Introduction
The gold standard following segmental mandibulectomy
is vascularized autologous bone grafts in the form of the fibula
flap (1,2). These autografts possess osteoinduction,
osteoconduction and osseointegration properties. However, the use
of autogenous bone does not necessarily guarantee a successful
outcome in bone reconstruction. In cases of large bone defects,
there are limits to the amount of cancellous bone grafts that can
be harvested from a patient's bones (3). Autogenous bone grafts may also increase
the risk of morbidity at the donor site (4,5).
Considering this, there is an interest in
allogeneic, xenogeneic and synthetic bone grafts for the
reconstruction of bone defects. However, allogeneic and xenogeneic
bone grafts have a risk of transmitting various diseases, the
potential to develop incompatibility reactions and chronic
granulomatous inflammation, and religious restrictions (6–8). The main
advantage of synthetic bone grafts is their biocompatibility and
bioresorption; however, they are unable to provide full bone
regeneration. This is due to insufficient vascularization, weak
osteoconductivity, inadequacy of bone formation, lower mechanical
resistance and stability of the graft (9,10).
In recent years, biological scaffold materials
composed of extracellular matrix (ECM) have been successfully used
for the remodeling of a variety of damaged soft tissues and bones,
including those in the maxillofacial region (11–16).
Tissue-specific ECM serves an important role in promoting tissue
regeneration and repair. In order to enhance the osteointegration
of bone ECM, bone marrow progenitor cells and platelet rich plasma
may be used (17).
Bone marrow stem cells (BMSCs) demonstrate excellent
potential for use in cellular therapy and regenerative medicine
(18). However, there is currently a
growing interest in the process of preservation of various cells by
lyophilization (19,20). BMSCs paracrine factors and their role
in the process of damaged tissue and organ restoration are
increasingly being studied (21–25).
The present study hypothesized that a biologically
active bone (BAB) graft may be developed by using freeze-dried BMSC
paracrine factors and a three-dimensional bone scaffold created
from bovine cancellous femoral bones. In the present study, the
method for producing BAB grafts and the possibility of applying
this product in patients needing reconstruction of large defects of
mandibular bone following tumor resection is presented and
discussed.
Materials and methods
Patient information
A total of one male and three female patients (age
range, 38–55 years) with primary tumors of the mandible, who
underwent surgical treatment between January 2008 and December 2015
in the Cancer Research Center of Tbilisi, Georgia, were enrolled
into the present study. All patients signed written informed
consent for the present study, which was conducted according to the
guidelines of the 1975 Declaration of Helsinki and approved by the
Ethics Committee of the Cancer Research Centre in Tbilisi,
Georgia.
Patients underwent preoperative orthopantomography,
bone scanning, computed tomography (CT) and magnetic resonance
imaging. The lesions were biopsied, and the histopathological
examination revealed osteoblastoma in three patients and
osteoblastic osteosarcoma in one patient. All patients underwent
hemimandibulectomy and following tumor resection, mandibular bone
defects were reconstructed with autogenous rib grafts in three
patients, and BAB graft was used in one patient. The graft-host
interfaces were covered with decellularized human amnion/chorion
membrane (dHACM) graft.
Fabrication of BAB grafts
Fresh samples of bovine femur bones were collected
from a slaughterhouse (Tbilisi, Georgia) 3–4 h subsequent to
slaughter. Following bacteriological analysis, the bone was
separated from the soft tissue. The femur bones were rinsed in
running water for 1 h and cut with a saw (JG210 Bone Cutting
machine; Shandong China Coal Industrial & Mining Supplies Group
Co., Ltd., Shandong, China) into bone fragments of 15×4×2 cm.
Bone fragments were placed in deionized water
solution containing 5,000 units/ml heparin (Sigma-Aldrich; EMD
Millipore, Billerica, MA, USA) for 24 h to remove blood components
present in the bone. Subsequently, the bone fragments were rinsed
with 800 ml 0.9% saline solution and frozen at −80°C for at least
12 h (fragments were fully submerged in the solution). The frozen
fragments of bone were thawed at 4°C and rinsed with
phosphate-buffered saline (PBS; Sigma-Aldrich; EMD Millipore) prior
to sequential washes in 0.01, 0.1 and 1% sodium dodecyl sulfate
(SDS; Sigma-Aldrich; EMD Millipore) solution for 72 h. Bone
fragments were rinsed with distilled H2O for 45 min,
followed by 1% Triton X-100 (Sigma-Aldrich; EMD Millipore) for 2 h
to remove the remaining SDS. Following Triton X-100 treatment, the
decellularized bone fragments were rinsed with PBS for 4 h.
The scaffolds were placed in a stirrer and rinsed
with a solution containing chloroform and ethanol, initially at a
ratio of 2:1 for 24 h, then at a ratio of 1:2 for the following 24
h. To remove the remaining chloroform and ethanol, the scaffolds
were placed in laboratory grade glass (600-ml PYREX™ Griffin
Beakers; Thermo Fisher Scientific, Inc., Waltham, MA, USA), then
deionized water (400 ml) was added, and the glass was placed on a
compact digital mini rotator (#88880026; Thermo Fisher Scientific,
Inc.), 37°C, with a gentle shaking speed of 50 rpm for 12 h.
Subsequently, the bone fragments were rinsed in fresh deionized
water for 2 h.
