Introduction
Endometrial cancer (EC) is the most common
gynecological malignancy, with almost 200,000 cases diagnosed every
year, and is a major cause of morbidity and mortality for women
worldwide (1,2). EC has been broadly classified into two
types, termed type I and type II, which possess different
etiologies and patient survival rates (3). Type I EC includes endometrioid
adenocarcinomas that represent 80–90% of EC arising from atypical
endometrial hyperplasia with unopposed estrogen exposure (4,5). The
remaining 10–20% of EC are classified as type II, including
papillary serous EC, clear cell EC and other histological variants.
Type II ECs are mostly poorly-differentiated, estrogen-independent
and aggressive, with a ~50% recurrence rate and mortality rate of
50–60% of patients with stage I–II disease (6). The majority of EC patients are diagnosed
at an early stage and are treated by surgery with favorable
outcomes. However, certain women are diagnosed at a late stage or
with type II EC, and often suffer from worse outcomes with limited
adjuvant treatment options and low survival rates, for example,
patients with low-grade (well-differentiated) type I ECs have a
5-year survival rate of ~90%, whereas patients with high-grade
(poorly-differentiated) type I ECs have a 5-year survival rate of
45–77% (6–10). By contrast, patients with type II ECs,
including papillary serous and clear cell ECs, have a 5-year
survival rate of 35–53% (4,6).
Previous studies have identified certain risk
factors for EC, such as nulliparity, early age at menarche, late
age at menopause, unopposed estrogen treatment, hereditary
non-polyposis colorectal cancer and polycystic ovarian syndrome
(11,12). However, EC pathogenesis remains poorly
understood. Additional attention has been paid to the tumor
microenvironment and mechanisms of immune evasion. For example,
programmed cell death protein 1 (PD-1) has emerged as a key player
in tumor immune evasion. PD-1 is a member of the B7/cluster of
differentiation (CD)28 family, which is an immune check-point
receptor expressed on T cells, natural killer cells, monocytes and
B cells (13–15). The ligands for PD-1, namely,
programmed death ligand 1 (PD-L1) and programmed death ligand 2
(PD-L2), interact with PD-1 to suppress T cell functions and induce
tumor immune evasion (16). PD-L1 is
expressed by tumor cells and tumor-infiltrating immune cells,
including macrophages, dendritic cells and T cells (17). PD-L1 and PD-L2 mRNAs are found in
human heart, placenta, spleen, lymph node and thymus tissues.
Additionally, PD-L2 mRNA, but not PD-L1 mRNA, is found in human
lung, liver, smooth muscle and pancreas tissues (18). In the tumor microenvironment, the
PD-1/PD-L1/PD-L2 immune inhibitory pathway plays a pivotal role in
the ability of tumor cells to evade the host's immune system by
inhibiting cytotoxic T lymphocyte proliferation, inducing apoptosis
of infiltrating T cells, and increasing the amount of regulatory T
cells (19,20). Based on the understanding of the
immunosuppressive function of the PD-1/PD-L1/PD-L2 axis, multiple
clinical trials have demonstrated that antibodies against PD-1 or
PD-L1 are effective therapeutics to block immune evasion and induce
tumor regression in patients with melanoma, non-small cell lung
cancer, and renal-cell cancer (21–27).
Therefore, the US Food and Drug Administration (FDA) has approved
the anti-PD-1 antibodies pembrolizumab (Keytruda®; Merck
& Co., Inc., Kenilworth, NJ, USA) (28) and nivolumab (Opdivo®;
Bristol-Myers Squibb, Princeton, NJ, USA) (29) for the treatment of patients with
unresectable or metastatic melanoma. Nivolumab has also been
approved for the treatment of patients with metastatic squamous
non-small cell lung cancer, with progression on or after
platinum-based chemotherapy (30).
The FDA has assigned a priority review designation to pembrolizumab
as a treatment for patients with advanced non-small cell lung
cancer (31). Since the anti-PD-L1
antibody MPDL3280A (Genentech, Inc., South San Francisco, CA, USA)
showed responsive rates of 13–26% in solid tumors, including
non-small cell lung cancer (17), the
FDA has assigned MPDL3280A a breakthrough therapy designation for
the treatment of PD-L1-positive non-small cell lung cancer that has
progressed during or subsequent to platinum-based chemotherapy, as
well as a targeted therapy for patients with epidermal growth
factor receptor-positive or anaplastic lymphoma kinase-positive
tumors, pending the outcomes of ongoing phase II and III trials
(32).
There have been an extremely limited number of
studies on PD-1 and EC (33,34), and neither of the previous two studies
has addressed the association between PD-1 expression and
clinicopathological characteristics of EC patients. Therefore, the
aim of the present study was to assess expression of PD-1, PD-L1
and PD-L2 in EC and to compare the expression of these proteins
with clinicopathological characteristics. Immunohistochemical (IHC)
staining was performed in 35 normal endometrium tissues and 75 EC
tissues. It was found that PD-1 expression was significantly
increased in EC than in normal endometrium and that expression of
PD-1, PD-L1 and PD-L2 in the tumor-infiltrating immune cells was
associated with differentiation status and histological type of
EC.
