Introduction
Hepatocellular carcinoma (HCC) is one of the most
malignant tumors worldwide (1).
Surgical therapies including hepatectomy and liver transplantation
are first-line treatments for HCC patients, but the unlikelihood of
early diagnosis and the high incidence of tumor recurrence and
metastasis following surgery remain major obstacles (2,3).
Non-surgical treatments including radiofrequency ablation, alcohol
injection and transcatheter arterial chemoembolization have made
great advances in recent years. However, they have substantial
limitations, including toxicity, insufficient tissue penetration
and poor tumor targeting, which together often result in incomplete
destruction of the tumors (4–6). Therefore, the development of novel
adjuvant therapies for the suppression of liver cancer growth and
metastasis is essential.
Researchers are now turning to bacterial treatments
and consider this a promising new strategy in cancer therapy.
Certain bacterial species including Salmonella,
Clostridia and Bifidobacteria have been observed to
preferentially replicate and accumulate in tumors (7–11). Through
observations and research over the past century, bacteria has been
noted to affect the tumor in the following ways (12,13): i) as
a tumoricidal agent; ii) as a vector for gene therapy; iii) as
toxins for cancer treatment; iv) as bacterial spores; and v) as an
immunotherapeutic agent. However, major problems with using
bacteria as anti-cancer agents include the systemic infection of
bacteria (7,14) and the inability to completely
eradicate cancer cells. Wild-type bacteria including
Salmonella may induce severe infection, which may result in
septic shock and high lethality (7).
Therefore, researchers use autotrophic mutations to attenuate the
virulence of Salmonella in diverse ways (15).
Previously, we engineered YB1, which was derived
from the attenuated Salmonella strain SL7207 (16–21).
Although SL7207 has aroA and other pathogenic gene
mutations, it is lethal for nude mice if administrated
intravenously. In YB1, the essential gene asd was engineered
to be controlled by the hypoxic promoter Pept and the antisense
aerobic promoter SodA. The asd gene is a key enzyme in the
synthesis of diaminopimelic acid (DAP), which is an essential
component for the gram-negative bacteria cell wall. A deficiency of
asd expression or shortage of extrinsic DAP supply in the
environment leads to bacterial lysis in a short period of time.
Therefore, without additional DAP, YB1 only survives under hypoxic
conditions (<0.5% oxygen) (20).
However, the normal functions of YB1 are not affected. In a nude
mouse breast tumor model, YB1 was rapidly eliminated from normal
organs due to their normoxia conditions. However, in tumors, YB1
accumulated inside the hypoxic region and caused tumor regression
(20).
In this study, we explored the potential anti-cancer
effect of YB1 on HCC using an orthotopic liver tumor animal model
with distant metastatic potential. We observed that liver tumor
growth was significantly inhibited following YB1 treatment.
Furthermore, YB1 also significantly decreased the incidence of lung
metastasis. These results indicate that YB1 has a great potential
for liver cancer therapy and it provides a new model to study the
mechanisms underlying the high efficacy of bacterial suppression of
liver cancer growth and metastasis.
Materials and methods
Orthotopic nude mice liver tumor
model
The orthotopic liver tumor model was established in
nude mice (male, 4–6 weeks old, 18–24 g) obtained from the Lab
Animal Unit of the University of Hong Kong (22). MHCC-97L cells (6×105; Liver
Cancer Institute, Fudan University, China) in 0.1 ml culture medium
were injected subcutaneously into the right flank of the nude mice.
Once the subcutaneous tumors reached 0.8 to 1 cm in diameter, they
were removed and cut into cubes 1 to 2 mm3 in size,
which were subsequently implanted into the left liver lobes of
another group of nude mice. Mice were housed at room temperature
(20–25°C) in a standard animal laboratory with free activity and
access to water and food. The mice were maintained under constant
environmental conditions with a 12-h light-dark cycle. All
operations were performed in clean conditions. The present study
was licensed according to Animal Ordinance Chapter 340 by the
Department of Health, Hong Kong Special Administrative Region (ref.
13–501 in DH/HA&P/8/2/3 Pt. 52).