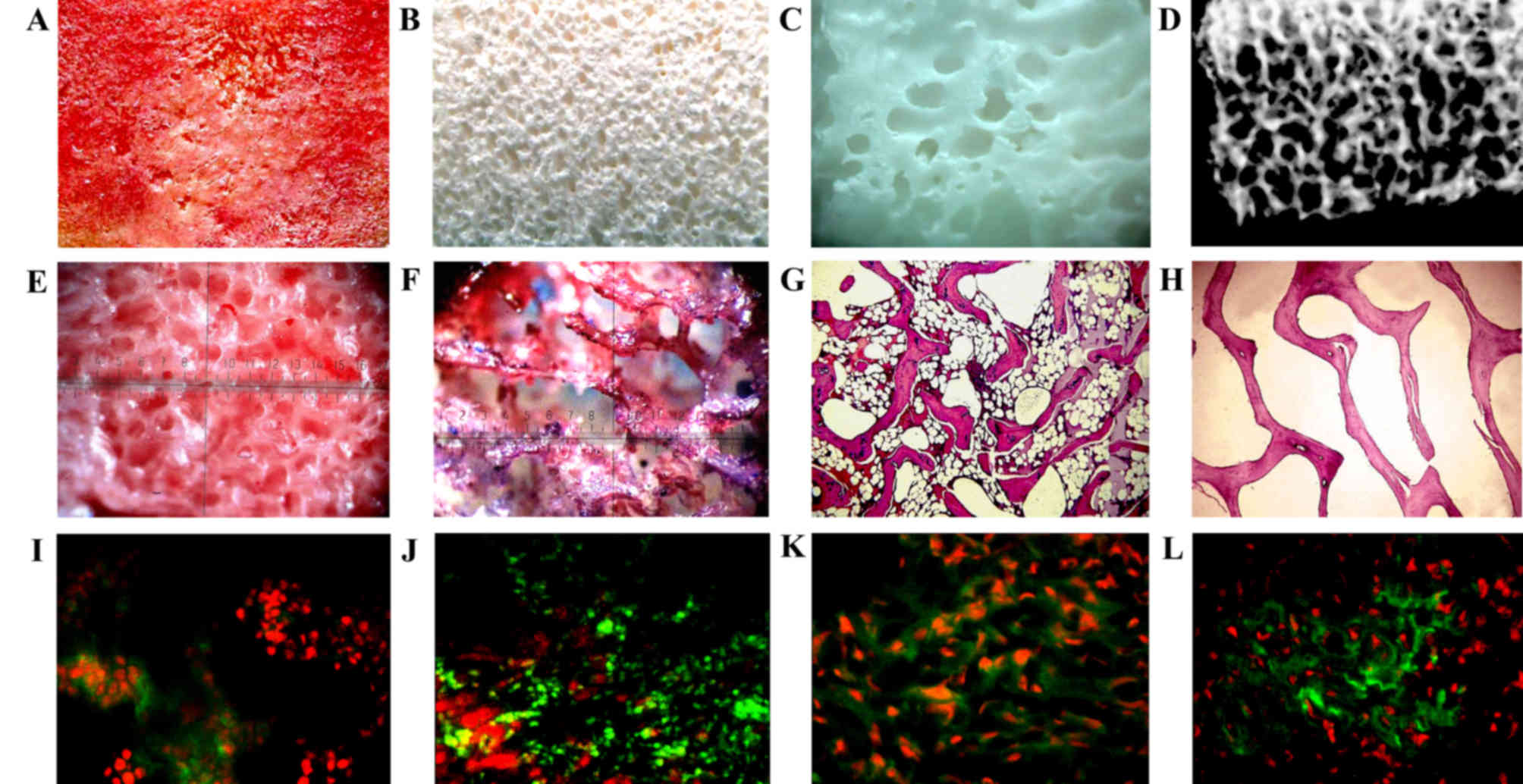
Decellularized bone fragments (Fig. 1A-D) were placed in a stirrer and
rinsed with 4% sodium hypochlorite for 24 h. To remove the
remaining solvent from the deproteinized bone, fragments placed in
laboratory grade glass (600-ml PYREX™ Griffin Beakers), deionized
water (400 ml) was added, and the glass was placed on a compact
digital mini rotator at 37°C, with a gentle shaking speed of 50
rpm, and left for 72 h.
dHACM graft was acquired through a previously
described decellularization protocol (26). The dHACM grafts that were created by
using this method, following lyophilization, were packed in a
disposable plastic bag and sterilized with gamma radiation (dose of
15 kGy). The dHACM grafts were stored aseptically at room
temperature until use. Prior to the transplantation, the dHACM was
rehydrated in a 0.9% saline solution for 30 min.
DNA quantification of BAB grafts
DNA was isolated from the BAB tissue in accordance
with manufacturer's protocol using a commercial extraction kit
(G-spin Total DNA Extraction Mini kit; iNtRON Biotechnology, Inc.,
Seongnam, South Korea). The total DNA was determined using a
spectrophotometer (NanoDrop 1000; Thermo Fisher Scientific, Inc.)
at 260 nm.
Collection of human autologous bone
marrow stem cells
A total of 4 days prior to the reconstruction of
mandibular defects, between 180–200 ml of bone marrow was aspirated
from the patient's anterior iliac crest under local anesthesia, and
placed in sterile tubes containing heparin. The aspirates were
diluted 1:2 with PBS. The mononuclear fraction was isolated by
density gradient centrifugation at 400 × g for 30 min at room
temperature using Ficoll Paque Plus or Ficoll Paque Premium
solution (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA).
Flow cytometry and viability
testing
The 0.5 ml final cell product was subjected to a
trypan blue dye exclusion test and flow cytometric analysis. The
viability test was performed using 0.4% trypan blue solution
(Sigma-Aldrich; EMD Millipore), according to the manufacturer's
protocol. For cell immunophenotyping, the cell suspensions were
incubated with anti-human cluster of differentiation (CD) 45
FiTC/CD34 PE (dilution, 1:200; #647821; BD Biosciences, Franklin
Lakes, NJ, USA), anti-human CD271 (dilution, 1:100; #560834; BD
Biosciences) and anti-human stromal cell surface marker (STRO-1)
antibodies (dilution, 1;100, #sc-47733; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), diluted in 0.5% bovine serum albumin/PBS
(Sigma-Aldrich; EMD Millipore) buffer, according to the
manufacturer's instructions. Flow cytometry analysis was performed
on a BD FACSCalibur flow cytometer (BD Biosciences). Mononuclear
CD45−/CD34−/CD271+/STRO-1+
cells were defined as bone marrow mesenchymal stem cells, and their
percentage and absolute count were recorded. Bone marrow
hematopoietic stem cells were determined as the
CD45+/CD34+ mononuclear cell population.