Materials and methods
Human endometrial tissue samples
In total, 35 samples of normal endometrium and 75
samples of ECs that were archived from surgeries performed between
January 2012 and December 2014, in the Department of Obstetrics and
Gynecology at Shijiazhuang Maternal and Child Health Care Hospital
and the Department of Obstetrics and Gynecology at Shijiazhuang
First Hospital (Shijiazhuang, Hebei, China) were retrospectively
collected. The samples were formalin-fixed and paraffin-embedded
tissue blocks and the pathological diagnoses were re-confirmed by a
pathologist. The present study was approved by the Institutional
Review Boards of Shijiazhuang Maternal and Child Health Care
Hospital and Shijiazhuang First Hospital. The procedures to obtain
human endometrial tissues were in accordance with the Ethical
Principles for Medical Research Involving Human Subjects, as
formulated in the World Medical Association Declaration of Helsinki
(2008 revision). The clinicopathological characteristics of the
patients were summarized in Table
I.
 | Table I.Clinicopathological characteristics of
patients. |
Table I.
Clinicopathological characteristics of
patients.
| Characteristics | Number |
|---|
| Normal
endometrium | 35 |
| Age, mean
± SD (years) | 45.3±5.6 |
| Endometrial
cancer | 75 |
| Age,
mean ± SD (years) | 57.3±10.1 |
| <60
years | 45 |
| ≥60
years | 30 |
|
Differentiation |
|
|
Well | 37 |
|
Moderate | 23 |
|
Poor | 15 |
| Stage |
|
| I | 62 |
| II | 4 |
|
III | 9 |
| Histological
type |
|
|
Endometrioid | 63 |
|
Papillary serous | 11 |
| Clear
cell | 1 |
| Vascular
invasion |
|
|
Yes | 7 |
| No | 68 |
Immunohistochemistry
Tissue sections (4-µm thick) were baked at 60°C for
60 min, deparaffinized in xylene and rehydrated through graded
ethanol solutions to water. The antigens were retrieved by heating
the tissue sections in 0.01 M ethylenediaminetetraacetic acid
buffer at 95°C for 5 min and then cooling down to room temperature
for 20 min. Endogenous peroxidase activity was blocked by 0.3%
H2O2 for 5 min. Non-specific binding was
blocked with 1.5% normal goat or horse serum (VECTASTAIN Elite ABC
kit; Vector Laboratories, Burlingame, CA, USA). The tissue sections
were incubated with primary antibodies in a humid chamber at 4°C
overnight. Rabbit anti-human PD-L1 polyclonal antibodies (dilution,
1:400; catalog no., ab58810; Abcam, Cambridge, MA, USA), rabbit
anti-human PD-L2 polyclonal antibodies (dilution, 1:800; catalog
no., SAB3500395-100UG; Sigma-Aldrich, St. Louis, MO, USA), and
rabbit anti-human CD279 (PD-1) affinity-purified and validated
polyclonal antibodies (dilution, 1:600; catalog no., PIPA520351;
Fisher Scientific; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) were used as the primary antibodies. Subsequent to being
washed 3 times in phosphate-buffered saline, the tissue sections
were incubated with goat anti-rabbit polyclonal secondary
antibodies (dilution, 1:200; catalog no., PK-6101; VECTASTAIN Elite
ABC kit; Vector Laboratories) for 2 h at room temperature. The
color was developed using 3,3′-diaminobenzidine substrate kit
(Vector Laboratories) following the manufacturer's protocol. The
tissue sections were then counterstained with hematoxylin. The
tissue sections that had previously stained positively for PD-1,
PD-L1 and PD-L2 in a pilot study were used as positive controls and
the tissue sections stained with non-immune serum (Vector
Laboratories) acted as negative controls. Positive staining for
PD-1, PD-L1 and PD-L2 appeared as brown particles at the
cytoplasmic membrane or in the cytoplasm. Under microscopy, 5
representative high-power fields (×400 magnification) per tissue
section were randomly selected and evaluated by two investigators,
who were blinded to the clinicopathological data. An average of the
scores obtained by the two examiners was used to represent each
case. A two-score system based on a proportion score and an
intensity score was used, as previously described by Allred et
al (35). The proportion scores
indicated the proportion of positive staining: 0, None; 1, less
than one-hundredth; 2, one-hundredth to one-tenth; 3, one-tenth to
one-third; 4, one-third to two-thirds; and 5, greater than
two-thirds. The intensity scores represented the estimated average
staining intensity of positive staining: 0, None; 1, weak; 2,
intermediate; and 3, strong. The overall scores (Allred scores)
were the sum of the proportion score and intensity score of each
case (range, 0–8).
