Introduction
Choroid plexus papillomas (CPPs) are rare,
histologically benign [World Health Organization (WHO) grade I]
intracranial neoplasms arising from the choroid epithelium. They
represent 0.4–0.6% of all primary intracranial tumors and account
for 1.5–4% of pediatric brain tumors (1,2). Magnetic
resonance imaging (MRI) and computed tomography (CT) are the main
radiological modality of choice for evaluating CPPs.
CPPs usually arise in the fourth ventricle in adults
and the lateral ventricles in children (3). Extraventricular CPPs are rare (3). In previous studies, typical imaging
findings of CPPs have been a lobulated, cauliflower-like or
mulberry-like mass that was homogeneous isointense or slightly
hypointense on T1-weighted imaging (T1WI) and heterogeneous
isointense or slightly hyperintense on T2WI (4–6). Following
contrast injection, they demonstrated notable homogeneous or
heterogeneous enhancement. Although the typical mass locations of
CPPs occurring intraventricularly have been extensively described,
extraventricular CPPs can be particularly misleading; as
extraventricular CPPs occur in atypical locations, it is typically
misdiagnosed as other tumors (7).
Extraventricular CPPs are uncommon (8). They may occur in cisterns, spinal
subarachnoid space and brain parenchyma. A differential diagnosis
between extraventricular CPPs and other intracranial entities is
crucial for the therapeutic approach and prognosis of patients.
However, few studies have showed the imaging findings of
extraventricular CPPs (4). For the
purpose of optimal diagnosis and differential diagnosis, in the
present study we retrospectively reviewed 11 masses of
extraventricular CPPs in 10 patients to provide more information
for a more accurate diagnosis of CPPs.
Materials and methods
Patient characteristics
Ten patients with extraventricular CPPs were
surgically treated, and the diagnosis was pathologically confirmed
at three hospitals located in Jiangsu Province (Huai'an First
People's Hospital, Huai'an; The Affiliated Hospital of Nanjing
University of Chinese Medicine, Nanjing, China) and Shanghai city,
China, during January 2003 and December 2013. All cases had
preoperative imaging pictures from MRI (n=9) or CT (n=1). The
institutional review board (Huai'an First People's Hospital)
approved this retrospective study, and the requirement for informed
consent was waived.
Clinical information
The clinical features of the 10 CPPs patients are
summarized in Table I. The average
age of the patients was 43.8±19.1 years (range, 8–74 years). The
patients included seven males and three females. The presenting
clinical symptoms included dizziness and headache (n=4), ataxia
(n=2), tinnitus (n=2), limb weakness (n=1), and gradual hearing
loss (n=1). The duration from onset to admission ranged from 8
months to 10 years; the median duration was one year. Eight of the
10 cases had been misdiagnosed prior to surgery as meningioma
(n=2), neurinoma (n=2), hemangioblastoma (n=1), ependymoma (n=1),
glioma with hemorrhage (n=1) and glomus jugulare tumor (n=1).
 | Table I.Radiological and pathological features
of 10 patients with extraventricular choroid plexus papillomas. |
Table I.
Radiological and pathological features
of 10 patients with extraventricular choroid plexus papillomas.
|
|
|
|
| MRI signal
intensity |
|
|
| Pathology |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Case no. | Gender/age (yrs) | Location | Contour | T1WI | T2WI | Hydrocephalus | Contrast
enhanced | Cystic | Hemorrhage | Calcification | Imaging
diagnosis |
|---|
| 1 | M/34 | CPA | Lobulated | Iso/hypo | Iso/hyper | − | Homogeneous
strong | + | − | − | Hemangioblastoma |
| 2 | M/40 | CPA | Round | Iso | Iso | + | Heterogeneous
strong | − | + | − | Neurinoma |
| 3 | F/25 | CPA | Lobulated | Slight hypo | Slight hyper | + | Homogeneous mild | − | + | + | Meningioma |
| 4 | M/35 | CPA | Mulberry -like | Slight hypo | Slight hyper | − | Heterogeneous
moderate | − | − | − | CPP |
| 5 | M/56 | CPA | Round | Slight hypo | Slight hyper | − | Heterogeneous
moderate | − | − | − | Neurinoma |
| 6 | M/74 | Mastoid, CPA | Lobulated | Mixed hyper | Mixed hyper | + | Heterogeneous
strong | − | + | − | Glomus jugulare
tumor |
| 7 | M/55 | Cisterna magna | Lobulated | Iso | Iso | + | Homogeneous
strong | − | − | − | Meningioma |
|
|
| Cerebellar
cistern | Ovoid | Iso | Iso | − | Homogeneous
strong | − | − | − |
|
| 8 | F/8 | Cisterna magna | Mulberry- like | Slight hypo | Slight hyper | +++ | Homogeneous
mild | − | − | + | CPP |
| 9 | F/53 | Periventricular
alba | Ovoid |
|
| − | Homogeneous
mild | + | − | + | Ependymoma |
| 10 | M/58 | Cerebellum | Round | Slight
hyper/hyper | Hyper/hypo | ++ | No enhancement | + | + | − | Glioma wirh
hemorrhage |
MRI examination
Nine patients were examined by MRI using a 3T system
(6 cases; Magnetom Verio, Siemens, Erlangen, Germany) or a 1.5T
system (3 cases; General Electric, Milwaukee, WI, USA). One patient
was examined only by CT. Axial spin-echo T1WI (TR/TE,
500–600/14–20), axial fast spin-echo T2WI (TR/TE, 2500–4500/90–110)
and axial or coronal T2-weighted fluid-attenuated inversion
recovery sequence (T2 FLAIR) images (TR/TE/TI, 8000/130/2200) were
acquired. Diffusion-weighted images (single-shot spin-echo
echoplanar sequence with b factors of 0 and 1,000 smm−2)
of the axial plane were also acquired for certain patients.