Cell seeding
The BAB graft was thoroughly rinsed in sterile PBS
for 30 min and immersed in RPMI-1640 medium (Sigma-Aldrich; EMD
Millipore) so that only the bottom half of the graft was covered by
the media.
In total, 1.2×108 mononuclear-enriched
cells, suspended in 10 ml saline, were seeded on the top surface of
the graft. After 15 min, the grafts were turned over, and the same
quantity of cells was seeded on the opposite side. This process was
repeated every 15 min for up to 1 h to facilitate uniform cell
distribution (Fig. 1E and F).
Decellularized bovine bone grafts with mononuclear enriched cells
were cultivated in a humidified incubator (37°C, 5% CO2)
for 4 days. The differentiation medium was as follows: Dulbecco's
modified Eagle's medium-low glucose (Sigma-Aldrich; EMD Millipore)
supplemented with 10% fetal bovine serum (Sigma-Aldrich; EMD
Millipore), 1 µM dexamethasone (Sigma-Aldrich; EMD Millipore), 50
µg/ml ascorbic acid (Sigma-Aldrich; EMD Millipore), 10 mM sodium
β-glycerophosphate (Sigma-Aldrich; EMD Millipore) and 1%
penicillin/streptomycin (Sigma-Aldrich; EMD Millipore).
Subsequently, the bone graft with the seeded mononuclear-enriched
cells was lyophilized.
Lyophilization protocol
The process of lyophilization consisted of two
stages: Deep freezing of objects and thawing in a vacuum. Following
seeding, the BAB graft with BMSCs complex was freeze-dried with a
lyophilizer (Heto PowerDry PL6000 Freeze Dryer; Sjia Lab, Shenzhen,
China). The temperature of the lyophilizer was set at −40°C, and
the vacuum was controlled under 10–15 Pa. The thawing procedure
lasted for 18–24 h. The chamber was warmed up to 15–20°C at a rate
of 0.2°C/min and sustained for 6–8 h.
Following lyophilization, the BAB grafts were packed
in disposable plastic bags (Wipak Medical, Bomlitz, Germany),
following which they were sterilized with gamma-ray doses of 15 kGy
and stored in sterile conditions at room temperature until use.
Histology and fluorescence
immunohistochemistry of BAB grafts containing freeze-dried
BMSCs
Samples of bovine bone were harvested prior to and
following decellularization, and were fixed in 10% neutral buffered
formalin, embedded in paraffin, sectioned and stained with
hematoxylin and eosin, and Masson's trichrome staining.
Fluorescence immunohistochemistry was performed
according to the following methods. For the staining of
decellularized bovine bone grafts containing freeze-dried BMSCs
with antibodies against CD105/endoglin, bone morphogenetic
protein-2 (BMP-2), collagen, type I α1 (Iα1) and fibronectin,
formalin-fixed paraffin-embedded tissue sections 5-µm thick were
cut on a rotary microtome, mounted on charged slides and baked
overnight at 50°C in an oven. All staining procedures were
performed at room temperature.
The slides were deparaffinized and rehydrated in
water. Antigen retrieval was performed using steam and proteinase K
digestion methods. Following antigen retrieval, the slides were
allowed to cool at room temperature for 20 min, following which the
slides were washed three times with PBS for 5 min each, and then
blocked with 3% H2O2. Subsequently, the
slides were incubated in the primary antibody: CD105/endoglin at
1:100 (#sc-19793; Santa Cruz Biotechnology, Inc.), BMP-2 at 1:100
(#sc-6895; Santa Cruz Biotechnology, Inc.) collagen Iα1 at 1:100
(#sc-25974; Santa Cruz Biotechnology, Inc.) and fibronectin at
1:200 (#sc-8422; Santa Cruz Biotechnology, Inc.) diluted with
IHC-Tek Antibody Diluent (IHC World, LLC, Woodstock, MD, USA) for 1
h at room temperature. The slides were subsequently washed three
times in PBS and incubated with a biotinylated secondary antibody
at 1:800 dilution (anti-rabbit IgG; #B7389; Sigma-Aldrich; EMD
Millipore) for 30 min. The slides were washed in PBS and incubated
with horseradish peroxidase-conjugated streptavidin (#S2438;
Sigma-Aldrich; EMD Millipore) for 30 min, prior to incubation with
3,3′-diaminobenzidine chromogen substrate solution (#D7304;
Sigma-Aldrich; EMD Millipore) for 5–10 min, washing with PBS and
counterstaining with Mayer's hematoxylin. Histological slides were
analyzed by upright microscope (E100; Nikon Corporation, Tokyo,
Japan) and bone fragment tissues by stereoscopic microscope (MBS 9;
Lomo, St. Petersburg, Russia). Immunofluorescence staining was
analyzed by fluorescence microscope (BH2-RFCA; Olympus Corporation,
Tokyo, Japan).
MicroCT
MicroCT scanning was conducted in a µCT 50 compact
cabinet microCT scanner (SCANCO Medical AG, Bassersdorf, Zurich,
Switzerland) using a protocol previously described in detail by Zhu
et al (27).
Scanning electron microscopy of BAB
grafts containing freeze-dried BMSCs
The BAB grafts were dehydrated by processing with an
ethanol solution prior to drying with a tousimis Samdri-780
Critical Point Dryer (Tousimis Research Corporation, Rockville, MD,
USA). Following drying, all tissues were sputter coated lightly
with gold and imaged on a Hitachi Scanning Electron microscope
(Hitachi, Ltd., Tokyo, Japan).
Gene expression analysis of BAB grafts
with freeze-dried BMSCs
The total RNA from the bone tissue was purified
using a miRNeasy mini kit, according to the manufacturer's
instructions (Qiagen GmbH, Hilden, Germany). cDNA was synthesized
using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Quantitative polymerase chain reaction (qPCR)
was performed using iTaq Universal SYBR Green supermix (Bio-Rad
Laboratories, Inc.) and a 7500 Fast Real-Time PCR system (Thermo
Fisher Scientific, Inc.). Thermal cycling conditions were as
follows: 15 min of denaturation at 95°C, followed by 40 cycles of
denaturation for 15 sec at 95°C, annealing for 30 sec at 60°C and
elongation for 20 sec at 72°C. The 18S rRNA was used as an internal
control for gene expression normalization, which is considered to
be the most reliable reference gene for normalization of qPCR data
(28).