Statistical analysis
Statistical analysis was performed using SPSS
version 16.0 for Windows (SPSS, Inc., Chicago, IL, USA). Patient
age was expressed as mean ± standard deviation. The comparison of
clinicopathological characteristics between different groups was
performed using the χ2 test. Spearman's correlation
coefficient was calculated to reveal the correlation between PD-1
scores and PD-L1 or PD-L2 scores. P<0.05 was considered to
indicate a statistically significant difference.
Results
PD-1, PD-L1 and PD-L2 are expressed in
endometrial cancer
IHC staining for PD-1, PD-L1 and PD-L2 was performed
using 35 normal endometrium tissues and 75 EC tissues.
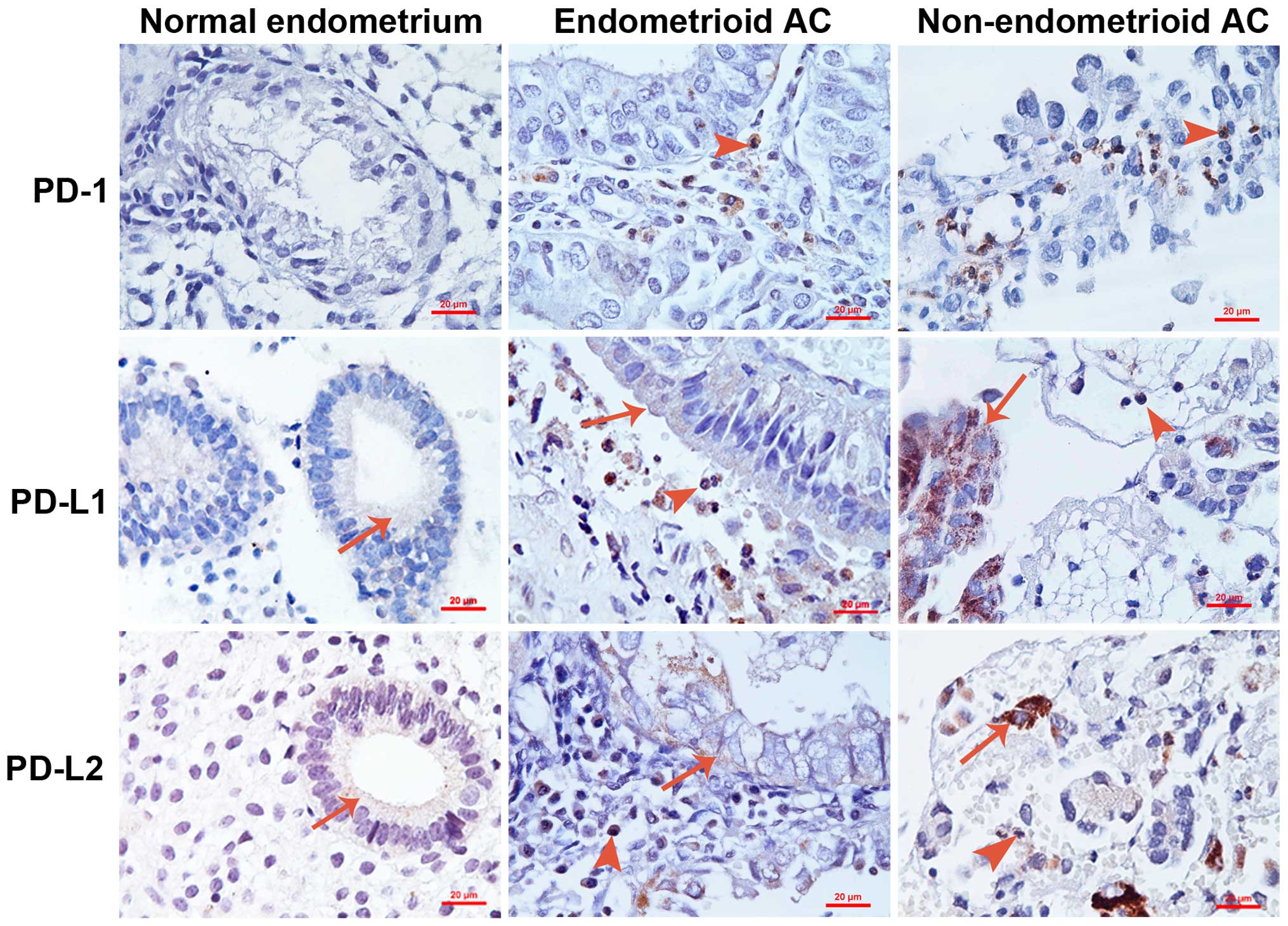
Representative photomicrographs of the stained samples are shown in
Fig. 1. Any sample was defined as
having positive staining if the Allred score was ≥1 and any sample
was defined as having negative staining if the Allred score was 0.
