Introduction
Liver cancer accounts for >5% of all cancers
worldwide and is the third most common cause of mortality globally,
being the fifth most common cause for men and eighth most common
cause for women (1). Since the
majority of patients with liver cancer are diagnosed at the
end-stage of liver dysfunction, the mortality rate and incidence of
liver cancer are similar (2). The
course of malignant cancer transformation involves the accumulation
of mutations and aberrations among the genes that govern
proliferation and apoptosis in cells, leading to apoptotic
avoidance, neo-angiogenesis and metastatic potential (3). During the multistep development process
in mature hepatocytes, genetic alterations in the hepatocytic
microenvironment lead to necrosis, inflammation and re-generation,
resulting in the cell populations becoming dysplastic nodules that
eventually develop into a prominent cancer (4). Currently, surgical resection is one
treatment modality used to treat liver cancer that decreases the
mortality rate to <5%, provided that liver function is
maintained at satisfactory levels (5). Inhibitors of tyrosine kinases provide
opportunities for curative resection when administered as a
preoperative neoadjuvant therapy (6).
Chemotherapeutic drugs, such as cyclophosphamide and busulphan,
which are used as a conditioning regimen, have immunosuppressive
and myeloablative effects and associated complications, including
mucositis, liver toxicity and hemorrhagic cystitis (7,8). In
addition, the chemotherapy may increase lipid peroxidation (LPO)
and malondialdehyde levels, leading to oxidative stress in patients
(9). Superoxide dismutase (SOD),
catalase (CAT) and reduced glutathione (GSH)-dependent enzymes
contribute to the enzymatic antioxidant system of cells, which
protects against reactive oxygen species (ROS) and oxidative damage
(10). However, circulating tumor
cells generally enter into the circulation and cause postoperative
recurrence and metastasis through an extremely complex process
(11).
Luteolin (LUT) is a naturally occurring polyphenolic
flavone that is present in the form of glycosides in numerous
fruits and vegetables. Epidemiological studies have suggested that
a LUT-rich plant derived diet may play an important role in the
reduction of numerous diseases through the pharmacological activity
of LUT, including antioxidant, anti-inflammatory, antimicrobial,
anticancer, anti-allergic and anti-platelet properties (12). LUT has been shown to have an
inhibitory effect on the growth of human pancreatic cancer cells
and was able to induce apoptosis of these cells, leading to DNA
damage (13). Absorption at the skin
surface and reaching deeper skin layers adds the ability of LUT to
be used as an antiphlogistic agent (14). Absorption of ultraviolet (UV) light by
LUT revealed the possibility of its use in sun protection products
that may potentially prevent skin cancer and skin aging problems
(15). Notably, food supplementation
with LUT increased the sensitivity of cancer cells to chemotherapy
(16). Since the beneficial effects
of LUT have been documented in various diseases, including numerous
cancers, the present study reports the anti-hepatocarcinogenic
effects of LUT in diethylnitrosamine (DN)-intoxicated mice.
Materials and methods
Drugs and chemicals
DN, LUT, 5,5′-dithiobis-2-nitrobenzoic acid and
malondialdehyde were purchased from Sigma-Aldrich (St. Louis, MO,
USA). All other chemicals used for the assays were of the highest
quality and analytical grade (Nanjing Jiancheng Bioengineering
Research Institute, Nanjing, China).
Animal experiment
In total, 24 male BALB/c mice weighing 20±2 g were
used for the experiments. The mice were procured from the
Laboratory Animal Center of Wuhan University (Wuhan, Hubei, China)
and were fed with standard pelleted feed and had free access to
purified sterile drinking water. The mice were acclimatized and
housed under conditions of controlled temperature with 12 h light
and dark cycles. The present protocol for animal experimentation
was approved by the Institutional Animal Care and Use Committee for
Laboratory Experiments at Hubei University of Medicine (Xiangyang,
Hubei, China).
The 6-week-old mice were divided into 4 groups, each
containing 6 mice. Group I acted as the normal control group and
group II acted as the drug control group, with an intraperitoneal
administration of 20 µg/kg of body weight LUT on each alternate day
between week 7 and the end of the experimental period at week 12.
In a separate group of DN-intoxicated animals, various doses of LUT
(1, 5, 10, 20 and 50 µg/kg of body weight) were administered to
optimize the dose for a maximum efficacy at a minimal dose (data
not shown) and a dose of 20 µg/kg of body weight was finalized.