Treatment regimen, imaging analysis
and sample collection
Two weeks after tumor implantation, a single dose of
SL7207 (5×107 CFU; lab stock; Stanford University School
of Medicine, Stanford, CA, USA), YB1 (5×107 CFU;
modified from SL7207) (20) or saline
(control group; Thai Otsuka. Pharmaceutical Co., Ltd., Bangkok,
Thailand) was injected via the tail vein. Five mice each from the
SL7207 and YB1 groups were used to observe the effect of bacteria
on mouse survival. The control group mice (n=9) and the remaining
nude mice from the YB1 group (n=14) were used to monitor liver
tumor growth and metastasis using a Xenogen in vivo imaging
system (PerkinElmer, Inc., Waltham, MA, USA) through the detection
of luminance signals from tumor cells. The mice were sacrificed 3
weeks after the YB1 or saline injection. The tumors, livers and
lungs were sampled for further research.
Hematoxylin and eosin (H&E) and
immunohistochemical (IHC) staining
The histological changes were assessed by H&E
staining (Vector Laboratories, Inc., Burlingame, CA, USA), and the
infiltration of neutrophils was assessed by IHC. The tissue samples
were fixed in 10% formalin and embedded in paraffin (Sigma-Aldrich,
St. Louis, MO, USA). Paraffin sections were dewaxed with xylene
(Merck Millipore, Darmstadt, Germany), rinsed in graded alcohol
(Merck Millipore) and rehydrated in water, and subsequently were
stained with H&E for histological examination. The distribution
of YB1 in liver tissue was detected by IHC staining. The initial
step was the same as H&E staining, but the paraffin sections
then underwent antigen retrieval with citrate buffer (pH 6.0;
Abcam, Cambridge, UK). Subsequently, the sections were blocked
using 10% fetal bovine serum (Life Technologies Ltd.; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 30 min, and rat anti-mouse
monoclonal primary antibody (dilution, 1:200; catalog no., 560454;
BD Biosciences, Franklin Lakes, NJ, USA) was applied and incubated
for 1 h. Following incubation, the sections underwent blocking with
3% peroxidase (Dako, Glostrup, Denmark) for 30 min, and then goat
anti-rat polyclonal secondary antibody (dilution, 1:100; catalog
no., 559286; BD Biosciences) was applied and incubated for 30 min.
The details of the H&E and IHC staining processes are described
in a previous study by the same group (22).
Cell lines
Human HCC cell lines MHCC-97L and PLC (Japanese
Cancer Research Bank; National Institutes of Biomedical Innovation,
Health and Nutrition, Osaka, Japan) were maintained in high-glucose
Dulbecco's modified Eagle's medium (Life Technologies Ltd.; Thermo
Fisher Scientific, Inc.) supplemented with 10% heat-inactivated
fetal bovine serum (Life Technologies Ltd.; Thermo Fisher
Scientific, Inc.), 100 mg/ml penicillin G and 50 µg/ml streptomycin
(Life Technologies Ltd., Thermo Fisher Scientific, Inc.) at 37°C in
a humidified atmosphere containing 5% CO2. MHCC-97L
cells were labeled via stable transfer of the luciferase gene in
chromosomes. Briefly, cells were transfected with pGL3 vector
(Promega Corporation, Madison, WI, USA), and positive clones were
selected according to luciferase activity in the Xenogen IVIS 100
imaging system (PerkinElmer, Inc., Waltham, MA, USA).
Salmonella invasion assay
YB1-incubated MHCC-97L and PLC cells were prepared
and co-cultured at a ratio of 1,000:1 for 2 h under anaerobic
(O2<0.5%) or aerobic conditions. Extracellular
bacteria were then removed by washing with phosphate-buffered
saline (USB Corporation; Affymetrix, Inc., Santa Clara, CA, USA)
and the cells were further cultured with gentamycin medium (Life
Technologies Ltd., Thermo Fisher Scientific, Inc.) for 24 h. Cells
were fixed in paraformaldehyde (4%) and stained with a rabbit
polyclonal anti-Salmonella antibody (dilution, 1:500;
catalog no., ab35156; Abcam) for 12 h at 4°C. Goat anti-rabbit
polyclonal immunoglobulin G Alexa Fluor® 488-conjugated
(dilution, 1:100; catalog no., ab150077) and polyclonal horseradish
peroxidase-conjugated (dilution, 1:100; catalog no., ab6721)
secondary antibodies (Abcam) were added and the cells were
incubated for 1 h at room temperature. Then CytoPainter
Phalloidin-iFluor 488 Reagent (dilution, 1:1,000; catalog no.,
ab176753; Abcam) was applied to indicate cell boundaries.
CytoPainter Phalloidin-iFluor 555 Reagent (dilution, 1:1,000;
catalog no., ab176756; Abcam) was also applied to demonstrate cell
boundaries. The cells were observed under a confocal microscope
(LSM 710; Carl Zeiss AG, Oberkochen, Germany).
