). Since
women that are aged <23 years old are more often protected
against HPV16 and 18, as a result of vaccination programs that
consist of girls and young women primarily, the proportion of other
HPV types among infected women may gradually rise in future
populations. Considering the increasing impact of vaccination
against certain HPV types, it is important to acknowledge the
shifting distribution of non-vaccine HPV types, predominantly
HPV51, 53, 56 and 66, which were observed to have one of the
highest incidences among the participants in the present study. A
reduction in the incidence of HPV31, due to the cross-protective
effects of the current vaccines, may also decrease the incidence of
HPV16 and 18 with continuing or intensified vaccination programs.
Although the present study expects that the proportion of HR-HPV
types not covered by vaccination may increase in the near future,
it remains unclear whether the absolute number of precancerous
lesions and cervical cancer may also increase in the absence of
HPV16 and 18. In a recently reported meta-analysis (27), HPV16 and 18 infections decreased
between pre-vaccination and post-vaccination by 68% in girls aged
13–19 years countries with a vaccination coverage of ≥50% (49), and the prevalence of HPV 31, 33 and 45
were also reduced. This probable effect of cross-protection was not
reported for countries with a vaccination coverage of <50% in
girls aged <20-years-old, but a reduction in HPV16 and 18 was
observed. Recently, it was demonstrated in a large cohort study
that, due to the negative interaction of HPV16 with other HR-HPVs
(35, 51, 56 and 58), the removal of HPV16 may enable other HR-HPVs
to become more prevalent (50). It
was previously discussed that the relatively higher prevalence of
HPV16 may result in under detection of low-copy HPV co-infections,
particularly when the same amplification primers are used for HPV16
and other HPV types (50). Using an
epidemiological approach, Tota et al (51) hypothesized that eradicating
vaccine-targeted HPV16 and 18 enhances the chance of other HPV
types not targeted by the vaccine to occupy the ecological niche
created by the extinction of HPV16 and 18. Due to cross-protection
against other HPV types with current vaccines and the upcoming
implementation of novel multivalent vaccines against the majority
of the HR-HPV types diminishes the risk of type replacement
(52).
Consequently, adequate prevention strategies against
HPV depend on adapted HPV testing, which should cover all non
LR-HPV types. Notably, certain FDA-approved used HPV tests, such as
Hybrid Capture 2 (Digene Corporation, Gaithersburg, MD, USA), do
not cover all HR-HPV types, which were detected with the test
platform used in the current study, including
Papillocheck® (Greiner Bio One Ltd., Stonehouse, UK) and
Linear Array® (Roche Diagnostics GmbH). The present
results indicate that ~9% of samples with HR-HPV infections may
have been incorrectly reported as HPV− by the Hybrid
Capture 2 test. The information that eight HPV types formerly
classified as low-risk or probably high-risk are indeed HR-HPV
types increases the percentage of non-vaccine HR-HPV to higher
values than previously expected (10). Accordingly, it may become increasingly
important to monitor non-vaccine types using capable detection
methods in future studies, and to investigate the possible increase
of several HR-HPV types.
In participants from the present cohort study, high
incidences of HR-HPV types (89.15%) and a high incidence of HPV16
(32.98%) were observed. In all grades of cytological diagnosis
(ASC-US, LSIL and HSIL), HPV16 was the most common HPV type
identified. The prevalence of specific HR-HPV infections increased
with a more severe CIN grade. The number of findings of HR-HPV
types, which may be suppressed directly (HPV16 and 18) or via
cross-protection (HPV31) following vaccination, was considerably
lower in participants aged ≤22 years old (HPV16, 28.57%; HPV18,
2.04%; HPV31, 6.12%), compared with those aged 23–29 years old
(HPV16, 45.71%; HPV18, 7.86%; HPV31, 22.86%). Consequently, in
HPV+ women of 16–22 years of age, who are more likely to
be protected against certain HPV types by vaccination, compared
with older woman, the present study observed an alteration in the
incidences of HPV type. Consequently, future studies are required
to investigate the risk of non-vaccine HR-HPV types for cervical
cancer.