It was found that all normal endometrial samples were negative for
PD-1 expression, whereas 61.3% of ECs were positive for PD-1
staining (Table II; P<0.001). In
total, 14.3% of normal endometrial samples were positive for PD-L1
staining, while 17.3% of ECs were positive for PD-L1 staining
(Table II; P=0.687). In addition,
20.0% of normal endometrial samples were positive for PD-L2
staining, while 37.3% of ECs were positive for PD-L2 staining
(Table II; P=0.069). It was found
that PD-1 was only expressed in the tumor-infiltrating immune
cells, but not in the tumor cells (Fig.
1). By contrast, PD-L1 and PD-L2 were expressed in the tumor
cells and infiltrating immune cells (Fig.
1).
 | Table II.Expression of PD-1, PD-L1 and PD-L2
in normal endometrium and EC. |
Table II.
Expression of PD-1, PD-L1 and PD-L2
in normal endometrium and EC.
|
|
| PD-1 | PD-L1 | PD-L2 |
|---|
|
|
|
|
|
|
|---|
| Group | n | Positive, n
(%) | P-value | Positive, n
(%) | P-value | Positive, n
(%) | P-value |
|---|
| Normal | 35 | 0 (0.0) | <0.001 | 5
(14.3) | 0.687 | 7
(20.0) | 0.069 |
| EC | 75 | 46 (61.3) |
| 13 (17.3) |
| 28 (37.3) |
|
PD-1 expression is associated with
differentiation status and histological type of EC
As shown in Table
III, the rate of positive PD-1 staining was 73.7% in the poorly
and moderately-differentiated ECs, which was significantly
increased compared with the well-differentiated ECs (48.6%;
P=0.026). The rate of positive PD-1 staining was 100% in the
non-endometrioid ECs, including 11 papillary serous ECs and 1 clear
cell EC, which was significantly increased compared with the
endometrioid ECs (54.0%; P=0.006; Table
III). However, PD-1 expression was not different among patients
with different ages, clinical stages or statuses of vascular
invasion in the tumors (Table
III).
 | Table III.PD-1 expression and
clinicopathological characteristics of patients with endometrial
cancer. |
Table III.
PD-1 expression and
clinicopathological characteristics of patients with endometrial
cancer.
|
Characteristics | n | Positive, n
(%) | P-value |
|---|
| Patients | 75 | 46 (61.3) | – |
| Age |
|
|
|
| <60
years | 45 | 26 (57.8) | 0.439 |
| ≥60
years | 30 | 20 (66.7) |
|
|
Differentiation |
|
|
|
|
Well | 37 | 18 (48.6) | 0.026 |
|
Poor/moderate | 38 | 28 (73.7) |
|
| Stage |
|
|
|
| I | 62 | 36 (58.1) | 0.204 |
|
II/III | 13 | 10 (76.9) |
|
| Histological
type |
|
|
|
|
Endometrioid | 63 | 34 (54.0) | 0.006 |
|
Non-endometrioid | 12 | 12
(100.0) |
|
| Vascular
invasion |
|
|
|
|
Yes | 7 | 4
(57.1) | 1.000 |
| No | 68 | 42 (61.8) |
|
PD-L1 expression in the
tumor-infiltrating immune cells is associated with the
differentiation status and histological type of EC
As shown in Table IV,
the rate of positive PD-L1 staining in the tumor-infiltrating
immune cells was ~73.7% in the poorly and moderately-differentiated
ECs, which was significantly increased compared with the
well-differentiated ECs (45.9%; P=0.014). The rate of positive
PD-L1 staining in the tumor-infiltrating immune cells was 100% in
the non-endometrioid ECs, which was significantly increased
compared with in the endometrioid ECs (52.4%; P=0.006; Table IV). However, PD-L1 expression in the
tumor-infiltrating immune cells was not different among patients
with different ages, clinical stages or statuses of vascular
invasion in the tumors (Table IV).
In addition, PD-L1 expression in the tumor cells was not
significantly different among patients with different ages,
differentiation statuses, clinical stages, histological types or
statuses of vascular invasion in the tumors (Table IV).
 | Table IV.Association between programmed death
ligand 1 expression and clinicopathological characteristics of
endometrial cancer patients. |
Table IV.