Detection of cell apoptosis
Cancer cell apoptosis and death induced by YB1 under
anaerobic conditions was detected using an Annexin V-propidium
iodide (PI) kit (BioVision, Inc., Milpitas, CA, USA) according to
the manufacturer's protocol. As demonstrated using flow cytometry
(FACSCalibur; (BD Biosciences) Annexin V1/PI- cells were apoptotic
and Annexin V1/PI1 cells were dead.
Statistics and data analyses
Continuous variables were expressed as the median
with a range. The t-test was used for statistical comparison. The
χ2 test was used to compare the incidence of lung
metastasis. P<0.05 was considered to indicate a statistically
significant difference. Calculations were performed using the SPSS
computer software version 16 (SPSS, Inc., Chicago, IL, USA).
Results
Effect and distribution of YB1 on nude
mice bearing orthotopic liver tumors
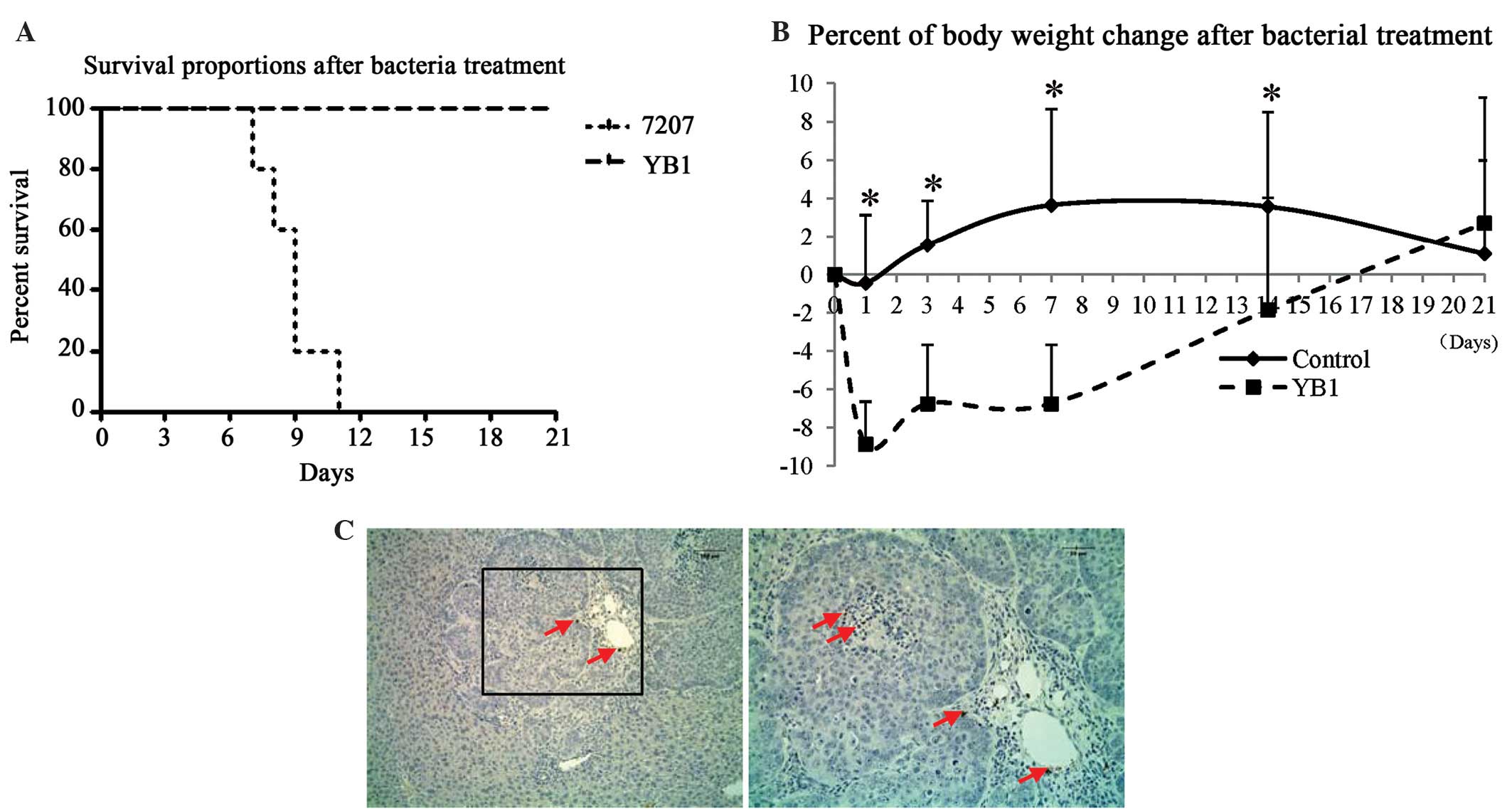
Orthotopic liver tumor-bearing mice were treated
with parental strain SL7207 or engineered strain YB1 for 3 weeks.