Group III animals were administered with DN at a dose of 100 mg/kg
of body weight every week, which was dissolved in sterile water and
administered using oral gavage for a maximum of 6 weeks. Group IV
animals were initially administered with DN, as in group III, for
the first 6 weeks and were then administered with LUT treatment
from week 7 onwards, as in group II, until the end of the
experimental period. At the end of the experimental period, all the
animals were sacrificed subsequent to overnight fasting for ≥12 h.
Plasma samples were collected for different assays and immediately
stored at −20°C until use. The collected liver tissues were
immediately rinsed with ice-cold physiological saline (0.9% NaCl;
Sigma-Aldrich) and were processed for analysis.
Marker enzymes and enzymatic
antioxidant assays
Assays for the measurement of the activity of AST
and ALT in the plasma and tissue were performed using a UV-visible
spectrophotometer (Lengguang Instrument Co., Ltd., Shanghai,
China), according to the manufacturer's protocol. The activity of
SOD and CAT was assayed using the manufacturer's instructions from
Calbiochem, Inc. (San Diego, CA, USA) using a UV-visible
spectrophotometer.
Assays for LPO and GSH
LPO was determined in the plasma and liver tissue
using the thiobarbituric acid reaction, as previously described by
Ohkawa et al (17). The level
of lipid peroxides was expressed as mmol thiobarbituric acid
reactive substances (TBARS)/ml of plasma or nmol TBARS/g of tissue.
The GSH level in the plasma and liver was determined by the method
previously reported by Ellman (18).
The amount of GSH is expressed as mg/dl of plasma or mg/g of liver
tissue.
Determination of the α-fetoprotein
(AFP) level in the plasma
The AFP level was determined in the plasma using an
Abbott chemiluminescence analyzer (Abbott Laboratories, Chicago,
IL, USA) as per the manufacturer's instructions. The technique was
based on immunoassay (19) and the
amount of relative light units was measured and expressed as
ng/ml.
Determination of the levels of IL-2
and IFN-γ by enzyme-linked immunosorbent assay (ELISA)
Levels of IL-2 and IFN-γ were determined using the
ELISA kit procured from Diaclone SAS, (Besancon, France), according
to the manufacturer's protocol.
Statistical analysis
All results were expressed as the mean ± standard
deviation of the group. Statistical comparisons were performed
using one-way analysis of variance and Tukey's post-hoc test. PRISM
software (version 7; GraphPad Software Inc., La Jolla, CA, USA) was
used for the analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Changes in body and liver weights
The body weight of the mice was measured at weeks 0
and 12 of the experimental protocol. The mean weight of the groups
was adjusted, without any significant difference present between
groups at week 0. At the end of the experimental period of 12
weeks, all the groups were weighed and a significant difference
(P=0.031) of 23.3% decrease in the body weight was identified in
the DN group compared with the control group mice (Table I). Similarly, a significant increase
in the liver weight (37.5%; P=0.021) was noted in the DN group
compared with the control. Although the decrease in body weight and
increase in liver weight of mice recovered towards normal levels
during LUT treatment, no significant difference was noted.
 | Table I.Body and liver weights of mice in
different experimental groups. |
Table I.
Body and liver weights of mice in
different experimental groups.