Acknowledgements
The authors of the present study would like to thank
Mrs. Andrea Becher for using the data included in her dissertation
thesis in the elaboration of the present manuscript. The authors
acknowledge the support of all the staff members of the Part Shared
Practice Molecular Pathology South Bavaria (Munich, Germany).
References
|
1
|
de Villiers EM, Fauquet C, Broker TR,
Bernard HU and zur Hausen H: Classification of papillomaviruses.
Virology. 324:17–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Villiers EM: Cross-roads in the
classification of papillomaviruses. Virology. 445:2–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiffman MH, Bauer HM, Hoover RN, Glass
AG, Cadell DM, Rush BB, Scott DR, Sherman ME, Kurman RJ, Wacholder
S, et al: Epidemiologic evidence showing that human papillomavirus
infection causes most cervical intraepithelial neoplasia. J Natl
Cancer Inst. 85:958–964. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kjaer SK, van den Brule AJ, Bock JE, Poll
PA, Engholm G, Sherman ME, Walboomers JM and Meijer CJ: Human
papillomavirus - the most significant risk determinant of cervical
intraepithelial neoplasia. Int J Cancer. 65:601–606. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bosch FX, Lorincz A, Muñoz N, Meijer CJ
and Shah KV: The causal relation between human papillomavirus and
cervical cancer. J Clin Pathol. 55:244–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
IARC: Latest World Cancer Statistics.
Global cancer burden rises to 14.1 million new cases in 2012:
Marked increase in breast cancers must be addressed. IARC/WHO Press
Release Dec 223. 2013.
|
|
8
|
Robert Koch-Institute: Cancer in Germany
2009–2010: Incidences and Trends. (9th). Robert Koch Institute and
the Association of Population-based Cancer Registries in Germany
(eds) (RKI-Hausdruckerei, Berlin). 2014.
|
|
9
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Halec G, Alemany L, Lloveras B, Schmitt M,
Alejo M, Bosch FX, Tous S, Klaustermeier JE, Guimerà N, Grabe N, et
al: Retrospective International Survey and HPV Time Trends Study
Group; Retrospective International Survey and HPV Time Trends Study
Group: Pathogenic role of the eight probably/possibly carcinogenic
HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J
Pathol. 234:441–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muñoz N, Bosch FX, de Sanjosé S, Herrero
R, Castellsagué X, Shah KV, Snijders PJ and Meijer CJ:
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group: Epidemiologic classification of human
papillomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Sanjose S, Quint WG, Alemany L, et al:
Retrospective International Survey and HPV Time Trends Study Group:
Human papillomavirus genotype attribution in invasive cervical
cancer: A retrospective cross-sectional worldwide study. Lancet
Oncol. 11:1048–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schiffman M, Clifford G and Buonaguro FM:
Classification of weakly carcinogenic human papillomavirus types:
Addressing the limits of epidemiology at the borderline. Infect
Agent Cancer. 4:82009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clifford GM, Smith JS, Plummer M, Muñoz N
and Franceschi S: Human papillomavirus types in invasive cervical
cancer worldwide: A meta-analysis. Br J Cancer. 88:63–73. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clifford GM, Gallus S, Herrero R, et al:
IARC HPV Prevalence Surveys Study Group: Worldwide distribution of
human papillomavirus types in cytologically normal women in the
International Agency for Research on Cancer HPV prevalence surveys:
A pooled analysis. Lancet. 366:991–998. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith JS, Lindsay L, Hoots B, Keys J,
Franceschi S, Winer R and Clifford GM: Human papillomavirus type
distribution in invasive cervical cancer and high-grade cervical
lesions: A meta-analysis update. Int J Cancer. 121:621–632. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lowy DR and Schiller JT: Prophylactic
human papillomavirus vaccines. J Clin Invest. 116:1167–1173. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
ECDC: Introduction of HPV vaccines in
European Union countries - an update. ECDC, Stockholm: 2012.
|
|
19
|
Robert Koch-Institute: Recommendations of
the Standing Committee on Vaccination (STIKO) at the Robert Koch
Institute/Effective: August 2014. Epid Bull. 34:305–339. 2014.
|
|
20
|
Paavonen J, Jenkins D, Bosch FX, et al:
HPV PATRICIA study group: Efficacy of a prophylactic adjuvanted
bivalent L1 virus-like-particle vaccine against infection with
human papillomavirus types 16 and 18 in young women: An interim
analysis of a phase III double-blind, randomised controlled trial.