All mice in the SL7207 group died within 11 days of treatment. All
of the mice were alive and healthy 3 weeks after YB1 injection
(Fig. 1). To monitor the healthy
condition of YB1 treated mice, the body weights were recorded after
injection. The results revealed that the body weight decreased by
~9% on day 1 after YB1 injection and recovered afterwards (Fig. 1B). Notably, we observed that YB1 had
low-level distribution in the tumors. This result is different from
that observed in our previous study in a breast tumor model
(20). On day 21 after treatment,
only low amounts of YB1 were identified in the shrunken tumor by
paraffin section staining (Fig. 1C).
In addition, YB1 was entirely eliminated from the kidney, lymph
nodes and spleen 3 weeks subsequent to injection. YB1 was
undetected in the blood throughout the experiment.
YB1 significantly suppresses liver
tumor growth and metastasis
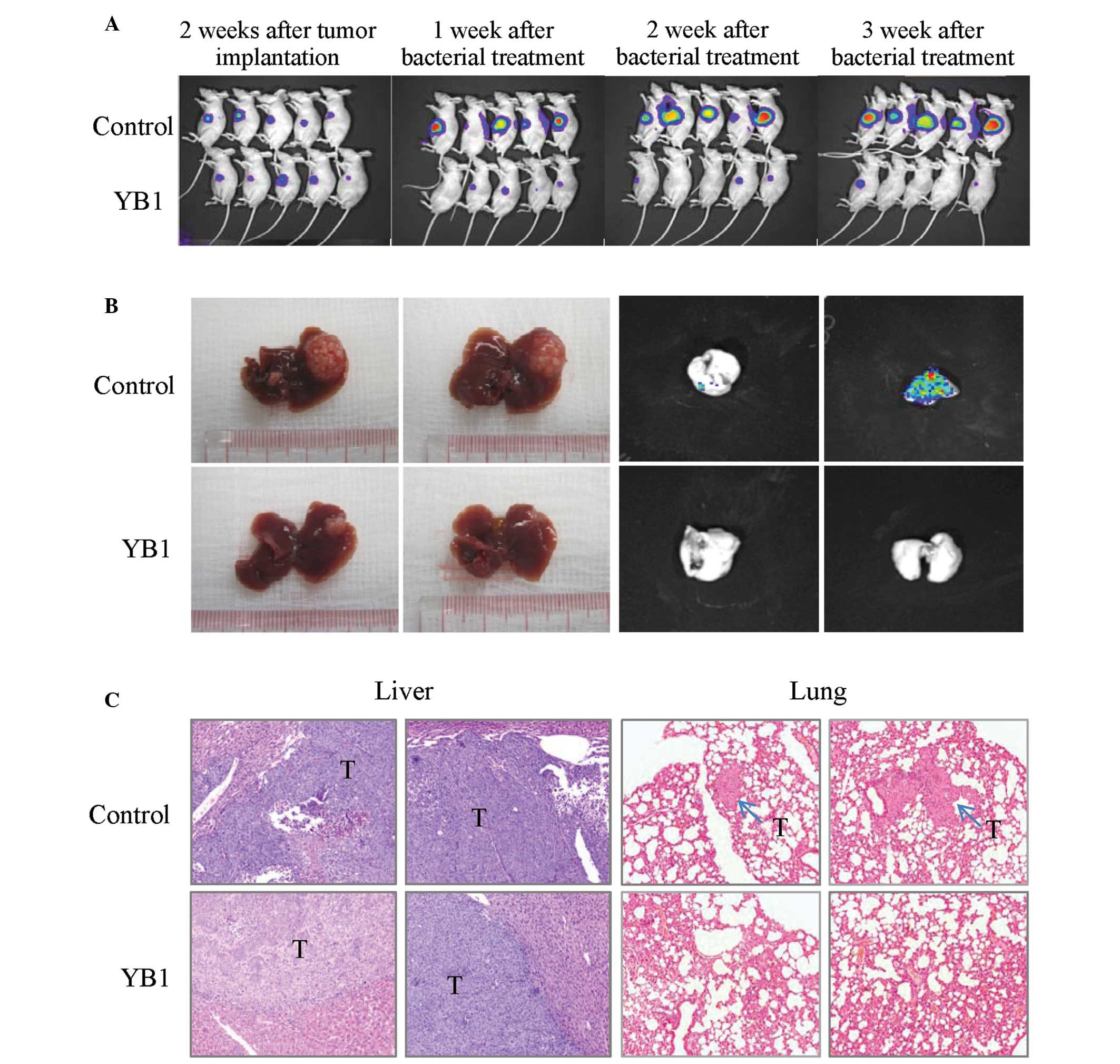
The effect of YB1 on liver tumor growth and
metastasis was studied using an orthotopic liver tumor model in
nude mice. Tumor-bearing mice were grouped randomly, and there were
no significant differences in the average size of liver tumors
between the treatment and control group when the experiment
started. Longitudinal monitoring of tumor growth and metastasis was
achieved by Xenogen IVIS and the tumor final volume was measured at
3 weeks after treatment. The results indicated that YB1
significantly reduced the size of the primary liver tumor from 1
week to 3 weeks after treatment (Fig.