Association between programmed death
ligand 1 expression and clinicopathological characteristics of
endometrial cancer patients.
|
|
| Tumor cells | Immune cells |
|---|
|
|
|
|
|
|---|
|
Characteristics | n | Positive, n
(%) | P-value | Positive, n
(%) | P-value |
|---|
| Patients | 75 | 13 (17.3) | – | 45 (60.0) | – |
| Age |
|
| 0.681 |
| 0.149 |
| <60
years | 45 | 7
(15.6) |
| 24 (53.3) |
|
| ≥60
years | 30 | 6
(20.0) |
| 21 (70.0) |
|
|
Differentiation |
|
| 0.141 |
| 0.014 |
|
Well | 37 | 4
(10.8) |
| 17 (45.9) |
|
|
Poor/moderate | 38 | 9
(23.7) |
| 28 (73.7) |
|
| Stage |
|
| 0.315 |
| 0.171 |
| I | 62 | 9
(14.5) |
| 35 (56.6) |
|
|
II/III | 13 | 4
(30.8) |
| 10 (77.0) |
|
| Histological
type |
|
| 0.237 |
| 0.006 |
|
Endometrioid | 63 | 9
(14.3) |
| 33 (52.4) |
|
|
Non-endometrioid | 12 | 4
(33.3) |
| 12
(100.0) |
|
| Vascular
invasion |
|
| 0.764 |
| 0.427 |
|
Yes | 7 | 2
(28.6) |
| 3
(42.9) |
|
| No | 68 | 11 (16.2) |
| 42 (61.8) |
|
PD-L2 expression in the
tumor-infiltrating immune cells is associated with the
differentiation status and histological type of EC
As shown in Table V,
the rate of positive PD-L2 staining in the tumor-infiltrating
immune cells was 73.7% in the poorly and moderately-differentiated
ECs, which was significantly higher than in the well-differentiated
ECs (51.4%; P=0.046). The rate of positive PD-L2 staining in the
tumor-infiltrating immune cells was 100% in the non-endometrioid
ECs, which was significantly higher than in the endometrioid ECs
(55.6%; P=0.003; Table V). However,
PD-L2 expression in the tumor-infiltrating immune cells was not
significantly different among patients with different ages,
clinical stages or statuses of vascular invasion in the tumors
(Table V). Additionally, PD-L2
expression in the tumor cells was not significantly different among
patients with different ages, differentiation statuses, clinical
stages, histological types or statuses of vascular invasion in the
tumors (Table V).
 | Table V.Association between programmed death
ligand 2 expression and clinicopathological characteristics of
endometrial cancer patients. |
Table V.
Association between programmed death
ligand 2 expression and clinicopathological characteristics of
endometrial cancer patients.
|
|
| Tumor cells | Immune cells |
|---|
|
|
|
|
|
|---|
|
Characteristics | n | Positive, n
(%) | P-value | Positive, n
(%) | P-value |
|---|
| Patients | 75 | 28 (37.3) | – | 47 (62.7) | – |
| Age |
|
| 0.380 |
| 0.119 |
| <60
years | 45 | 15 (33.3) |
| 25 (55.6) |
|
| ≥60
years | 30 | 13 (43.3) |
| 22 (73.3) |
|
|
Differentiation |
|
| 0.698 |
| 0.046 |
|
Well | 37 | 13 (35.1) |
| 19 (51.4) |
|
|
Poor/moderate | 38 | 15 (39.5) |
| 28 (73.7) |
|
| Stage |
|
| 1.000 |
| 0.393 |
| I | 62 | 23 (37.1) |
| 37 (59.7) |
|
|
II/III | 13 | 5
(38.5) |
| 10 (76.9) |
|
| Histological
type |
|
| 0.188 |
| 0.003 |
|
Endometrioid | 63 | 21 (33.3) |
| 35 (55.6) |