Enhanced T1WIs in the axial, coronal and sagittal planes were
acquired following intravenous injection of
gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA, 0.1
mmol/kg; Magnevist, Bayer HealthCare Pharmaceuticals, Leverkusen,
Germany) in all cases. The MRI findings, including the tumor
location, size, contour, signal intensity, cystic degeneration and
necrosis, degree of enhancement and associated hydrocephalus were
analyzed. Tumor size was recorded as the maximal diameter on MRI.
Tumor signals were divided into hypointense, slightly hypointense,
isointense, slightly hyperintense and hyperintense, relative to the
cerebral gray matter. The degree of enhancement was classified as
mild, moderate or strong. Mild and moderate enhancements were
defined as low enhancement relative to the typical strong
enhancement of CPPs. Hydrocephalus was divided into mild, moderate
and severe. All images were reviewed by two experienced
radiologists and consensus was reached.
Pathological analysis
The tissues were fixed in 10% neutral buffered
formalin, embedded in paraffin and sliced for hematoxylin and eosin
(HE) staining according to standard procedures. The tissue sections
of the 11 masses in the 10 CPP patients were reviewed by an
experienced pathologist to evaluate the presence of hemorrhage,
psammomatous bodies, dystrophic calcifications and other gross
features that might correspond to macroscopic imaging features on
MRI. Psammomatous bodies were defined as round, concentric
calcified bodies within the interstitium.
Results
Imaging findings of extraventricular
CPPs
The radiological features of the 11 extraventricular
masses in the 10 CPP patients are summarized in Tables I and II. The tumors were located in the
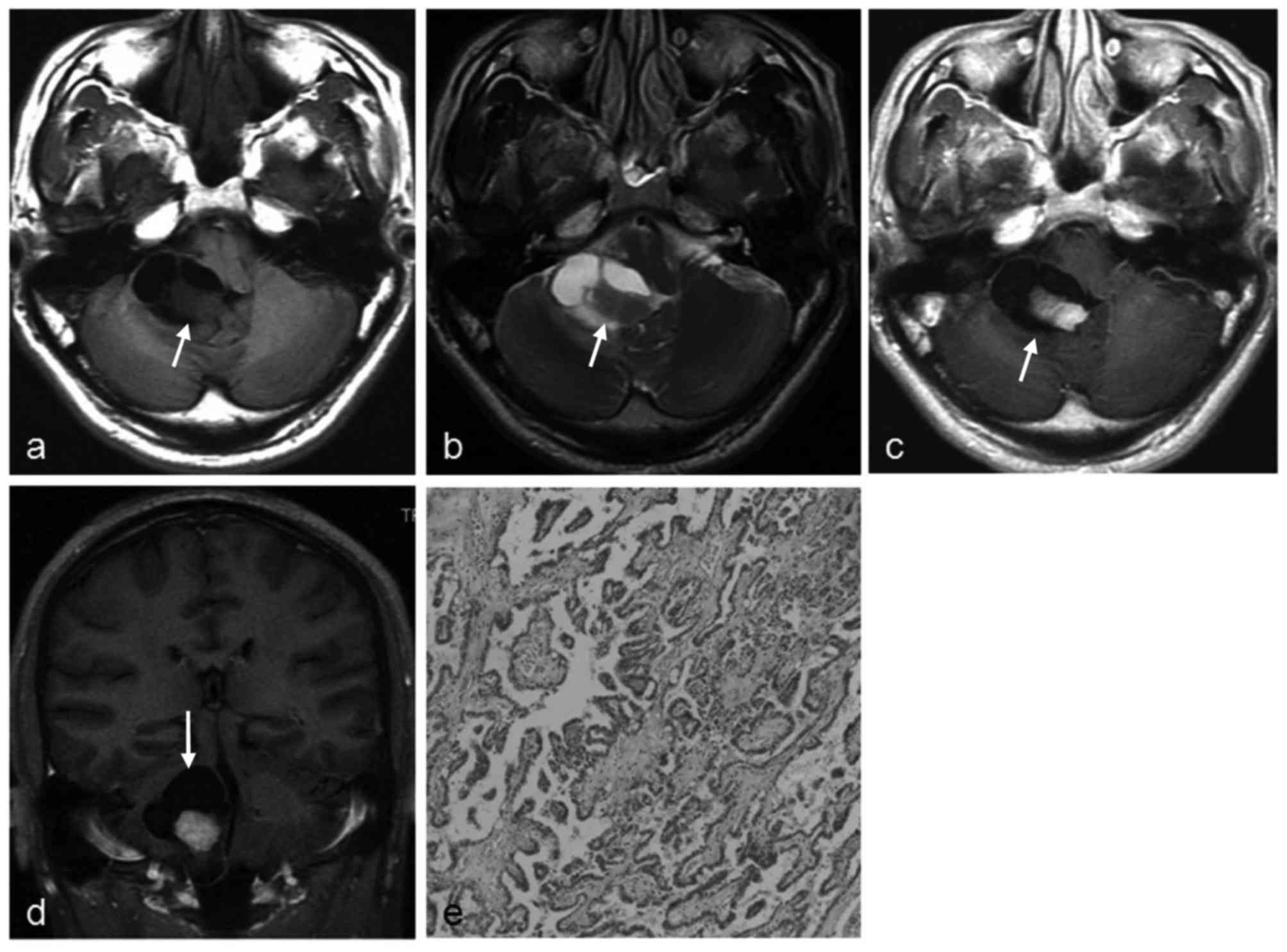
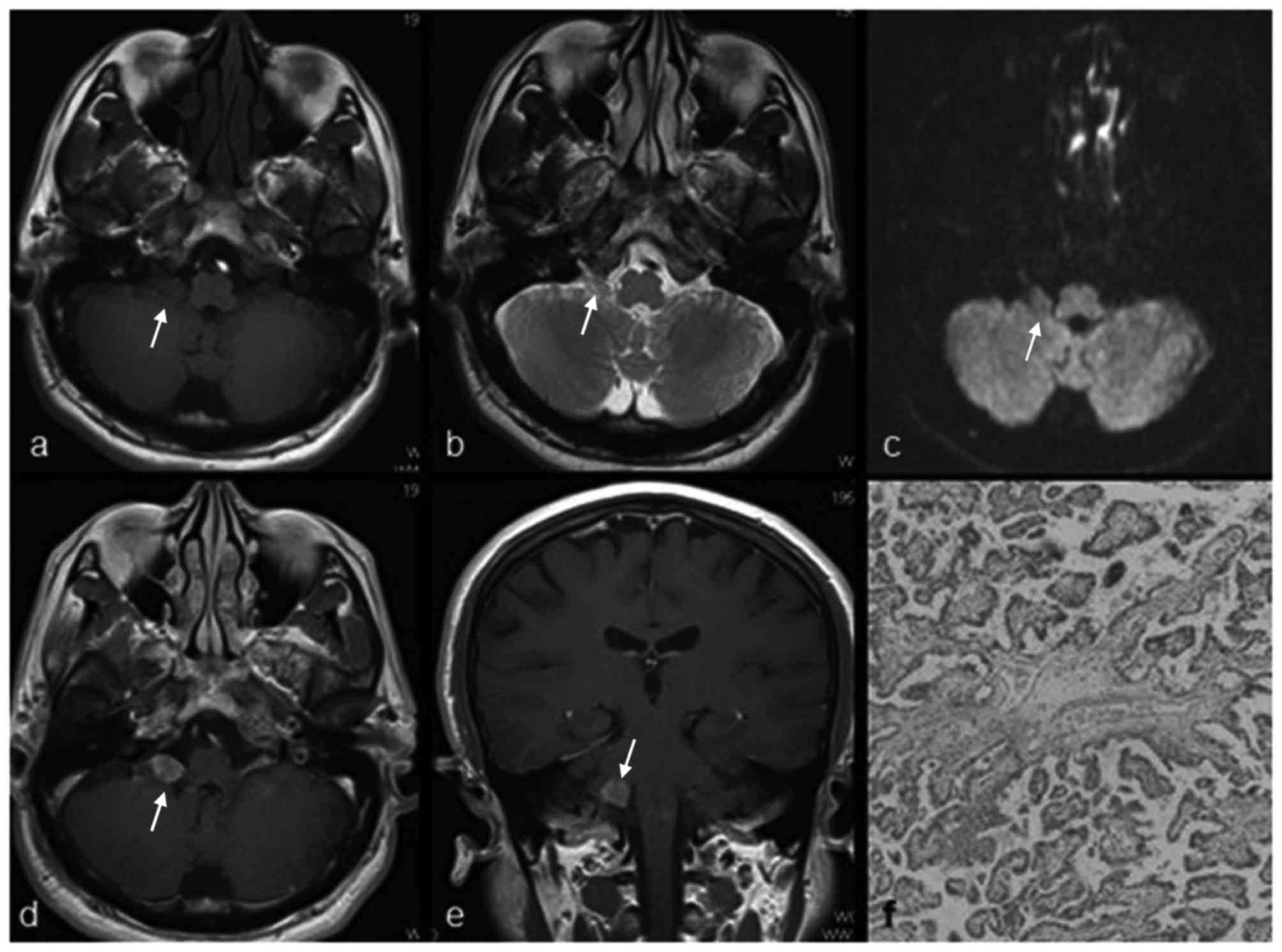
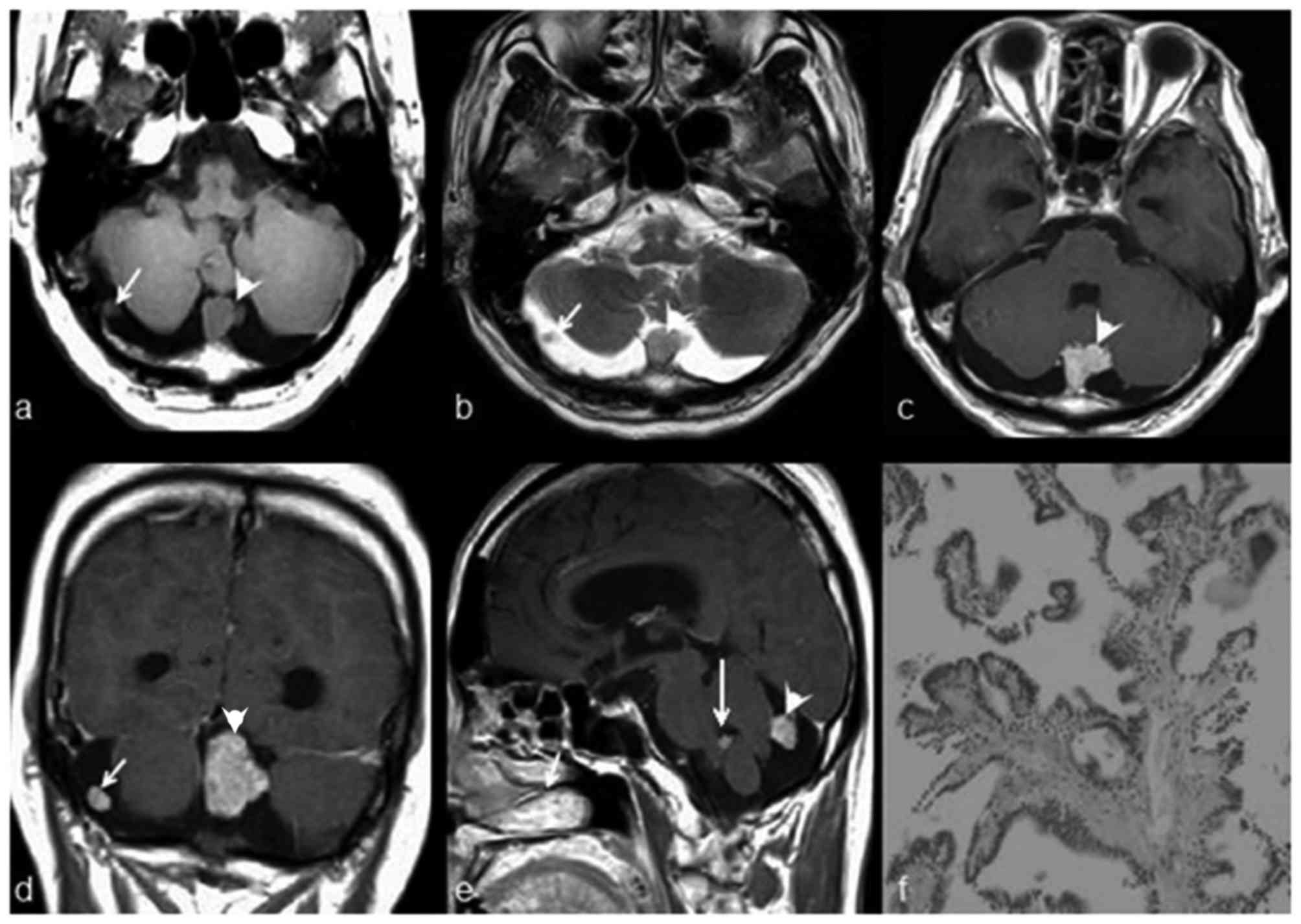
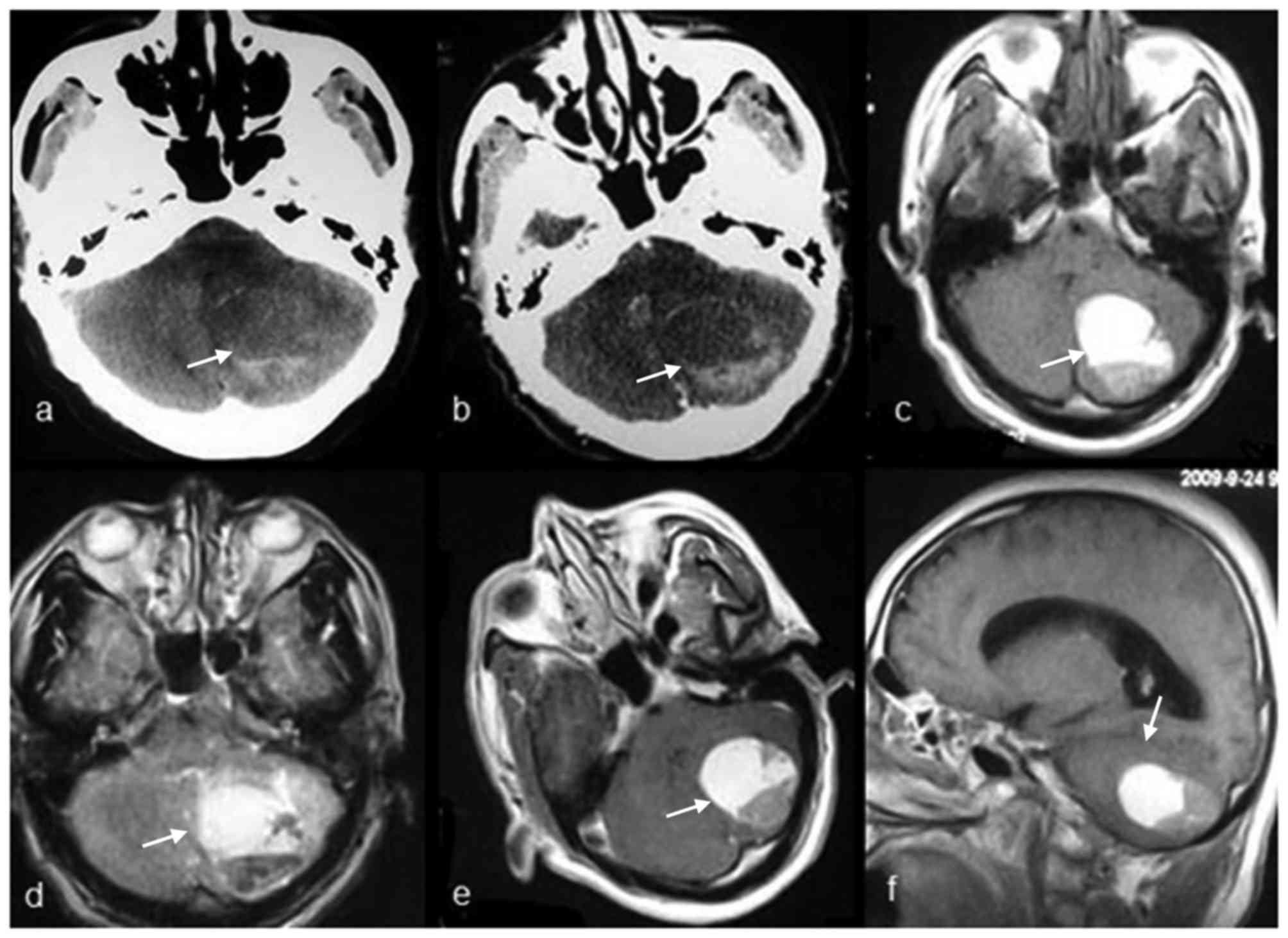
cerebellopontine angle (CPA; six cases, Fig. 1), cisterna magna (two cases, Figs. 2–3),
cerebellum (two cases, Fig. 4), and
periventricular alba (one case), respectively. Three of the six
tumors in the CPA were partly in the foramen of Luschka with