The polymerase chain reaction (PCR) amplification
was performed using the following primer sets: BMP7 forward,
5′ACAGACCAAGCACCTCTCCT-3′ and reverse, 5′-CGGTGTGCTCAGGTTTCTAA-3′;
BMP8a forward, 5′-ATTATGGTGGTCAGGGCATT-3′ and reverse,
5′-GCACCGTTATACCTGGCTCT-3′; epidermal growth factor (EGF) forward,
5′-ACTGGGAGCAGACAGAAGGT-3′ and reverse, 5′-ATTAGCCGTGGAGACAGGAG-3′;
vascular endothelial growth factor (VEGF) forward,
5′-AGATTCTGCAAGAGCACCCT-3′ and reverse, 5′-CCTAGGCTCCTCAGAAGTGG-3′;
fibroblast growth factor forward, 5′-ACCGGTACCTGGCTATGAAG-3′ and
reverse, 5′-GTGCCACATACCAACTGGAG-3′; osteopontin forward,
5′-TCCAGGAGTTTCCCTGTTTC-3′ and reverse, 5′-TGACCTTGATAGCCTCATCG-3′;
osteocalcin forward, 5′-CTGCATTCTGCCTCTCTGAC-3′ and reverse,
5′-CCGGAGTCTATTCACCACCT-3′; bone sialoprotein forward,
5′-GAGACAACGGAGAAGAAGCC-3′ and reverse, 5′-CCATAACTGGTCAGCTCCCT-3′;
18S rRNA forward, 5′-TAAAGGAATTGACGGAAGGG-3′ and reverse,
5′-CTGTCAATCCTGTCCGTGTC-3′ (29).
Results
Characterization of BAB graft
Following decellularization, cancellous bone
fragments acquired from bovine femur bones are a light yellow
color; they have a desired three-dimensional porous structure and
may be repopulated by host bone-forming cells (30). The DNA content of the fresh samples of
bovine femur bones prior to treatment was 482 µg/ml. Following the
decellularization procedure, the residual DNA content was <1.4%.
Histological investigation indicated the presence of osteocytes in
the native tissue, whereas in the decellularized tissues, the
presence of cellular material was not observed (Fig. 1G and H). Following decellularization,
the mesh of collagen fibers had retained their structure and was
similar to natural bone. However, the collagen arrangements were