Lancet. 369:2161–2170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paavonen J, Naud P, Salmerón J, Wheeler
CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC,
Skinner SR, et al: HPV PATRICIA Study Group: Efficacy of human
papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical
infection and precancer caused by oncogenic HPV types (PATRICIA):
Final analysis of a double-blind, randomised study in young women.
Lancet. 374:301–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
FUTURE II Study Group: Quadrivalent
vaccine against human papillomavirus to prevent high-grade cervical
lesions. N Engl J Med. 356:1915–1927. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lehtinen M, Paavonen J, Wheeler CM, et al:
Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade
3 or greater cervical intraepithelial neoplasia: 4-year
end-of-study analysis of the randomised, double-blind PATRICIA
trial. Lancet Oncol. 13:89–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tjalma WA: There are two prophylactic
human papillomavirus vaccines against cancer, and they are
different. J Clin Oncol. 33:964–965. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Malagón T, Drolet M, Boily MC, Franco EL,
Jit M, Brisson J and Brisson M: Cross-protective efficacy of two
human papillomavirus vaccines: A systematic review and
meta-analysis. Lancet Infect Dis. 12:781–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wheeler CM, Castellsagué X, Garland SM, et
al: HPV PATRICIA Study Group: Cross-protective efficacy of
HPV-16/18 AS04-adjuvanted vaccine against cervical infection and
precancer caused by non-vaccine oncogenic HPV types: 4-year
end-of-study analysis of the randomised, double-blind PATRICIA
trial. Lancet Oncol. 13:100–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Drolet M, Bénard É, Boily MC, Ali H,
Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JM, Cummings
T, Donovan B, et al: Population-level impact and herd effects
following human papillomavirus vaccination programmes: a systematic
review and meta-analysis. Lancet Infect Dis. 15:565–580. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Plummer M, Vaccarella S and Franceschi S:
Multiple human papillomavirus infections: the exception or the
rule? J Infect Dis. 203:891–893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wagner D: Munich nomenclature II for
gynaecologic cytodiagnosis. Acta Cytol. 34:900–901. 1990.
|
|
30
|
Solomon D, Davey D, Kurman R, Moriarty A,
O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T Jr, et
al: The 2001 Bethesda System: Terminology for reporting results of
cervical cytology. JAMA. 287:2114–2119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Apgar BS, Zoschnick L and Wright TC Jr:
The 2001 Bethesda System terminology. Am Fam Physician.
68:1992–1998. 2003.PubMed/NCBI
|
|
32
|
WHO: WHO Guidelines: Guidelines for
screening and treatment of precancerous lesions for cervical cancer
prevention. World Health Organization. Geneva: 2013.
|
|
33
|
Cannavo I, Benchetrit M, Loubatier C,
Michel G, Lemichez E and Giordanengo V: Characterization of a
cluster of oncogenic mutations in E6 of a human papillomavirus 83
variant isolated from a high-grade squamous intraepithelial lesion.
J Gen Virol. 92:2428–2436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Robert Koch Institute: Vaccination against
human papillomavirus among girls aged 12–17 years - recommendation
and explanatory statement. Epidemiol Bull. 12:97–103. 2007.(In
German).
|
|
35
|
Bruni L, Diaz M, Castellsagué X, Ferrer E,
Bosch FX and de Sanjosé S: Cervical human papillomavirus prevalence
in 5 continents: Meta-analysis of 1 million women with normal
cytological findings. J Infect Dis. 202:1789–1799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meloni A, Pilia R, Campagna M, Usai A,
Masia G, Caredda V and Coppola RC: Prevalence and molecular
epidemiology of human papillomavirus infection in italian women
with cervical cytological abnormalities. J Public Health Res.