extension to the CPA, and other three cases did not have tumors in
the foramen of Luschka. Multifocal CPPs were observed in one
patient who had three nodes in the cisterna magna, cerebellar
cistern and foramen of Luschka (not shown).
 | Table II.Summary of imaging characteristics of
11 masses in 10 patients with extraventricular choroid plexus
papilloma. |
Table II.
Summary of imaging characteristics of
11 masses in 10 patients with extraventricular choroid plexus
papilloma.
| Characteristic | n | % | Characteristic | n | % |
|---|
| Location |
|
| Enhancement |
|
|
|
Cerebellopontine angle | 5 |
|
Typical | 5 | 45.5% (5/11) |
|
Cisterna magna | 1 |
|
Strong | 5 |
|
|
Periventricular alba | 1 |
|
Atypical | 6 | 54.5% (6/11) |
|
Cerebellum | 1 |
|
Mild | 3 |
|
|
Mastoid | 1 |
|
Moderate | 2 |
|
|
Multiple sites | 1 |
|
None | 1 |
|
| Tumor contour |
|
| Hydrocephalus |
|
|
|
Typical | 6 | 54.5% (6/11) |
Moderate or
severe | 2 | 20.0% (2/10) |
|
Lobulated | 4 |
|
Mild or local | 4 | 40.0% (4/10) |
|
Mulberry-like | 2 |
|
None | 4 | 40.0% (4/10) |