disturbed, with gaps between the collagen fibers.
A cross-section of the BAB graft following cell
seeding indicated the presence of the cells at depth in the
scaffolds, in addition to along the sides of the scaffolds.
However, there were separate areas in the central regions of the
BAB graft where the cells were not homogenously distributed.
Immunohistochemical investigation demonstrated the presence of
CD105/endoglin, BMP-2, collagen Iα1 and fibronectin in the BAB
grafts containing freeze-dried BMSCs (Fig. 1I-L).
Low magnification scanning electron micrographs
demonstrated decellularized bovine bone, observed as a mesh of
collagen fibers that were intact and which were similar to the
structure of natural bone. Freeze-dried BMSCs (Fig. 2A-D) that were seeded on the BAB graft
were scattered between the collagen fibers (Fig. 2E-H), and their arrangement was compact
and orderly.
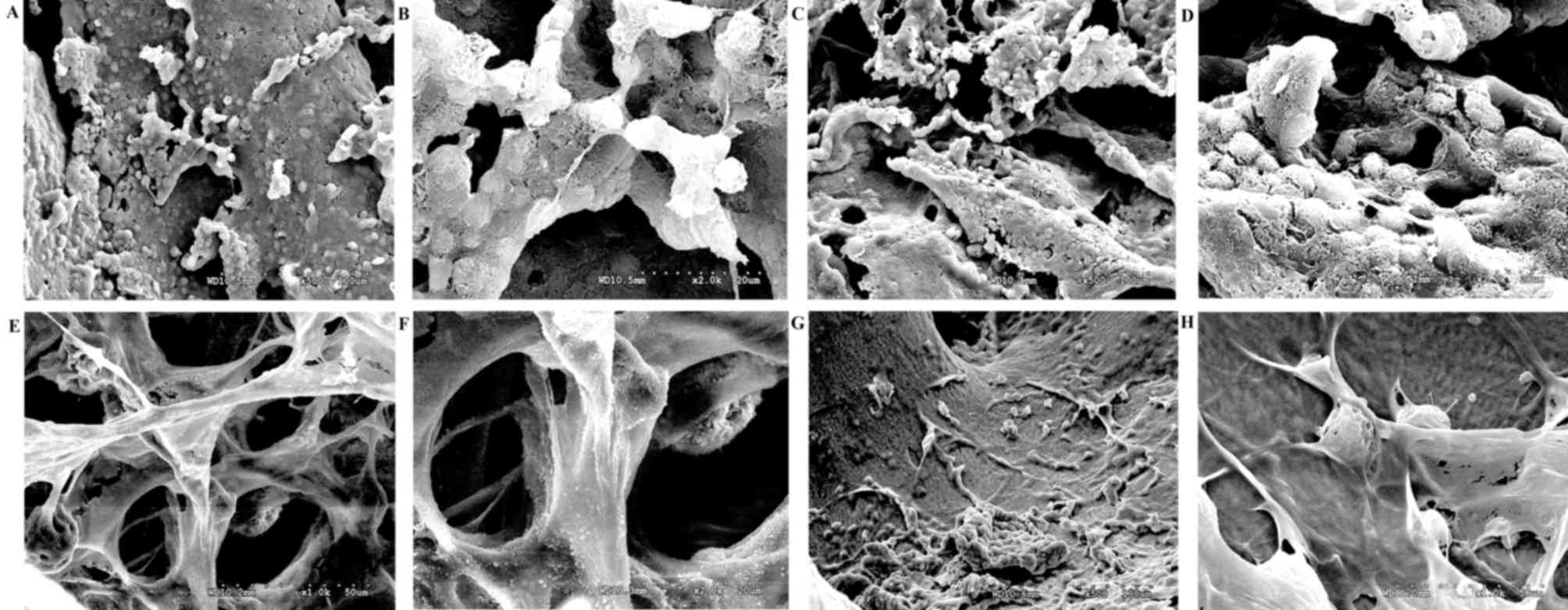 | Figure 2.Scanning electron microscopy of bone
graft and freeze-dried human bone marrow stem cells. (A and B)
Human bone marrow stem cell prior to (magnification, ×500 and
×2,000, respectively) and (C and D) following freeze-drying
(magnification, ×500 and ×2,000, respectively). (E and F) Scanning
electron micrograph of the bone graft (magnification ×1,000 and
×2,000, respectively). (G and H) Scanning electron micrograph of
bone marrow stem cells seeded on the decellularized bone graft
(magnification, ×500 and ×1,000, respectively). |
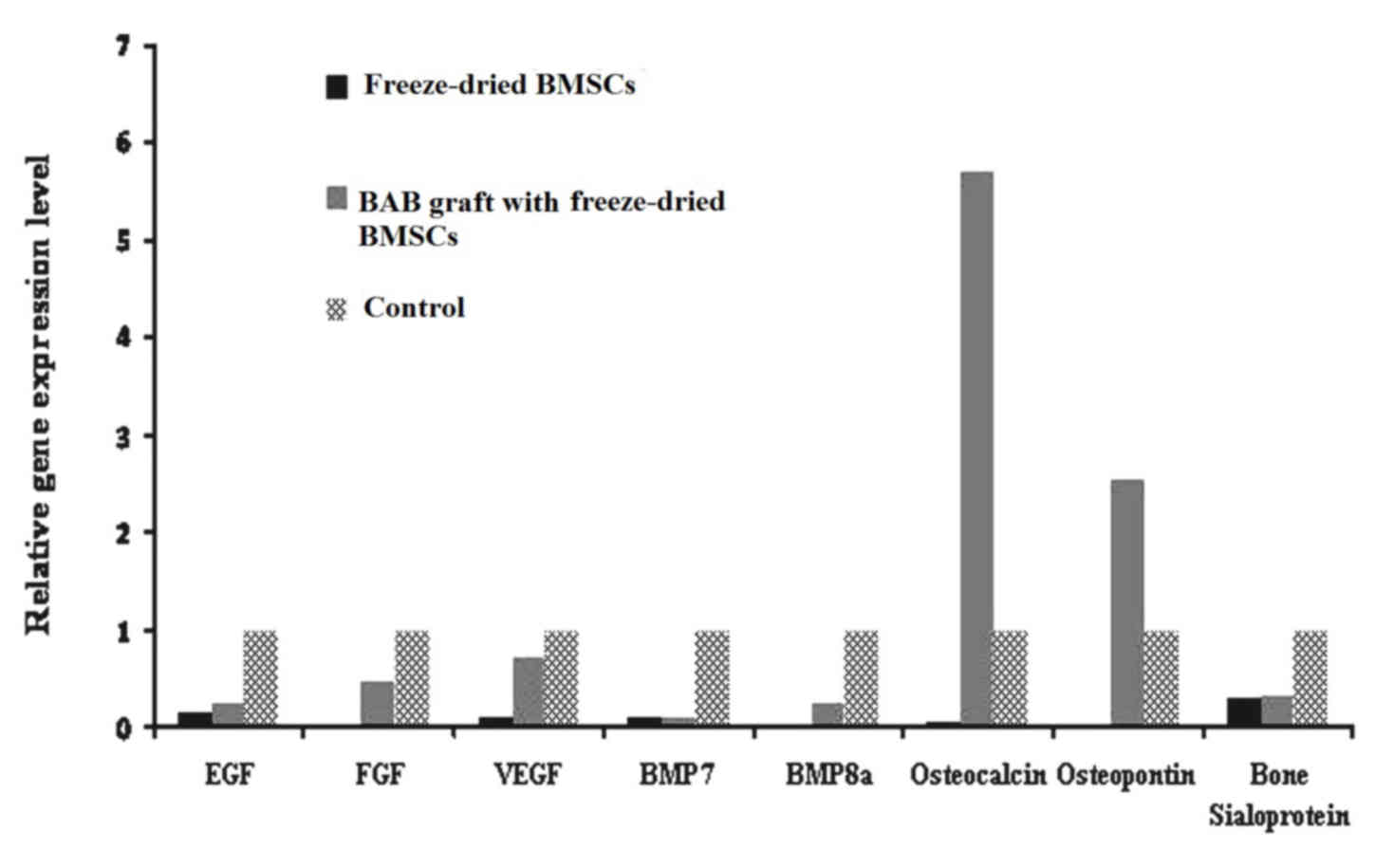
Gene expression analysis demonstrated that the BAB
grafts containing freeze-dried BMSCs expressed a large number of
varying growth factors, in particular osteocalcin and osteopontin,
which may enhance the osteogenic process (Fig. 3).