2A-B). After 3 weeks, the tumors in the control and YB1-treated
groups demonstrated significant differences (580.1 mm3
vs. 61.2 mm3; P=0.000; Table
I). Lung metastasis was totally repressed by YB1 treatment (0
of 14), however, the control group still demonstrated a 55.6% (5 of
9; P=0.004) lung metastasis rate (Table
I). These results were additionally confirmed by histological
examination (Fig. 2C).
 | Table I.Comparison of tumor size and lung
metastasis in mice with or without YB1 treatment. |
Table I.
Comparison of tumor size and lung
metastasis in mice with or without YB1 treatment.
| Variable | Control (n=9) | Treatment (n=14) | P-value |
|---|
| Tumor volume
(mm3)a | 580.1±218.4 | 61.2±32.1 | 0.000 |
| Lung metastasis | 5/9 (55.6%) | 0/14 (0.0%) | 0.004 |
YB1 enhances neutrophil infiltration
in liver tissue
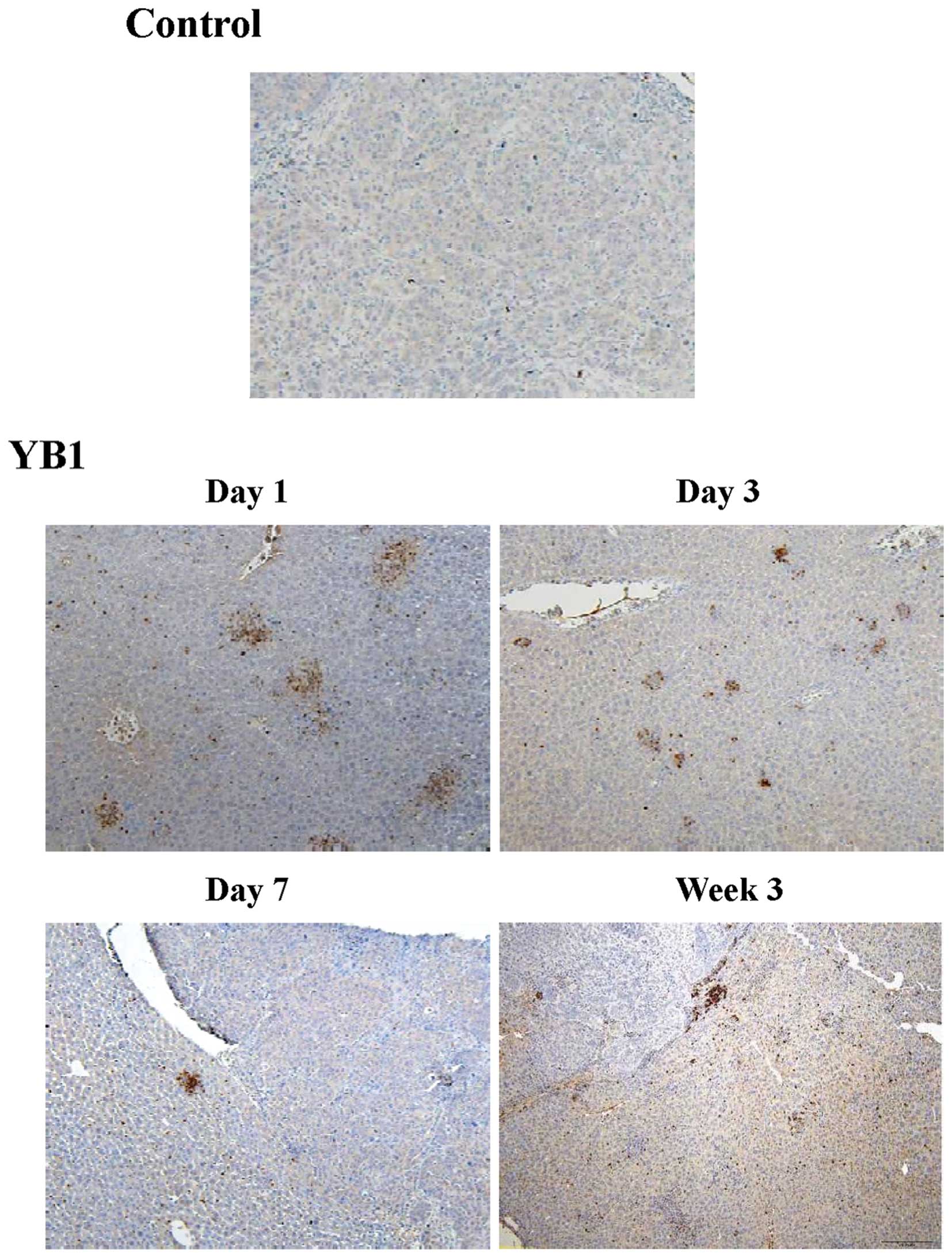
During inflammation of bacterial treatment,
circulating neutrophils are recruited to the site of inflammation,
which releases a number of various cytokines and enhances the
innate immune response (19). In
order to investigate the mechanism of YB1 suppression of tumor
growth and metastasis, the present study compared the infiltration
of neutrophils in the liver between the YB1 treatment and control
group. In the control group, only a small number of Gr1-positive
cells were detected during the entire experiment (Fig. 3). Large amounts of Gr1-positive cells
were detected on day 1 to day 21 following YB1 treatment (Fig. 3).
YB1 effectively invades tumor cells
and induces tumor cell apoptosis and death
To test the interaction between YB1 and liver
cancer, MHCC-97L and PLC cell lines were incubated with YB1 or
SL7207 in vitro under anaerobic conditions (oxygen level
below 0.5%). Following the removal of extracellular bacteria and
further culturing, it was demonstrated that SL7207 and YB1
effectively invaded the liver cancer cells (Fig. 4A). The effect of SL7207 and YB1 on
tumor cells was further investigated by measuring tumor cell
apoptosis and death. The results of the present study revealed that
an increasing number of dying and apoptotic MHCC-97L cells were
detected following co-culture with SL7207 or YB1, relative to the