|
Atypical | 5 | 45.5% (5/11) | Capsule |
|
|
|
Round | 3 |
|
Yes | 11 | 100% (11/11) |
|
Relatively
regular | 2 |
| No | 0 |
|
| T1WI & T2WI
signals |
|
| Imaging
diagnosis |
|
|
|
Typical | 7 | 70.0% (7/10) |
Correct | 2 | 20.0% (2/10) |
|
Iso T1WI & iso
T2WI | 3 |
|
Misdiagnosed | 8 | 80.0% (8/10) |
|
Slight hypo T1WI
& slight hyper T2WI | 4 |
| Tumor size, in
cm |
|
|
|
Atypical | 3 | 30.0% (3/10) | <3
cm | 4 | 36.4% (7/11) |
|
Iso-/hypo T1WI
& iso-/hyper T2WI | 1 |
| ≥3
cm | 7 | 63.6% (7/11) |
|
Mixed T1WI &
mixed T2WI | 1 |
|
|
|
|
|
Slight
hyper-/hyper T1WI & hypo-/hyper T2WI | 1 |
|
|
|
|
| Cystic
formation | 1 |
|
|
|
|
Yes | 3 | 27.3% (3/11) |
|
|
|
| No | 8 | 72.7% (8/11) |
|
|
|
|
The mean maximal diameter was 4.5±2.8 cm (range, 0.9
cm to 7.5 cm). Tumors of less than 3 cm in size accounted for 36.4%
(4/11) of the total amount. The tumors presented with typical
lobulated or mulberry-like contours in six masses (54.5%, 6/11) and
atypical contours in five (45.5%, 5/11) masses, including round in
three cases and ovoid in two cases.