Clinical data
Out of three female patients that were enrolled in
the present study, two underwent right hemimandibulectomy and one
underwent left hemimandibulectomy. The mandibular resections were
all lateralized and none crossed the symphysis menti. Surgical
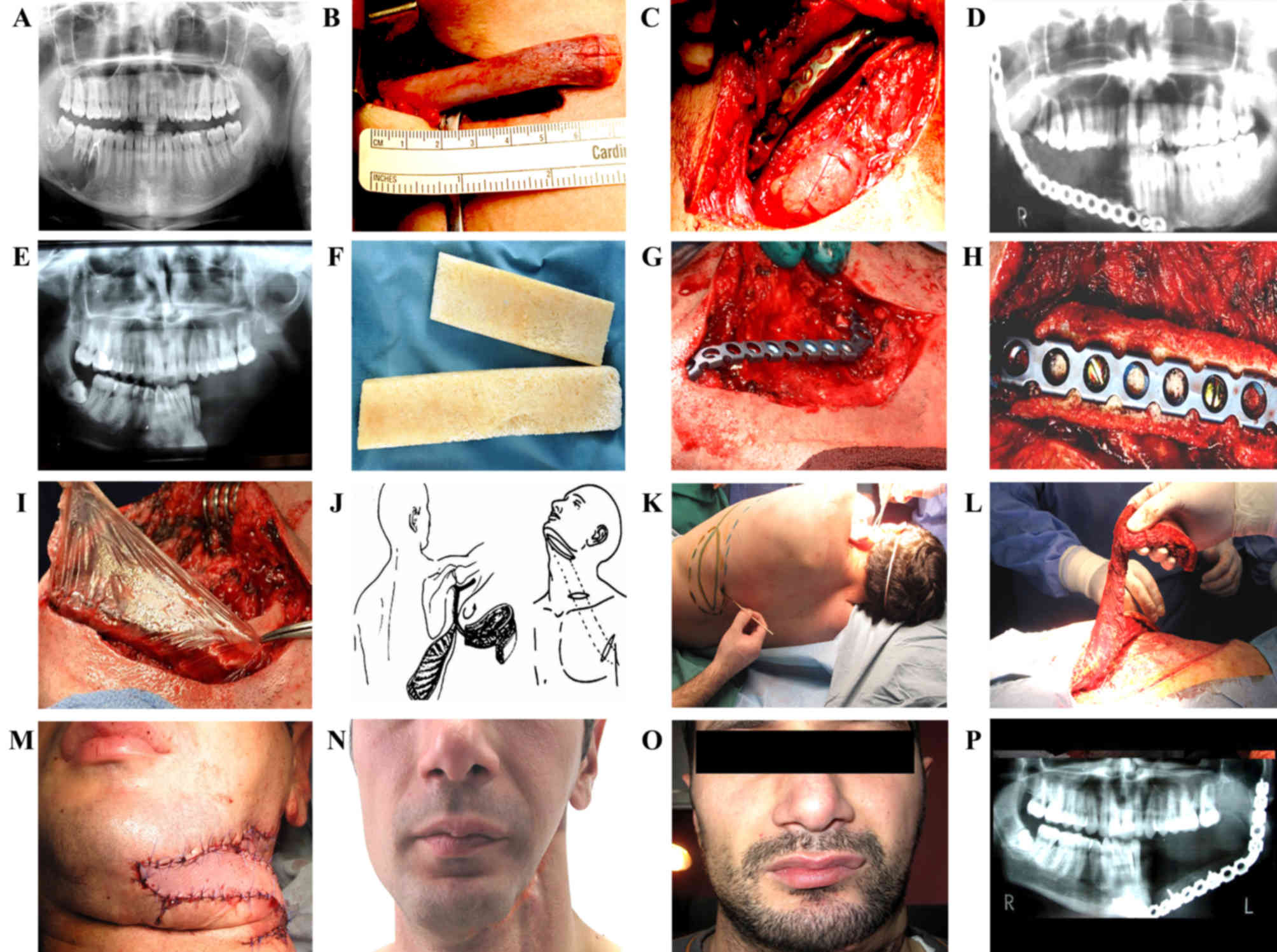
defects were restored with autogenous rib grafts (Fig. 4A and B). Following the stabilization
of the host mandible bone and autologous rib grafts with a titanium
plate, the dHACM graft was placed around the defect (Fig. 4C and D). The dimensions of the dHACM
graft were 10×4 cm, and placement ensured that a 2 cm portion of
the graft overlapped with the intact portions beyond the proximal
and distal defect interfaces. The dHACM graft was then secured in
place (stretched in all directions) with a 2/0 vicryl suture with
measures to assure that no vessels or muscles were included.
In one male patient, aged 38 years, the solid tumor
involved the whole of the left mandible, including the body and
ramus. The lesion was biopsied which indicated osteoblastic
osteosarcoma. The left hemimandible was removed en bloc with the
tumor (Fig. 4E).
Therefore, the initial plan to bridge the bony gap
created with a titanium plate was not possible and the wound was
closed primarily without reconstruction. Following 5 years of
follow-up, complete remission was observed in this patient. For the
reconstruction of the mandible bone defect, the BAB graft
containing freeze-dried BMSC paracrine factors was selected
(Fig. 4F). The titanium plate was
used to stabilize the host bone (Fig. 4G
and H), and additionally a dHACM wrap was used to cover the
defect (Fig. 4I). The thoracodorsal
flap on the vascular pedicle was used for the complete closure of
the soft tissue without tension (Fig.
4J-M). The patient was followed-up every five months following
the reconstruction of the mandible, and no complications were
observed (Fig. 4N and O). A total of
5 months subsequent to mandible bone reconstruction, x-ray imaging
demonstrated bone volume maintenance (Fig. 4P).
Discussion
Bones exhibit an excellent ability for healing via
the natural mechanisms of bone repair (4). However, the healing process in patients
following bone tumor resection may be slow or inadequate (31).
Previous investigations showed that autogenous rib
graft demonstrates a positive effect on the reconstruction of
mandibular bone defects, as the autogenous grafts activate the
mechanisms of bone formation, including osteoconduction,
osteinduction and osteogenesis (32–35).
However, in cases with large defects of the
mandible, reconstruction with autogenous bone grafts represents a
great challenge, as the structure and large size of the graft slows
the revascularization process (36).
A previous study reported non-vascularized graft failure in 17% of
cases where the defect size was 6 cm, and in 75% of cases where the
defect was ≥12 cm (37).
Decellularized bone scaffolds have been considered
as an alternative to autogenous grafts (38–40). The
main drawback for these materials is that they have osteoinductive
and osteoconductive characteristics; however, they lack the
osteogenic properties that are present in autologous grafts
(41). Following the seeding of
freeze-dried BMSC paracrine factors on BAB grafts, which possess
osteoinductive and osteoconductive features, these grafts
additionally exhibited osteogenic properties. The complete absence
or the presence of only minimal DNA following decellularization
means BAB grafts are biocompatible and do not induce
antigen-antibody reactions (41,42).
Once implanted or injected, the BMSCs at the site of
the lesion differentiate into several cell types (43,44).