3:1572014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Klug SJ, Hukelmann M, Hollwitz B, Düzenli
N, Schopp B, Petry KU and Iftner T: Prevalence of human
papillomavirus types in women screened by cytology in Germany. J
Med Virol. 79:616–625. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iftner T, Eberle S, Iftner A, Holz B,
Banik N, Quint W and Straube AN: Prevalence of low-risk and
high-risk types of human papillomavirus and other risk factors for
HPV infection in Germany within different age groups in women up to
30 years of age: An epidemiological observational study. J Med
Virol. 82:1928–1939. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Argyri E, Papaspyridakos S, Tsimplaki E,
Michala L, Myriokefalitaki E, Papassideri I, Daskalopoulou D,
Tsiaoussi I, Magiakos G and Panotopoulou E: A cross sectional study
of HPV type prevalence according to age and cytology. BMC Infect
Dis. 13:532013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Plummer M, Schiffman M, Castle PE,
Maucort-Boulch D and Wheeler CM: ALTS Group: A 2-year prospective
study of human papillomavirus persistence among women with a
cytological diagnosis of atypical squamous cells of undetermined
significance or low-grade squamous intraepithelial lesion. J Infect
Dis. 195:1582–1589. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Heard I, Tondeur L, Arowas L, Falguières
M, Demazoin MC and Favre M: Human papillomavirus types distribution
in organised cervical cancer screening in France. PLoS One.
8:e793722013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Resende LS, Rabelo-Santos SH, Sarian LO,
Alves Figueiredo RR, Ribeiro AA, Zeferino LC and Derchain S: A
portrait of single and multiple HPV type infections in Brazilian
women of different age strata with squamous or glandular cervical
lesions. BMC Infect Dis. 14:2142014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cobo F, Concha A and Ortiz M: Human
papillomavirus (HPV) type distribution in females with abnormal
cervical cytology. A correlation with histological study. Open
Virol J. 3:60–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
García-Espinosa B, Moro-Rodríguez E and
Alvarez-Fernández E: Genotype distribution of human papillomavirus
(HPV) in histological sections of cervical intraepithelial
neoplasia and invasive cervical carcinoma in Madrid, Spain. BMC
Cancer. 12:5332012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
van der Marel J, Berkhof J, Ordi J, Torné
A, Del Pino M, van Baars R, Schiffman M, Wentzensen N, Jenkins D
and Quint WG: Attributing oncogenic human papillomavirus genotypes
to high-grade cervical neoplasia: Which type causes the lesion? Am
J Surg Pathol. 39:496–504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kuznetsov AV, Müller RA, Ruzicka T,
Herzinger T and Kuznetsov L: Knowledge of sexually transmitted HPV
infection, genitoanal warts, cancer and their prevention among
young females after vaccine introduction in Germany. J Eur Acad
Dermatol Venereol. 27:1527–1534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rieck T, Feig M, Deleré Y and Wichmann O:
Utilization of administrative data to assess the association of an
adolescent health check-up with human papillomavirus vaccine uptake
in Germany. Vaccine. 32:5564–5569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Deleré Y, Remschmidt C, Leuschner J,
Schuster M, Fesenfeld M, Schneider A, Wichmann O and Kaufmann AM:
Human Papillomavirus prevalence and probable first effects of
vaccination in 20 to 25 year-old women in Germany: A
population-based cross-sectional study via home-based
self-sampling. BMC Infect Dis. 14:872014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Paul P and Fabio A: Literature review of
HPV vaccine delivery strategies: Considerations for school- and
non-school based immunization program. Vaccine. 32:320–326. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang Z, Cuzick J, Hunt WC and Wheeler CM:
Concurrence of multiple human papillomavirus infections in a large
US population-based cohort. Am J Epidemiol. 180:1066–1075. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tota JE, Ramanakumar AV, Jiang M, Dillner
J, Walter SD, Kaufman JS, Coutlée F, Villa LL and Franco EL:
Epidemiologic approaches to evaluating the potential for human
papillomavirus type replacement postvaccination. Am J Epidemiol.
178:625–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Safaeian M and Rodriguez AC: Invited
commentary: Multiple human papillomavirus infections and type
replacement-anticipating the future after human papillomavirus
vaccination. Am J Epidemiol. 180:1076–1081. 2014. View Article : Google Scholar : PubMed/NCBI
|