| Specification | Control | LUT | DN | DN+LUT |
|---|
| Body weight, g |
|
|
|
|
| Week
0 |
18.9±1.10a |
19.2±0.80a |
19.1±1.40a |
19.4±0.60a |
| Week
12 |
24.5±1.90a |
25.9±3.70a |
18.8±1.90 |
21.5±2.40a |
| Liver weight, g |
0.8±0.01a |
0.8±0.02a |
1.1±0.06 |
1.0±0.09a |
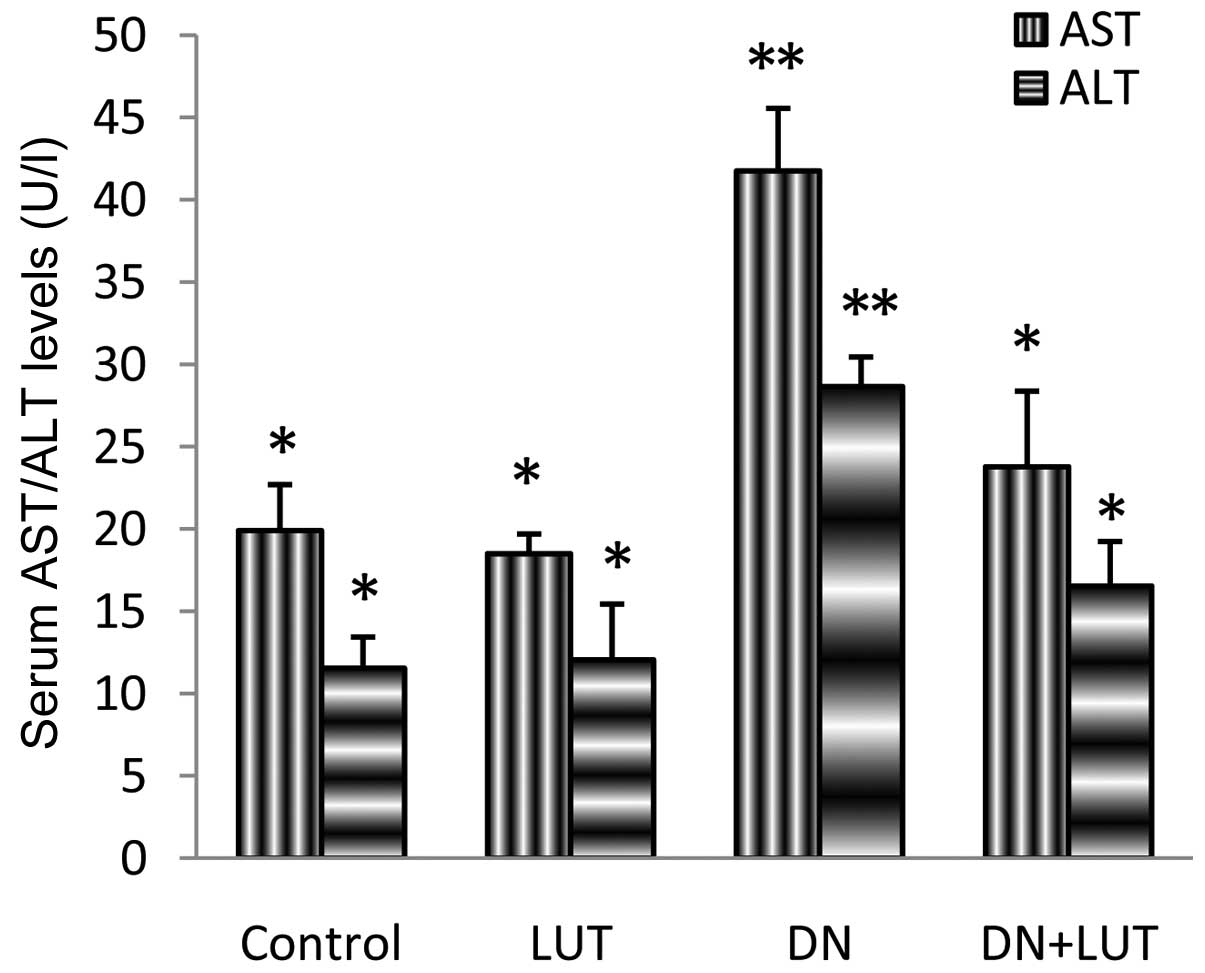
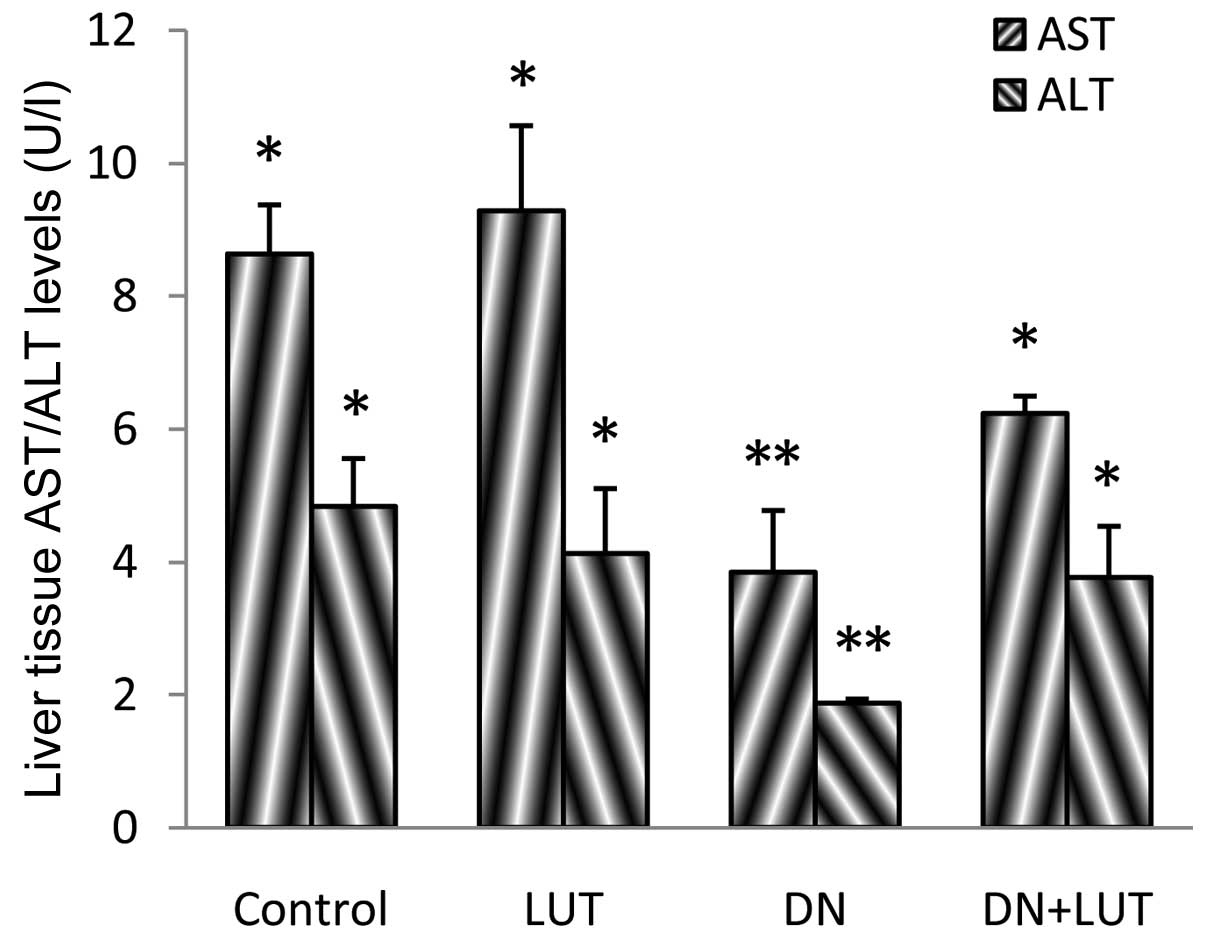
Activity of marker enzymes in the
plasma and liver
Marker enzymes, such as aspartate aminotransferase
(AST) and alanine aminotransferase (ALT) were assayed in the plasma
and liver tissues of the experimental mice (Figs. 1 and 2).
Significant differences were noted between groups (P=0.042), and
the levels of AST and ALT in the plasma were 109.8 and 148.3%,
respectively, and the levels of AST and ALT in the liver tissue
were 55.5 and 61.3%, respectively. All these alterations returned
to the normal levels during LUT treatment.
Activity of enzymatic antioxidants in
the liver
Enzymatic antioxidants, including SOD and CAT,
defend against oxidative stress (20). The activity of the enzymatic
antioxidants SOD and CAT was significantly decreased by 38.6 and
12.5%, respectively, in DN intoxication (Table II). During LUT treatment, the
decrease in activity was restored to normal. No significant
difference was identified between the activity in the group
administered with LUT alone and the group treated with DN and
LUT.
 | Table II.Activity of SOD and CAT in the liver
of mice in different experimental groups. |
Table II.