However, the mechanism of differentiation currently remains to be
elucidated. Recently, BMSC paracrine factors and their role in the
restoration of damaged tissues and organs have received increased
interest (45–47).
BMSC paracrine factors stimulate tissue regeneration
via effects on homing, immunosuppression, differentiation,
angiogenesis, stimulation of endogenous cells and potential
regulation of specific metabolic signaling pathways (25). Following freeze-drying, BMSCs have
been observed to retain >80% of paracrine factors, including
VEGF-1, insulin-like growth factor 1, EGF, hepatocyte growth
factor, keratinocyte growth factor, angiopoietin-1, stromal
cell-derived factor-1, monocyte chemoattractant protein-1 and
erythropoietin (24,48–50).
In order to prevent the invasion of fibrous tissue
between the autologous bone or BAB graft, and the host mandible
bone, dHACM has been used as a barrier membrane. In examined cases
in the present study, it was observed that using dHACM for the
reconstruction of mandibular bone defects enhanced osseointegration
and provided solid protection against fibrous tissue invasion
between the bone graft and host mandible bone. Similar results have
also been previously reported (51–55).
In a previous study (26), the present authors demonstrated that
the dHACM contained type III collagen, glycoproteins and numerous
growth factors, including epidermal growth factor, basic fibroblast
growth factor, keratinocyte growth factor, VEGF, TGFα, TGFβ, PDGF,
hepatocyte growth factor and nerve growth factor. Following the
decellularization of the dHACM, the majority of the growth factors
and cytokines, and the structural and mechanical properties were
preserved. In addition, dHACM is easy to prepare and handle, and by
its use, fibrous tissue invasion can be prevented (51,56).
Micromovements between the host mandibular bone and
any type of donor bone graft prevent bone formation, due to fibrous
tissue invasion (52,53). Non-rigidly fixed defects with no
membrane showed ingrowths of fibroblasts and fibrous nonunions
(54–56).
In conclusion, BAB grafts seeded with freeze-dried
BMSC paracrine factors hold great promise for the future of
mandible defect reconstruction following tumor resection. As a
result, this type of composite graft should be able negate the need
for harvesting donor bone. Preliminary clinical investigations
indicated that BAB grafts containing freeze-dried BMSCs paracrine
factors may be used for the reconstruction of large mandibular bone
defects following tumor resection. The dHACM graft efficiently
induces the bone formation, and protects against fibrous tissue
invasion between the bone grafts and host bone.
References
|
1
|
Chim H, Salgado CJ, Mardini S and Chen HC:
Reconstruction of mandibular defects. Semin Plast Surg. 24:188–197.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yap YL, Lim J, Ong WC, Yeo M, Lee H and
Lim TC: Stabilization of mobile mandibular segments in mandibular
reconstruction: Use of spanning reconstruction plate.
Craniomaxillofac Trauma Reconstr. 5:123–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clokie CM and Sándor GK: Reconstruction of
10 major mandibular defects using bioimplants containing BMP-7. J
Can Dent Assoc. 4:67–72. 2008.
|
|
4
|
Frohlich M, Grayson WL, Wan LQ, Marolt D,
Drobnic M and Vunjak-Novakovic G: Tissue engineered bone grafts:
Biological requirements, tissue culture and clinical relevance.
Curr Stem Cell Res Ther. 3:254–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finkemeier CG: Bone-grafting and
bone-graft substitutes. J Bone Joint Surg Am 84-A. 454–464. 2002.
View Article : Google Scholar
|
|
6
|
Kaláb M, Karkoška J, Kamínek M and Šantavý
P: Successful three-year outcome in a patient with allogenous
sternal bone graft in the treatment of massive post-sternotomy
defects. Int J Surg Case Rep 7C. 6–9. 2014.
|
|
7
|
Schleicher I, Lips KS, Sommer U, Schappat
I, Martin AP, Szalay G and Schnettler R: Allogenous bone with
collagen for repair of deep osteochondral defects. J Surg Res.
185:667–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gomes KU, Carlini JL, Biron C, Rapoport A
and Dedivitis RA: Use of allogeneic bone graft in maxillary
reconstruction for installation of dental implants. J Oral
Maxillofac Surg. 66:2335–2358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oryan A, Alidadi S, Moshiri A and Maffulli
N: Bone regenerative medicine: Classic options, novel strategies
and future directions. J Orthop Surg Res. 9:182014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Friesenbichler J, Maurer-Ertl W, Sadoghi
P, Pirker-Fruehauf U, Bodo K and Leithner A: Adverse reactions of
artificial bone graft substitutes: Lessons learned from using
tricalcium phosphate geneXR. Clin Orthop Relat Res. 472:976–682.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han F, Dong Y, Su Z, Yin R, Song A and Li
S: Preparation, characteristics and assessment of a novel
gelatin-chitosan sponge scaffold as skin tissue engineering
material. Int J Pharm. 476:124–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Noth U, Schupp K, Heymer A, Kall S, Jakob
F, Schütze N, Baumann B, Barthel T, Eulert J and Hendrich C:
Anterior cruciate ligament constructs fabricated from human
mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy.
7:447–455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mohan N, Nair PD and Tabata Y: A 3D
biodegradable protein based matrix for cartilage tissue engineering
and stem cell differentiation to cartilage. J Mater Sci Mater Med.
20:(Suppl 1). S49–S60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fröhlich M, Grayson WL, Marolt D, Gimble
JM, Kregar-Velikonja N and Vunjak-Novakovic G: Bone grafts
engineered from human adipose-derived stem cells in perfusion
bioreactor culture. Tissue Eng Part A. 16:179–189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Ahmad S, Shu XZ, Sanders RK,
Kopesec SA and Prestwich GD: Accelerated repair of cortical bone
defects using a synthetic extracellular matrix to deliver human
demineralized bone matrix. J Orthop Res. 24:1454–1462. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kinoshita Y and Maeda H: Recent
developments of functional scaffolds for craniomaxillofacial bone
tissue engineering applications. ScientificWorldJournal.