Of the 10 masses undergoing MRI examination, seven
masses (70.0%) presented typical signal intensity (homogeneous
isointensity or slight hypointensity on T1WI and heterogeneous
isointensity or slight hyperintensity on T2WI), and three masses
(30.0%) presented atypical signal intensity (one mass had mixed
signal intensity on T1WI and T2WI, one had slight
hyper-/hyperintensity on T1WI and hypo-/hyperintensity on T2WI, and
one had iso-/hypointensity on T1WI and iso-/hyperintensity on T2WI.
Cystic degeneration was observed in three masses. In addition,
irregular enhancement was noted in six masses (54.5%, 6/11),
including moderate enhancement in two masses, mild enhancement in
three masses and no enhancement in one mass. Of these six masses,
three masses had homogeneous enhancement and two masses had
heterogeneous enhancement. Hydrocephalus was absent in four
patients (40.0%, 4/10); mild or local hydrocephalus was observed in
four patients (40.0%, 4/10). Only two patients had moderate or
severe hydrocephalus.
Pathological appearance of
extraventricular CPPs
The pathological features of the 11 masses in 10
patients with CPPs are summarized in Table I. Histological examinations revealed a
characteristic branching growth pattern of the papilla in all
tumors. The individual papilla was lined with a single layer of
cuboidal and cylindrical epithelium cells covering the
fibrovascular connective tissue. Occasionally, tubular or solid
growth was observed adjacent to the papillary structures. Among the
11 masses, hemorrhage was observed in four masses (36.4%), of which
one mass had notable hemorrhage and three masses had focal
hemorrhage. Psammomatous bodies and/or calcification were observed
in three masses. In one case, the mass had invaded the adjacent
petrous bone tissue.
Discussion
This retrospective study reveals the imaging
findings of extraventricular CPPs. In addition to typical imaging
findings (including well-defined lobulated or mulberry-like
intraventricular masses with solid slight hypointensity or
isointensity on T1WI and isointense or slight hyperintensity on
T2WI, as well as strong intense enhancement accompanied with
hydrocephalus (4–6,9) atypical
imaging findings, including round or oval contours, mixed
hyperintense signals on T1WI and T2WI or slightly hyperintense
signals on T1WI or hypo-/hyperintense signals on T2WI, low
enhancement and absence of hydrocephalus were also observed in
extraventricular CPPs.
CPPs are rare primary intracranial tumors, usually
occurring intraventricularly. Their most frequent location is the
lateral ventricle, followed by the fourth and third ventricles
(10). Their characteristic MRI
appearance has been previously described in a number of studies
(4–6,11). In
addition, following contrast injection, they demonstrate notable
homogeneous or heterogeneous enhancement (11). Hydrocephalus has been noted in a high
percentage of patients with CPPs (5,12).
Metathetic or extraventricular CPPs are uncommon (8,13,14). The imaging findings of
extraventricular CPPs are seldom reported (4,15), and
these CPPs have often been misdiagnosed as other intracranial
tumors or lesions (11,16). They may occur in cisterns, the spinal
subarachnoid space and the brain parenchyma. Direct extension of
the intraventricular tumor and dissemination along the
cerebrospinal fluid (CSF) pathways by an intraventricular tumor are
the common pathogeneses of metathetic CPPs (8). They may also occur primarily in the
extraventricular region, which is extremely rare and considered to
originate from the choroid plexus, extending from the ventricle or
ectopic choroid plexus (8).
Multifocal CPPs are also extremely rare. They may be a result of
dissemination along the CSF pathways by an intraventricular tumor
(17,18). In one of our cases, although the tumor
in the foramen of Luschka was extremely small, early dissemination
to the cisterna magna and spinal subarachnoid space had
occurred.