|
|
Non-endometrioid | 12 | 7
(58.3) |
| 12
(100.0) |
|
| Vascular
invasion |
|
| 0.095 |
| 0.413 |
|
Yes | 7 | 5
(71.4) |
| 3
(60.0) |
|
| No | 68 | 23 (33.8) |
| 44 (64.7) |
|
PD-1 expression is associated with
PD-L1 and PD-L2 expression in the tumor cells and infiltrating
immune cells
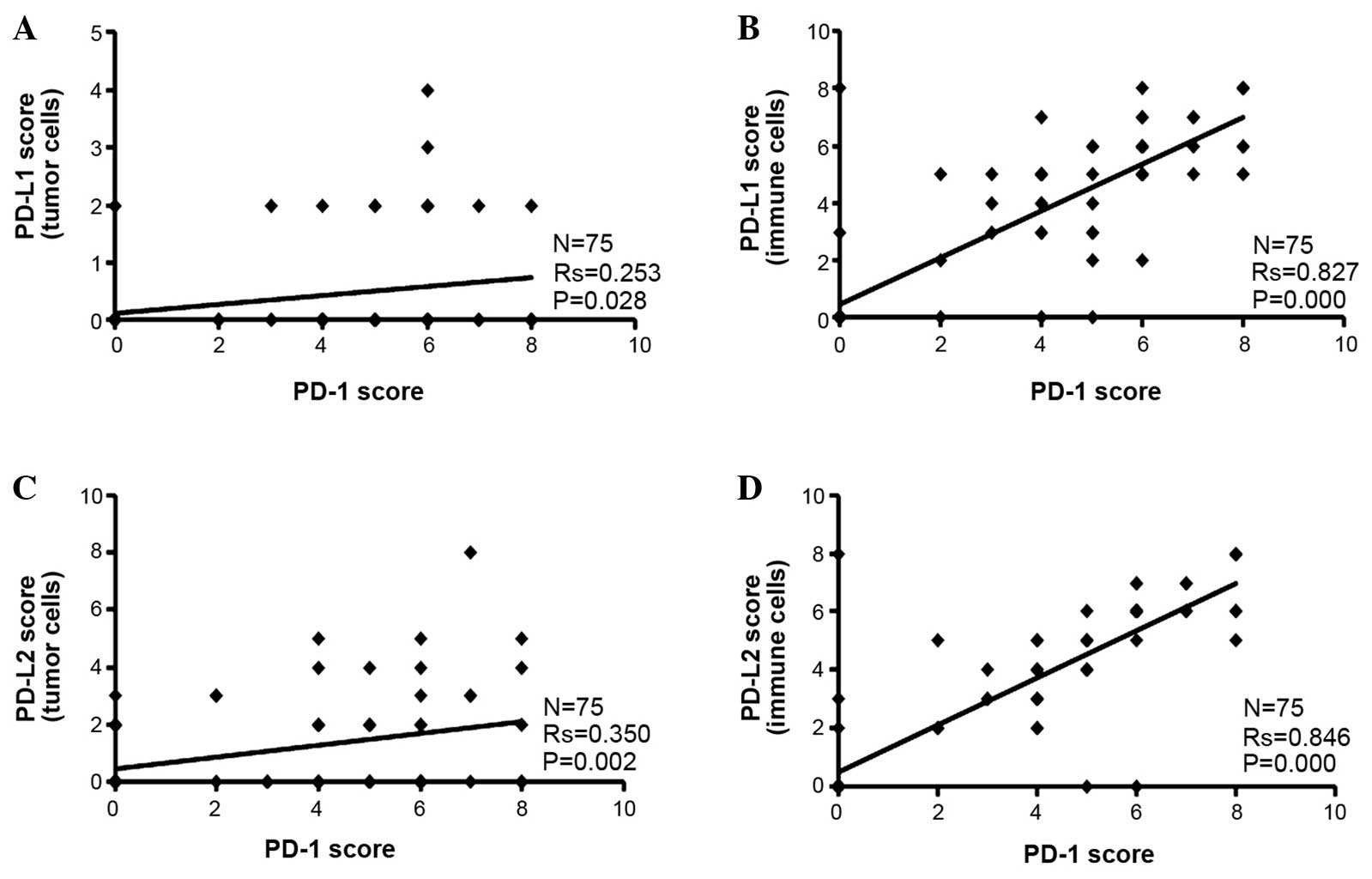
It was found that the PD-L1 and PD-L2 Allred
staining scores were higher in the tumor-infiltrating immune cells
than the tumor cells alone (Fig. 2).
Since PD-1 was only expressed in the tumor-infiltrating immune
cells, Spearman's correlation analysis was performed between PD-1
scores and PD-L1 or PD-L2 scores. It was found that the PD-1 score
was positively associated with the PD-L1 or PD-L2 score in the
tumor cells and infiltrating immune cells (Fig. 2).
Discussion
The present study found that 61.3% of human ECs
stained positive for PD-1, which was almost exclusively found in
the tumor-infiltrating immune cells. By contrast, PD-1 was not
expressed in the tumor cells or normal endometrial tissues. It was
also found that 14.3% of normal endometria and 17.3% of ECs were
positive for PD-L1 expression, while 20.0% of normal endometria and
37.3% of ECs were positive for PD-L2 expression, though there was
no statistically significant difference between normal endometrium
and EC. Vanderstraeten et al (34) reported that PD-1 expression was
present in all 15 cases of normal endometria, PD-L1 expression was
present in 81% of 16 cases of normal endometria, and PD-L2
expression was present in 47% of 15 cases of normal endometria. In
primary ECs, Vanderstraeten et al found that PD-1 expression
was present in 100% of 30 cases of ECs, PD-L1 expression was
present in 83% of 29 cases of ECs, and PD-L2 expression was present
in 40% of 30 cases of ECs. The discrepancy between the present
study and the study by Vanderstraeten et al (34) may be due to different antibodies and
IHC protocols used. It may also be due to the different EC patients
studied. For example, the study by Vanderstraeten et al
included 28 cases of papillary serous and clear cell ECs who were
compared with 16 cases of endometrioid ECs (34). By contrast, the present study compared
12 cases of papillary serous and clear cell ECs with 63 cases of
endometrioid ECs. Therefore, it is expected that Vanderstraeten
et al should find more PD-1, PD-L1 and PD-L2-positive cases
than the present study, since the present study showed that
non-endometrioid ECs were 100% positive for PD-1, PD-L1 and PD-L2
expression, if we did not distinguish between the tumor cells and
infiltrating immune cells. In addition, Howitt et al
(33) reported that PD-1 was
overexpressed in tumor-infiltrating lymphocytes of 81% of
polymerase ε-mutated ECs and 28% of microsatellite-instable ECs. In
peritumoral lymphocytes, PD-1 was overexpressed in 90% of
polymerase ε-mutated ECs and 28% of microsatellite-instable ECs.