control. The YB1 and SL7207 groups exhibited significant damage to
cancer cells under hypoxic conditions (Fig. 4B). Similar results were observed in
the PLC cell line (Fig. 4B).
Discussion
In the present study, the significant effects of YB1
on the inhibition of HCC growth and metastasis were observed in an
orthotopic liver tumor model with distant metastatic potential.
With the application of Xenogen IVIS, a notable suppression of
liver tumor growth by YB1 treatment was demonstrated longitudinally
at different time points. In addition to the inhibition of primary
liver tumor growth, YB1 also significantly decreased the incidence
of lung metastasis. In contrast with the SL7207 group, all mice in
the YB1 group were alive 3 weeks after YB1 injection. Although body
weights decreased for a short period, they were restored to their
original states within days. The data suggested that YB1 has no
severe adverse effects and hence may be a new option in the therapy
of HCC patients.
Compared with our previous studies, YB1 treatment in
the liver cancer model demonstrated better results, with the
primary tumor being greatly repressed and lung metastasis totally
eliminated. To explore the underlying mechanism, we investigated
two potential directions. First, the innate immune response induced
by YB1. A tumor has the ability to escape the immune system due to
the development of tolerance. Numerous methods have been used in
attempts to alert the immune system to the presence of tumors. In
previous studies, immunotherapeutic strategies employ bacteria
including Salmonella to enhance the antigenicity of tumor
cells (23–25). Studies by Avogadri et al and Al
Ramadi et al revealed that attenuated S. typhimurium
infects malignant cells and triggers an anti-cancer immune response
(26,27). Furthermore, S. typhimurium
plays a significant role in the induction of polymorphonuclear
leukocyte migration through its Salmonella invasion protein
A (SipA) (28). The present study
confirmed that YB1 enhances innate immune response by increasing
the infiltration of neutrophils. The results indicate that the
suppression effect of YB1 on tumor growth and metastasis may be due
to its ability to enhance the innate immune response.