Activity of SOD and CAT in the liver
of mice in different experimental groups.
| Specification | Control | LUT | DN | DN+LUT |
|---|
| SOD, U/mg
protein |
45.9±3.8a |
44.8±4.6a |
28.2±4.9 |
35.3±5.2a |
| CAT, U/mg
protein |
31.9±2.5a |
32.4±3.8a |
27.9±3.1 |
31.3±1.8a |
Level of LPO for oxidative stress
LPO was assessed by the levels of the thiobarbituric
acid reactive substance malondialdehyde in the plasma and tissue,
which was used to quantitate the oxidative stress due to DN
administration (Tables III and
IV). Significant increases (P=0.016)
in the level of LPO of ~7-fold and ~2-fold were identified in the
plasma and liver tissues, respectively. These increases were
returned to the normal level during LUT treatment.
 | Table III.Levels of plasma LPO and GSH in mice
in different experimental groups. |
Table III.
Levels of plasma LPO and GSH in mice
in different experimental groups.
| Specification | Control | LUT | DN | DN+LUT |
|---|
| LPO, mM
TBARS/ml |
11.48±1.10a |
12.73±1.10a |
92.82±6.90 |
61.32±7.90c |
| GSH, mg/dl |
1.95±0.09a |
2.08±0.06a |
0.67±0.05 |
1.17±0.15a |
 | Table IV.Levels of liver LPO and GSH in mice
in different experimental groups. |
Table IV.
Levels of liver LPO and GSH in mice
in different experimental groups.
| Specification | Control | LUT | DN | DN+LUT |
|---|
| LPO, nM TBARS/g
tissue |
30.8±1.60a |
31.7±2.10a |
89.3±5.20 |
71.5±4.60 |
| GSH, mg/g
tissue |
4.6±0.79a |
4.9±0.57a |
2.6±0.09 |
3.8±0.19a |
Levels of GSH indicated the redox
status
GSH level indicates the redox status and health of
cells. In the present study, 65.6 and 43.5% decreases in the GSH
level were identified in the plasma and liver, respectively, during
DN intoxication, which was then significantly (P=0.036) normalized
during LUT treatment (Tables III
and IV). Notably, a significant
increase (6.7%; P=0.027) in the plasma GSH level was noted in the
group administered with LUT alone.
AFP levels in the plasma
AFP is a circulating tumor marker. A 12-fold
increase in the AFP level in the plasma of mice treated with DN
indicated the progression of neoplastic disease in the animals
(Fig. 3). The significant decrease of
the AFP level during LUT treatment indicated the suppression of the
disease.
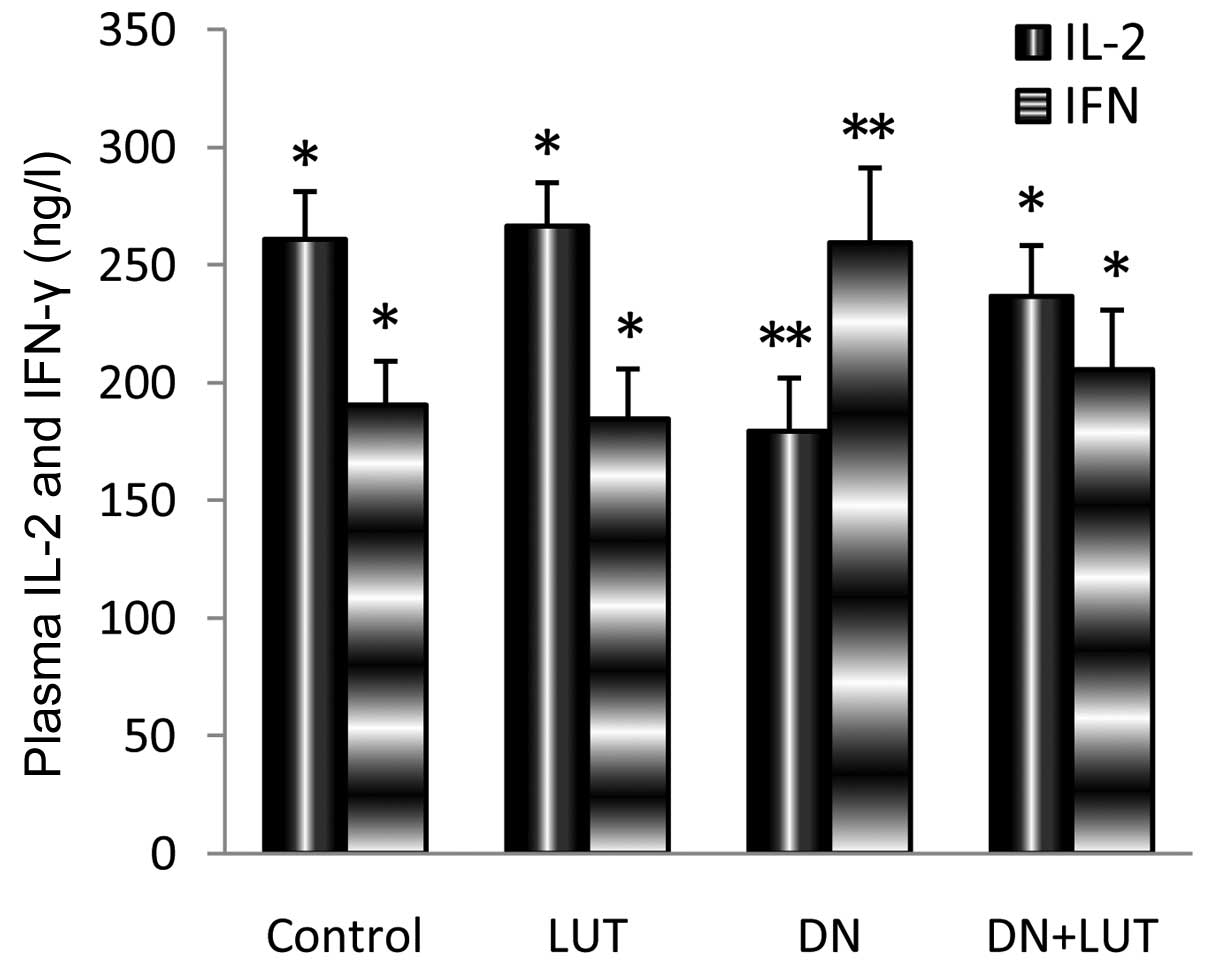
Levels of inflammatory markers in the
plasma
IL-2 and IFN-γ are inflammatory markers that
indicate the progression of disease and the status of the immune
response of the host. A significant (P=0.037) 31.2% decrease in the
IL-2 level and 36.2% increase in the IFN-γ level were identified
during DN intoxication when compared with the control (Fig. 4). The return of these alterations to
the normal level during LUT treatment indicated the protective
effect of LUT against tumor development.