PD-L1 expression was infrequently noted in the tumor cells, but was
common in intraepithelial immune cells and more frequent in
polymerase ε-mutated ECs (39%) than in microsatellite-instable ECs
(13%; P=0.02) (33). The present
study also showed that PD-L1 and PD-L2 expression was less
frequently found in the tumor cells than in the tumor-infiltrating
immune cells, which is consistent with the findings of Howitt et
al (33).
The present study went beyond the previous two
studies (33,34) to show the correlation between the
expression of PD-1, PD-L1 and PD-L2 and clinicopathological
characteristics of ECs. It was shown that PD-1 expression in the
tumor-infiltrating immune cells was more frequently found in the
moderately and poorly-differentiated ECs and non-endometrioid (type
II) ECs than in the well-differentiated ECs and endometrioid (type
I) ECs. Similarly, it was shown that PD-L1 and PD-L2 expression in
the tumor-infiltrating immune cells was more frequently found in
the moderately and poorly-differentiated ECs and non-endometrioid
(type II) ECs than in the well-differentiated ECs and endometrioid
(type I) ECs. It is known that moderately and poorly-differentiated
ECs and type II ECs have a lower 5-year survival rate than the
well-differentiated ECs and type I ECs (6–10).
Therefore, the present findings suggest that more frequent
expression of PD-1, PD-L1 and PD-L2 in the moderately and
poorly-differentiated ECs and type II ECs may cause
immunosuppression to favor tumor growth, thus negatively affecting
the patient's survival. Future studies may address whether
expression of PD-1, PD-L1 and PD-L2 may be used as an independent
predictor of patient survival, once the follow-up data for the
present patients are available. The follow-up data are not
available at this time due to the short period subsequent to
inclusion of the cases. By contrast, it is reasonable to speculate
that the moderately and poorly-differentiated ECs and type II ECs
may be more sensitive to anti-PD-1 or anti-PD-L1 antibodies-based
therapies, since it has been demonstrated in clinical trials that
PD-L1-positive tumors tend to be more responsive to anti-PD-1 or
anti-PD-L1 therapies (17,26).
In summary, the present study demonstrates that the
expression of PD-1, PD-L1 and PD-L2 is associated with moderately
and poorly-differentiated endometrial cancer and type II
endometrial cancer. Frequent expression of the PD-1/PD-L1/PD-L2
axis in these subgroups of endometrial cancers may be potentially
correlated with their aggressive progression and poor patient
survival. The present findings indicate a possible improved outcome
for future treatment with Keytruda and Opdivo in these subgroups of
endometrial cancers with frequent expression of the
PD-1/PD-L1/PD-L2 axis.
Acknowledgements
Z.Y. was supported partially by National Institutes
of Health (grant nos. R01CA174714 and P20GM103518), Department of
Defense (grant nos. W81XWH-14-1-0050, W81XWH-14-1-0149,
W81XWH-14-1-0458 and W81XWH-15-1-0444), Developmental Fund of
Tulane Cancer Center, Louisiana Cancer Research Consortium Fund,
and Tulane's Institute of Integrated Engineering for Health and
Medicine. Z.M., Z.C. and S.Y. were supported by the Service Center
for Experts and Scholars of Hebei Province, China to pursue
research in the USA. L.L. was partially supported by National
Natural Science Foundation of China (grant nos. NSFC 81172236 and
NSFC 81372505) and Key Science and Technology Program of Sichuan
Province, China (grant no. 2013SZ0005). J.M. was a visiting scholar
at Tulane University School of Medicine sponsored by the China
Scholarship Council (grant no. 201406240145).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Loos AH, Oostindier M and
Weiderpass E: Geographic and temporal variations in cancer of the
corpus uteri: Incidence and mortality in pre- and postmenopausal
women in Europe. Int J Cancer. 117:123–131. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mendivil A, Schuler KM and Gehrig PA:
Non-endometrioid adenocarcinoma of the uterine corpus: A review of
selected histological subtypes. Cancer Control. 16:46–52.