Secondly, due to the ability of YB1 to penetrate
deep inside the tumor and gain direct contact with cancer cells, we
also further explore the direct effect of YB1 on HCC cells. In the
present study, our results revealed that YB1 effectively invades
HCC cells and induces tumor cell apoptosis and death. This is
consistent with our previous results (20). A previous study reveals that S.
typhimurium may activate specific apoptotic enzymes including
caspase-3 through S. typhimurium effector SipA (29). The activation of caspase-3 in turn
increases the infectivity of this pathogen by enhancing the
secretion of effectors (28,29). Furthermore, the activation of cleaved
caspase-3 is linked to tumor cell apoptosis (30). Therefore, these data suggested that
the suppressive effect of YB1 on tumor growth and metastasis might
be attributed to the induction of apoptosis. However, the precise
mechanism of YB1 on induced tumor cell apoptosis requires further
investigation.
In conclusion, YB1 suppresses liver tumor growth and
metastasis by inducing HCC cell apoptosis and enhancing the innate
immune response, suggesting that it may be a promising candidate
for potential adjuvant therapies for HCC patients.
Acknowledgements
The present study was supported by the Collaborative
Research Fund (HKU1/CRF/10 and HKU3/CRF11R) of the Research Grant
Council Hong Kong, the National Basic Research Program of China
(973 Program, 2014CB745200) from the Ministry of Science and
Technology of PRC, the CRCG Seed Funding Program for Applied
Research, and the National Science Foundation of China (NSFC) grant
no. 31200639.
References
|
1
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: worldwide incidence and trends.
Gastroenterology. 127:S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Doi K, Horiuchi T, Uchinami M, Tabo T,
Kimura N, Yokomachi J, Yoshida M and Tanaka K: Hepatic
ischemia-reperfusion promotes liver metastasis of colon cancer. J
Surg Res. 105:243–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Bilt JD, Kranenburg O, Nijkamp MW,
Smakman N, Veenendaal LM, Te Velde EA, Voest EE, van Diest PJ and
Rinkes IH Borel: Ischemia/reperfusion accelerates the outgrowth of
hepatic micrometastases in a highly standardized murine model.
Hepatology. 42:165–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bruix J, Llovet JM, Castells A, Montañá X,
Brú C, Ayuso MC, Vilana R and Rodés J: Transarterial embolization
versus symptomatic treatment in patients with advanced
hepatocellular carcinoma: results of a randomized, controlled trial
in a single institution. Hepatology. 27:1578–1583. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahn J and Flamm SL: Hepatocellular
carcinoma. Dis Mon. 50:556–573. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wall DM, Srikanth CV and McCormick BA:
Targeting tumors with salmonella Typhimurium - potential for
therapy. Oncotarget. 1:721–728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pawelek JM, Low KB and Bermudes D:
Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res.
57:4537–4544. 1997.PubMed/NCBI
|
|
8
|
Nauts HC, Fowler GA and Bogatko FH: A
review of the influence of bacterial infection and of bacterial
products (Coley's toxins) on malignant tumors in man; a critical
analysis of 30 inoperable cases treated by Coley's mixed toxins, in
which diagnosis was confirmed by microscopic examination selected
for special study. Acta Med Scand Suppl. 276:1–103. 1953.PubMed/NCBI
|
|
9
|
Barbé S, Van Mellaert L and Anné J: The
use of clostridial spores for cancer treatment. J Appl Microbiol.
101:571–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Mellaert L, Barbé S and Anné J:
Clostridium spores as anti-tumour agents. Trends Microbiol.
14:190–196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sasaki T, Fujimori M, Hamaji Y, Hama Y,
Ito K, Amano J and Taniguchi S: Genetically engineered
Bifidobacterium longum for tumor-targeting enzyme-prodrug therapy
of autochthonous mammary tumors in rats. Cancer Sci. 97:649–657.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Fu GF, Fan YR, Liu WH, Liu XJ, Wang
JJ and Xu GX: Bifidobacterium adolescentis as a delivery system of
endostatin for cancer gene therapy: selective inhibitor of
angiogenesis and hypoxic tumor growth. Cancer Gene Ther.