Discussion
LUT has numerous biological roles in prophylactic
and therapeutic modes of treatment. Alterations in the body and
liver weights during DN intoxication indicate the progression of
disease, and the reversal of DN intoxication resulted in the
normalization of the body and liver weights. Pathological changes
leading to increased production or decreased scavenging of free
radicals may play a crucial role in tissue injury (21). The cellular mechanism of toxicity
produced by DN may involve the marker enzymes (AST and ALT), and
may result in the elevation of these enzymes in the plasma and
decrease of the marker enzymes in the liver (8,22). A
significant normalization of the level of the marker enzymes during
LUT treatment may be beneficial, as indicated by the protective
role of the drug. Increased free radical production stimulates LPO
and is the source of the degradation of DNA, lipids and
carbohydrates (23). Numerous
flavonoid aglycones have been shown to have chain-breaking
antioxidant properties and to quench free radicals, thereby
exerting protective effects on macromolecules (24). The cellular level of total GSH content
determines the total redox potential of a cell (25). The restoration of a protective level
of GSH and suppression of LPO in the plasma and liver tissues
depicts the protective role of LUT against ROS and oxidative
damage. This is consistent with previous studies (12,16,20).
Notably, a significant increase in the plasma GSH level was
identified in the group that was administered with LUT alone, which
indicates the increased health of the normal animals, due to the
antioxidant efficacy of GSH.
Enzymatic antioxidants, such as SOD and CAT, and the
non-protein thiol GSH are involved in primary cellular antioxidant
defense mechanisms (20). Antioxidant
enzymes, including SOD and CAT, may either directly detoxify the
ROS produced or facilitate antioxidant reactions using GSH as a
reducing agent (26). CAT is
responsible for the decomposition of peroxides in a cell (27). In the present study, disturbances in
the balance between the ROS levels and expression of antioxidant
defenses were indicated and LUT was revealed to efficiently resolve
this imbalance.
AFP is an oncoglycoprotein of unknown function that
is more prevalent in Italian patients with cirrhosis and
hepatocellular carcinoma. The level of AFP is considered to
differentiate HCC from chronic liver disease (28). Additionally, AFP mRNA acts as a
circulating marker that may be used as a more reliable indicator of
liver cancer (29,30). The high level of AFP present during DN
administration was significantly decreased during the subsequent
LUT treatment, and this reduction demonstrated the protective
effects of LUT.