Multi-lobulated contours are a typical morphological
characteristic of CPPs, and are often said to have a
cauliflower-like or mulberry-like shape (19). However, for extraventricular CPPs, we
observed that a number of contours were atypical, with a round or
relatively regular shape, accounting for 45.5% of cases in our
series (Table II). CPPs have
relatively homogeneous MRI signals in most cases (19). Occasionally, hypointense signals on
T1WI and T2WI have been noted due to rich vessels or small
calcification (4,6). In our study, 70% of extraventricular
CPPs demonstrated a typical T1WI and T2WI signal, which indicated
that there may be no notable difference on MRI signals between
intraventricular CPPs and extraventricular CPPs. However, atypical
MRI signals of extraventricular CPPs were also observed. Two
extraventricular CPPs with a mulberry-like shape and typical MRI
signal were corrected diagnosed in this study, which suggested that
contours and MRI signals may be useful in the diagnosis of
extraventricular CPPs.
The presence of necrosis has been considered as
malignancy criteria (20,21). None of the cases in our series had
notable necrosis, either on the MRI or under a microscope, which is
consistent with the benign nature of CPPs.
Typical CPPs are known as hypervascular tumors, thus
characterized by marked enhancement on enhanced MRI. Peritumoral or
intratumoral rich signal voids may also be observed. However,
certain studies also reported that mild enhancement (22), moderate enhancement (4) or no enhancement (23) could be observed on MRI of
extraventricular CPPs. These authors considered that
extraventricular CPPs do not have the same rich blood supply as
intraventricular CPPs. However, Stafrace and Molloy (15) revealed that notable enhancement could
be also observed in CPP located in the CPA. In our series, atypical
enhancement (none, mild or moderate enhancement) was observed in
six of the 11 masses. Our study reveals that weak or strong
enhancement may occur in extraventricular CPPs.
Radiological hydrocephalus is a characteristic
imaging finding in CPPs. This could be due to overproduction of CSF
by the tumor or obstruction of the CSF pathway by compressing the
ventricle (4). CPPs are also
characterized by significant vessel hyperplasia under the
microscope (24). However, certain
CPPs are not accompanied by hydrocephalus, regardless of the size
of the tumor (4,23,25). In
our study, extraventricular CPPs without hydrocephalus were
observed in four patients. Hydrocephalus was also observed in six
patients with extraventricular CPPs. We speculated that
extraventricular CPPs may also cause notable local hydrocephalus of
the ventricle by compressing the route between the temporal horn or
postcornu and body of lateral ventricle, without apparent dilation
of other parts of the ventricle.
Highly suspected extraventricular CPPs should be
differentiated from common tumors at this location; for example,
neurinoma and meningioma in the CPA. Neurinomas, the most common
tumor in the CPA, usually have more frequent necrosis, cystic
change and low signal intensity on T1WI (26). Their oval shape, broad dural base,
strong homogeneous enhancement and ‘dural tail sign’ are useful in
determining the diagnosis of meningiomas (27). CPPs in cerebral parenchyma are
extremely rare and usually have other radiologically atypical
appearances. It is difficult to differentiate these CPPs from other
parenchymatous tumors.
The present study has certain deficiencies and
limitations. Firstly, as a retrospective study performed in several
hospitals, the imaging examinations in certain cases were not
comprehensive enough. For example, the MRI sequences were not
integrated in all cases, and the sequence exhibiting calcification
was not used. Secondly, the number of cases was low, and the varied
imaging appearances of CPPs need to be further summarized and
analyzed.
In conclusion, the misdiagnosis rate of
extraventricular CPPs is high. They manifest a variety of typical
and atypical MRI findings, and may be easily misdiagnosed as other
diseases, including meningioma, neurinoma and ependymoma. In
addition to the typical imaging findings, round or oval contours,
hyperintense signals on T1WI and T2WI or slightly hyperintense
signals on T1WI or hypo-/hyperintense signals on T2WI, low
enhancement and absence of hydrocephalus are among the atypical
imaging findings of extraventricular CPPs. Understanding the
imaging appearances of extraventricular CPPs allows a more accurate
preoperative diagnosis and differential diagnosis.
References
|
1
|
Khoddami M and Gholampour Shahaboddini R:
Choroid plexus papilloma of the cerebellopontine angle. Arch Iran
Med. 13:552–555. 2010.PubMed/NCBI
|
|
2
|
Wolff JE, Sajedi M, Brant R, Coppes MJ and
Egeler RM: Choroid plexus tumours. Br J Cancer. 87:1086–1091. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan LA, Fontes RB and Byrne RW:
Retrosigmoid approach for resection of an extraventricular choroid
plexus papilloma in the cerebellopontine angle. Neurosurg Focus.