2009.PubMed/NCBI
|
|
5
|
Rasool N, Fader AN, Seamon L, Neubauer NL,
Shahin FA, Alexander HA, Moore K, Moxley K, Secord AA, Kunos C, et
al: Stage I, grade III endometrioid adenocarcinoma of the
endometrium: An analysis of clinical outcomes and patterns of
recurrence. Gynecol Oncol. 116:10–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goff BA, Kato D, Schmidt RA, Ek M, Ferry
JA, Muntz HG, Cain JM, Tamimi HK, Figge DC and Greer BE: Uterine
papillary serous carcinoma: Patterns of metastatic spread. Gynecol
Oncol. 54:264–268. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hidaka T, Kato K, Yonezawa R, Shima T,
Nakashima A, Nagira K, Nakamura T and Saito S: Omission of
lymphadenectomy is possible for low-risk corpus cancer. Eur J Surg
Oncol. 33:86–90. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soslow RA, Bissonnette JP, Wilton A,
Ferguson SE, Alektiar KM, Duska LR and Oliva E: Clinicopathologic
analysis of 187 high-grade endometrial carcinomas of different
histologic subtypes: Similar outcomes belie distinctive biologic
differences. Am J Surg Pathol. 31:979–987. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petignat P, Usel M, Gauthier P, Popowski
Y, Pelte MF, Bouchardy C and Verkooijen HM: Outcome of uterine
clear cell carcinomas compared to endometrioid carcinomas and
poorly-differentiated endometrioid carcinomas. Eur J Gynaecol
Oncol. 29:57–60. 2008.PubMed/NCBI
|
|
10
|
Hamilton CA, Cheung MK, Osann K, Chen L,
Teng NN, Longacre TA, Powell MA, Hendrickson MR, Kapp DS and Chan
JK: Uterine papillary serous and clear cell carcinomas predict for
poorer survival compared to grade III endometrioid corpus cancers.
Br J Cancer. 94:642–646. 2006.PubMed/NCBI
|
|
11
|
Brinton LA, Berman ML, Mortel R, Twiggs
LB, Barrett RJ, Wilbanks GD, Lannom L and Hoover RN: Reproductive,
menstrual, and medical risk factors for endometrial cancer: Results
from a case-control study. Am J Obstet Gynecol. 167:1317–1325.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McPherson CP, Sellers TA, Potter JD,
Bostick RM and Folsom AR: Reproductive factors and risk of
endometrial cancer. The iowa women's health study. Am J Epidemiol.
143:1195–1202. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of pd-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992.PubMed/NCBI
|
|
14
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: Pd-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharpe AH and Freeman GJ: The b7-cd28
superfamily. Nat Rev Immunol. 2:116–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blank C, Gajewski TF and Mackensen A:
Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T
cells as a mechanism of immune evasion: Implications for tumor
immunotherapy. Cancer Immunol Immunother. 54:307–314. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Latchman Y, Wood CR, Chernova T, Chaudhary
D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al:
PD-L2 is a second ligand for PD-1 and inhibits T cell activation.
Nat Immunol. 2:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Day CL, Kaufmann DE, Kiepiela P, Brown JA,
Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C,
et al: PD-1 expression on HIV-specific T cells is associated with
T-cell exhaustion and disease progression. Nature. 443:350–354.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okazaki T and Honjo T: PD-1 and PD-1
ligands: From discovery to clinical application. Int Immunol.
19:813–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lipson EJ, Sharfman WH, Drake CG, Wollner
I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, et al: Durable
cancer regression off-treatment and effective reinduction therapy
with an anti-PD-1 antibody. Clin Cancer Res. 19:462–468. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robert C, Schachter J, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med.
372:2521–2532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
FDA News Release: FDA approves Keytruda
for advanced melanoma. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm412802.htmAccessed.
January. 2016
|
|
29
|
FDA News Release: FDA approves Opdivo to
treat advanced form of kidney cancer. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm473971.htmAccessed.
January. 2016
|
|
30
|
Nivolumab (Opdivo). http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm436566.htmAccessed.
January. 2016
|
|
31
|
http://www.onclive.com/web-exclusives/fda-grants-priority-review-to-pembrolizumab-in-lung-cancerAccessed.
January. 2016
|
|
32
|
Cha E, Wallin J and Kowanetz M: PD-L1
inhibition with MPDL3280A for solid tumors. Semin Oncol.
42:484–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Howitt BE, Shukla SA, Sholl LM,
Ritterhouse LL, Watkins JC, Rodig S, Stover E, Strickland KC,
D'Andrea AD, Wu CJ, et al: Association of polymerase e-mutated and
microsatellite-instable endometrial cancers with neoantigen load,
number of tumor-infiltrating lymphocytes, and expression of PD-1
and PD-L1. JAMA Oncol. 1:1319–1323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vanderstraeten A, Luyten C, Verbist G,
Tuyaerts S and Amant F: Mapping the immunosuppressive environment
in uterine tumors: Implications for immunotherapy. Cancer Immunol
Immunother. 63:545–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allred DC, Clark GM, Elledge R, Fuqua SA,
Brown RW, Chamness GC, Osborne CK and McGuire WL: Association of
p53 protein expression with tumor cell proliferation rate and
clinical outcome in node-negative breast cancer. J Natl Cancer
Inst. 85:200–206. 1993. View Article : Google Scholar : PubMed/NCBI
|