10:105–111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patyar S, Joshi R, Byrav DS, Prakash A,
Medhi B and Das BK: Bacteria in cancer therapy: a novel
experimental strategy. J Biomed Sci. 17:212010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Minton NP: Clostridia in cancer therapy.
Nat Rev Microbiol. 1:237–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bacon GA, Burrows TW and Yates M: The
effects of biochemical mutation on the virulence of bacterium
typhosum: the induction and isolation of mutants. Br J Exp Pathol.
31:702–713. 1950.
|
|
16
|
Forbes NS, Munn LL, Fukumura D and Jain
RK: Sparse initial entrapment of systemically injected Salmonella
typhimurium leads to heterogeneous accumulation within tumors.
Cancer Res. 63:5188–5193. 2003.PubMed/NCBI
|
|
17
|
Loessner H, Endmann A, Leschner S,
Westphal K, Rohde M, Miloud T, Hämmerling G, Neuhaus K and Weiss S:
Remote control of tumour-targeted Salmonella enterica serovar
Typhimurium by the use of L-arabinose as inducer of bacterial gene
expression in vivo. Cell Microbiol. 9:1529–1537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Royo JL, Becker PD, Camacho EM, Cebolla A,
Link C, Santero E and Guzmán CA: In vivo gene regulation in
Salmonella spp. by a salicylate-dependent control circuit. Nat
Methods. 4:937–942. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Westphal K, Leschner S, Jablonska J,
Loessner H and Weiss S: Containment of tumor-colonizing bacteria by
host neutrophils. Cancer Res. 68:2952–2960. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu B, Yang M, Shi L, Yao Y, Jiang Q, Li X,
Tang LH, Zheng BJ, Yuen KY, Smith DK, et al: Explicit hypoxia
targeting with tumor suppression by creating an ‘obligate’
anaerobic Salmonella Typhimurium strain. Sci Rep. 2:4362012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hoiseth SK and Stocker BA:
Aromatic-dependent Salmonella typhimurium are non-virulent and
effective as live vaccines. Nature. 291:238–239. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li CX, Shao Y, Ng KT, Liu XB, Ling CC, Ma
YY, Geng W, Fan ST, Lo CM and Man K: FTY720 suppresses liver tumor
metastasis by reducing the population of circulating endothelial
progenitor cells. PLoS One. 7:e323802012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saccheri F, Pozzi C, Avogadri F, Barozzi
S, Faretta M, Fusi P and Rescigno M: Bacteria-induced gap junctions
in tumors favor antigen cross-presentation and antitumor immunity.
Sci Transl Med. 2:44ra57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishikawa H, Sato E, Briones G, Chen LM,
Matsuo M, Nagata Y, Ritter G, Jäger E, Nomura H, Kondo S, et al: In
vivo antigen delivery by a Salmonella typhimurium type III
secretion system for therapeutic cancer vaccines. J Clin Invest.
116:1946–1954. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
al-Ramadi BK, Fernandez-Cabezudo MJ,
El-Hasasna H, Al-Salam S, Bashir G and Chouaib S: Potent anti-tumor
activity of systemically-administered IL2-expressing Salmonella
correlates with decreased angiogenesis and enhanced tumor
apoptosis. Clin Immunol. 130:89–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Avogadri F, Martinoli C, Petrovska L,
Chiodoni C, Transidico P, Bronte V, Longhi R, Colombo MP, Dougan G
and Rescigno M: Cancer immunotherapy based on killing of
Salmonella-infected tumor cells. Cancer Res. 65:3920–3927. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Al-Ramadi BK, Fernandez-Cabezudo MJ,
El-Hasasna H, Al-Salam S, Attoub S, Xu D and Chouaib S: Attenuated
bacteria as effectors in cancer immunotherapy. Ann N Y Acad Sci.
1138:351–357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wall DM, Nadeau WJ, Pazos MA, Shi HN,
Galyov EE and McCormick BA: Identification of the Salmonella
enterica serotype typhimurium SipA domain responsible for inducing
neutrophil recruitment across the intestinal epithelium. Cell
Microbiol. 9:2299–2313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Srikanth CV, Wall DM, Maldonado-Contreras
A, Shi HN, Zhou D, Demma Z, Mumy KL and McCormick BA: Salmonella
pathogenesis and processing of secreted effectors by caspase-3.
Science. 330:390–393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghavami S, Hashemi M, Ande SR, Yeganeh B,
Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ and
Los M: Apoptosis and cancer: mutations within caspase genes. J Med
Genet. 46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|