36(1): Suppl. 12014. View Article : Google Scholar
|
|
4
|
Shin JH, Lee HK, Jeong AK, Park SH, Choi
CG and Suh DC: Choroid plexus papilloma in the posterior cranial
fossa: MR, CT and angiographic findings. Clin Imaging. 25:154–162.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Menon G, Nair SN, Baldawa SS, Rao RB,
Krishnakumar KP and Gopalakrishnan CV: Choroid plexus tumors: an
institutional series of 25 patients. Neurol India. 58:429–435.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Girardot C, Boukobza M, Lamoureux JP,
Sichez JP, Capelle L, Zouaoui A, Bencherif B and Metzger J: Choroid
plexus papillomas of the posterior fossa in adults: MR imaging and
gadolinium enhancement. Report of four cases and review of the
literature. J Neuroradiol. 17:303–318. 1990.(In English and
French). PubMed/NCBI
|
|
7
|
McEvoy AW, Harding BN, Phipps KP, Ellison
DW, Elsmore AJ, Thompson D and Harkness W: Management of choroid
plexus tumours in children: 20 years experience at a single
neurosurgical centre. Pediatr Neurosurg. 32:192–199. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borota O Casar, Jacobsen EA and Scheie D:
Bilateral atypical choroid plexus papillomas in cerebellopontine
angles mimicking neurofibromatosis 2. Acta Neuropathol.
111:500–502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahta A, Kim RY and Kesari S: Fourth
ventricular choroid plexus papilloma. Med Oncol. 29:1285–1286.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonneville F, Savatovsky J and Chiras J:
Imaging of cerebellopontine angle lesions: an update. Part 2:
Intra-axial lesions, skull base lesions that may invade the CPA
region and non-enhancing extra-axial lesions. Eur Radiol.
17:2908–2920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coates TL, Hinshaw DB, Peckman N, Thompson
JR, Hasso AN, Holshouser BA and Knierim DS: Pediatric choroid
plexus neoplasms: MR, CT and pathologic correlation. Radiology.
173:81–88. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugiyama K and Kurisu K: Choroid plexus
tumor-choroid plexus papilloma and choroid plexus carcinoma.
Ryoikibetsu Shokogun Shirizu. 28:57–64. 2000.(In Japanese).
|
|
13
|
DeMarchi R, Al Khalidi H, Fazl M and
Bilbao JM: Primary choroid plexus papilloma of the cauda equina. A
case report. Can J Neurol Sci. 37:416–418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gelabert-Gonzalez M, Serramito-Garcia R,
Arcos-Algaba A, Santin-Amo JM and Allut AG: Extraventricular
choroid plexus papilloma. Rev Neurol. 48:559–560. 2009.(In
Spanish). PubMed/NCBI
|
|
15
|
Stafrace S and Molloy J: Extraventricular
choroid plexus papilloma in a neonate. Pediatr Radiol. 38:5932008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tena-Suck ML, Lopez-Gomez M, Salinas-Lara
C, Arce-Arellano RI, Biol AS and Renbao-Bojorquez D: Psammomatous
choroid plexus papilloma: three cases with atypical
characteristics. Surg Neurol. 65:604–610. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scholsem M, Scholtes F, Robe PA, Bianchi
E, Kroonen J and Deprez M: Multifocal choroid plexus papilloma: a
case report. Clin Neuropathol. 31:430–434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McCall T, Binning M, Blumenthal DT and
Jensen RL: Variations of disseminated choroid plexus papilloma: 2
case reports and a review of the literature. Surg Neurol. 66:62–67.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jaiswal AK, Jaiswal S, Sahu RN, Das KB,
Jain VK and Behari S: Choroid plexus papilloma in children:
diagnostic and surgical considerations. J Pediatr Neurosci.
4:10–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wyatt-Ashmead J, Kleinschmidt-DeMasters B,
Mierau GW, Malkin D, Orsini E, McGavran L and Foreman NK: Choroid
plexus carcinomas and rhabdoid tumors: phenotypic and genotypic
overlap. Pediatr Dev Pathol. 4:545–549. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barreto AS, Vassallo J and Lde S Queiroz:
Papillomas and carcinomas of the choroid plexus: histological and
immunohistochemical studies and comparison with normal fetal
choroid plexus. Arq Neuropsiquiatr. 62:600–607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao A, Xu J, He X and You C:
Extraventricular choroid plexus papilloma in the brainstem. J
Neurosurg Pediatr. 12:247–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Talacchi A, De Micheli E, Lombardo C,
Turazzi S and Bricolo A: Choroid plexus papilloma of the
cerebellopontine angle: a twelve patient series. Surg Neurol.
51:621–629. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shogan P, Banks KP and Brown S: AJR
teaching file: intraventricular mass. Am J Roentgenol. 189:S55–S57.
2007. View Article : Google Scholar
|
|
25
|
Li S and Savolaine ER: Imaging of atypical
choroid plexus papillomas. Clin Imaging. 20:85–90. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo B, Sun G, Zhang B, Liang K, Wen J and
Fang K: Neuroradiological findings of intracranial schwannomas not
arising from the stems of cranial nerves. Br J Radiol.
77:1016–1021. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wilms G, Lammens M, Marchal G, Van
Calenbergh F, Plets C, Van Fraeyenhoven L and Baert AL: Thickening
of dura surrounding meningiomas: MR features. J Comput Assist
Tomogr. 13:763–776. 1989. View Article : Google Scholar : PubMed/NCBI
|