IL-2 is an important inflammatory cytokine. An
increased level of IL-2 is involved in efficient cellular immune
function (31). Previous studies have
shown that a locally-secreted high level of IL-2 generates fewer
side effects compared with a systemically administered high dose of
IL-2 (31,32). In the present study, the low level of
IL-2 present during the formation of liver lesions was
significantly increased during treatment with LUT. Due to this
increase in the IL-2 level, treatment with LUT may be beneficial
for the development of the immune response against the tumor in
vivo. IFN-γ is one of the important inflammatory cytokines that
trigger liver damage during various stresses, and the depletion of
IFN-γ may result in a marked reduction in injury and inflammation
(33). In the present study,
treatment with LUT decreased the elevated level of IFN-γ and
thereby suppressed the inflammatory response, which is consistent
with previously studies in the literature (34). Significant reversal of the
pathological alterations during LUT treatment indicates the
treatment efficiency of LUT and may aid the triggering of the host
immune response against the tumor. However, additional information
on the underlying mechanism of the suppression of inflammatory
cytokines and protective action of LUT is required from additional
investigation.
References
|
1
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality and survival trends
in the United States from 1995 to 2005. J Clin Oncol. 27:1485–1491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma and cirrhosis: Incidence and
risk factors. Gastroenterology. 127(5): Suppl 1. S35–S50. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faloppi L, Scartozzi M, Maccaroni E, Di
Pietro Paolo M, Berardi R, Del Prete M and Cascinu S: Evolving
strategies for the treatment of hepatocellular carcinoma: From
clinical-guided to molecularly-taylored therapeutic options. Cancer
Treat Rev. 37:169–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borzio M, Fargion S, Borzio F, Fracanzani
AL, Croce AM, Stroffolini T, Oldani S, Cotichini R and Roncalli M:
Impact of large regenerative, low grade and high grade dysplastic
nodules in hepatocellular carcinoma development. J Hepatol.
39:208–214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pawlik TM, Poon RT, Abdalla EK, Ikai I,
Nagorney DM, Belghiti J, Kianmanesh R, Ng IO, Curley SA, Yamaoka Y,
et al: Hepatectomy for hepatocellular carcinoma with major portal
or hepatic vein invasion: Results of a multicenter study. Surgery.
137:403–410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Irtan S, Chopin-Laly X, Ronot M, Faivre S,
Paradis V and Belghiti J: Complete regression of locally advanced
hepatocellular carcinoma induced by sorafenib allowing curative
resection. Liver Int. 31:740–743. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petersen FB, Buckner CD, Appelbaum FR,
Clift RA, Sanders JE, Bensinger WI, Storb R, Witherspoon RP,
Sullivan KM and Bearman SI: Busulfan, cyclophosphamide and
fractionated total body irradiation as a preparatory regimen for
marrow transplantation in patients with advanced hematological
malignancies: A phase I study. Bone Marrow Transplant. 4:617–623.
1989.PubMed/NCBI
|
|
8
|
Senthilkumar S, Devaki T, Manohar BM and
Babu MS: Effect of squalene on cyclophosphamide-induced toxicity.
Clin Chim Acta. 364:335–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clemens MR, Ladner C, Schmidt H, Ehninger
G, Einsele H, Bühler E, Waller HD and Gey KF: Decreased essential
antioxidants and increased lipid hydroperoxides following high-dose
radiochemotherapy. Free Radic Res Commun. 7:227–232. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hosakote YM, Liu T, Castro SM, Garofalo RP
and Casola A: Respiratory syncytial virus induces oxidative stress
by modulating antioxidant enzymes. Am J Respir Cell Mol Biol.
41:348–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Li J, Cao L, Xu W and Yin Z:
Circulating tumor cells in hepatocellular carcinoma: Detection
techniques, clinical implications and future perspectives. Semin
Oncol. 39:449–460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lopez-Lazaro M: Distribution and
biological activities of the flavonoid luteolin. Mini Rev Med Chem.
9:31–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai X, Lu W, Ye T, Lu M, Wang J, Huo J,
Qian S, Wang X and Cao P: The molecular mechanism of
luteolin-induced apoptosis is potentially related to inhibition of
angiogenesis in human pancreatic carcinoma cells. Oncol Rep.
28:1353–1361. 2012.PubMed/NCBI
|
|
14
|
Merfort I, Heilmann J, Hagedorn-Leweke U
and Lippold BC: In vivo skin penetration studies on chamomile
flavones. Pharmazie. 49:509–511. 1994.PubMed/NCBI
|
|
15
|
Plaschke K: Composition comprising one or
more flavonoids, method of obtaining such composition and use there
of as UV-absorbing agent. US Patent 6,409,996 B1. Filed November
19, 1998; issued June 25. 2002.
|
|
16
|
Graefe EU, Derendorf H and Veit M:
Pharmacokinetics and bioavailability of the flavonols quercetin in
humans. Int J Clin Pharmacol Ther. 37:219–233. 1999.PubMed/NCBI
|
|
17
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ellman GL: Tissue sulfhydryl groups. Arch
Biochem Biophys. 82:70–77. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morota K, Komori M, Fujinami R, Yamada K,
Kuribayashi K, Watanabe N, Sokoll LJ, Elliott D, Chan DW, Martens
F, et al: Improvement and multicenter evaluation of the analytical
performance of an automated chemiluminescent immunoassay for alpha
fetoprotein. Int J Biol Markers. 27:39–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rice-Evans CA, Miller NJ and Paganga G:
Structure-antioxidant activity relationships of flavonoids and
phenolic acids. Free Radic Biol Med. 20:933–956. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halliwell B and Gutteridge JM: Lipid
peroxidation, oxygen radicals, cell damage and antioxidant therapy.
Lancet. 1:1396–1397. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Senthilkumar S, Ebenezar KK, Sathish V,
Yogeeta S and Devaki T: Modulation of tissue defense system by
squalene in cyclophosphamide induced toxicity in rats. Arch Med
Sci. 2:94–100. 2006.
|
|
23
|
Quinlan GJ and Gutteridege JM: Hydroxyl
radical generation by the tetracycline antibodies with free radical
damage to DNA, lipids, carbohydrates in the presence of iron and
copper salts. Free Radic Biol Med. 5:341–348. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Poppel G, Verhoeven DT, Verhagen H and
Goldbohm RA: Brassica vegetables and cancer prevention.
Epidemiology and mechanisms. Adv Exp Med Biol. 472:159–168. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Østergaard H, Tachibana C and Winther JR:
Monitoring disulfide bond formation in the eukaryotic cytosol. J
Cell Biol. 166:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Senthilkumar S, Yogeeta SK, Subashini R
and Devaki T: Attenuation of cyclophosphamide induced toxicity by
squalene in experimental rats. Chem Biol Interact. 160:252–260.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tafazoli S and O'Brien PJ: Peroxidases: A
role in the metabolism and side effects of drugs. Drug Discov
Today. 10:617–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trevisani F, D'Intino PE, Morselli-Labate
AM, Mazzella G, Accogli E, Caraceni P, Domenicalli M, De Notariis
S, Roda E and Bernardi M: Serum alpha-fetoprotein for diagnosis of
hepatocellular carcinoma in patients with chronic liver disease:
Influence of HBsAg and anti- HCV status. J Hepatol. 34:570–575.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wright LM, Kreikemeier JT and Fimmel CJ: A
concise review of serum markers for hepatocellular cancer. Cancer
Detect Prev. 31:35–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chiappini F: Circulating tumor cells
measurements in hepatocellular carcinoma. Int J Hepatol.
2012:6848022012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang Gui-zhen: Boundary immunology.
Beijing: Science Press; 2002, View Article : Google Scholar
|
|
32
|
Tagawa M: Cytokine therapy for cancer.
Curr Pharm Des. 6:681–699. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kusters S, Gantner F, Künstle G and Tiegs
G: Interferon-ã plays a critical role in T cell-dependent liver
injury in mice initiated by concanavalin A. Gastroenterology.
111:462–471. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Haqqi TM, Anthony DD, Gupta S, Ahmad N,
Lee MS, Kumar GK and Mukhtar H: Prevention of collagen-induced
arthritis in mice by a polyphenolic fraction from green tea. Proc
Natl Acad Sci USA. 96:4524–4529. 1999. View Article : Google Scholar : PubMed/NCBI
|