2013:8631572013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang SZ, Qian H, Wang Z, Fan JL, Zhou Q,
Chen GM, Li R, Fu S and Sun J: Preliminary study on the
freeze-drying of human bone marrow-derived mesenchymal stem cells.
J Zhejiang Univ Sci B. 11:889–894. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fisher JN, Peretti GM and Scotti C: Stem
cells for bone regeneration: From cell-based therapies to
decellularised engineered extracellular matrices. Stem Cells Int.
2016:93525982016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ward MA, Kaneko T, Kusakabe H, Biggers JD,
Whittingham DG and Yanagimachi R: Long-term preservation of mouse
spermatozoa after freeze-drying and freezing without
cryoprotection. Biol Reprod. 69:2100–2108. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Natan D, Nagler A and Arav A:
Freeze-drying of mononuclear cells derived from umbilical cord
blood followed by colony formation. PLoS One. 4:e52402009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Linero I and Chaparro O: Paracrine effect
of mesenchymal stem cells derived from human adipose tissue in bone
regeneration. PLoS One. 9:e1070012014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nowotny J, Farack J, Vater C, Johnsen M,
Gelinsky M, Tonn T and Kasten P: Translation of cell therapy into
clinical practice: Validation of an application procedure for bone
marrow progenitor cells and platelet rich plasma. J Appl Biomater
Funct Mater. 14:e1–e8. 2015.
|
|
23
|
Liang X, Ding Y, Zhang Y, Tse HF and Lian
Q: Paracrine mechanisms of mesenchymal stem cell-based therapy:
Current status and perspectives. Cell Transplant. 23:1045–1059.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng Y, Xuan M, Zou J, Liu H, Zhuo Z, Wan
Y and Cheng B: Freeze-dried rat bone marrow mesenchymal stem cell
paracrine factors: A simplified novel material for skin wound
therapy. Tissue Eng Part A. 21:1036–1046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Burdon TJ, Paul A, Noiseux N, Prakash S
and Shum-Tim D: Bone marrow stem cell derived paracrine factors for
regenerative medicine: Current perspectives and therapeutic
potential. Bone Marrow Res. 2011:2073262011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kakabadze Z, Mardaleishvili K, Loladze G,
Javakhishvili I, Chakhunashvili K, Karalashvili L, Sukhitashvili N,
Chutkerashvili G, Kakabadze A and Chakhunashvili D: Clinical
application of decellularized and lyophilized human amnion/chorion
membrane grafts for closing post-laryngectomy pharyngocutaneous
fistulas. J Surg Oncol. 113:538–543. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu S, Zhu Q, Liu X, Yang W, Jian Y, Zhou
X, He B, Gu L, Yan L, Lin T, et al: Three-dimensional
reconstruction of the microstructure of human acellular nerve
allograft. Sci Rep. 6:306942016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim BR, Nam HY, Kim SU, Kim SI and Chang
YJ: Normalization of reverse transcription quantitative-PCR with
housekeeping genes in rice. Biotechnol Lett. 25:1869–1872. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–88. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lawrence BJ and Madihally SV: Cell
colonization in degradable 3D porous matrices. Cell Adh Migr.
2:9–16. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sterling JA and Guelcher SA: Biomaterial
scaffolds for treating osteoporotic bone. Curr Osteoporos Rep.
12:48–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zou W and Chen X: Osteogenesis and
ototoxicity of a novel preparation of autogenous bone cement:
Implications for mastoid obliteration. Otolaryngol Head Neck Surg.
151:1020–1027. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tde M Tera, Prado RF, De Marco AC,
Santamaria MP and Jardini MA: The RANK/RANKL/OPG interaction in the
repair of autogenous bone grafts in female rats with estrogen
deficiency. Braz Oral Res. 28:S1806-83242014000100261. 2014.
|
|
34
|
Bastos AS, Spin-Neto R, Conte-Neto N,
Galina K, Boeck-Neto RJ, Marcantonio C, Marcantonio E and
Marcantonio E Jr: Calvarial autogenous bone graft for maxillary
ridge and sinus reconstruction for rehabilitation with dental
implants. J Oral Implantol. 40:469–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Al-Nawas B and Schiegnitz E: Augmentation
procedures using bone substitute materials or autogenous bone-a
systematic review and meta-analysis. Eur J Oral Implantol. 7:(Suppl
2). S219–S234. 2014.PubMed/NCBI
|
|
36
|
Koerdt S, Siebers J, Bloch W, Ristow O,
Kuebler AC and Reuther T: Immunohistochemial study on the
expression of von Willebrand factor (vWF) after onlay autogenous
iliac grafts for lateral alveolar ridge augmentation. Head Face
Med. 9:402013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pogrel MA, Podlesh S, Anthony JP and
Alexander J: A comparison of vascularized and nonvascularized bone
grafts for reconstruction of mandibular continuity defects. J Oral
Maxillofac Surg. 55:1200–1206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pennington EC, Dionigi B, Gray FL, Ahmed
A, Brazzo J, Dolinko A, Calderon N, Darrah T, Zurakowski D,
Nazarian A, et al: Limb reconstruction with decellularized,
non-demineralized bone in a young leporine model. Biomed Mater.
10:0150212015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gawlitta D, Benders KE, Visser J, van der
Sar AS, Kempen DH, Theyse LF, Malda J and Dhert WJ: Decellularized
cartilage-derived matrix as substrate for endochondral bone
regeneration. Tissue Eng Part A. 21:694–703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hoffman MD, Van Hove AH and Benoit DS:
Degradable hydrogels for spatiotemporal control of mesenchymal stem
cells localized at decellularized bone allografts. Acta Biomater.
10:3431–3441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gardin C, Ricci S, Ferroni L, Guazzo R,
Sbricoli L, De Benedictis G, Finotti L, Isola M, Bressan E and
Zavan B: Decellularization and delipidation protocols of bovine
bone and pericardium for bone grafting and guided bone regeneration
procedures. PLoS One. 10:e01323442015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Quan TM, Vu DN, My NTN and Ha TLB:
Decellularization of xenogenic bone grafts for potential use as
tissue engineering scaffolds. International Journal of Life Science
and Medical Research Aug. 4:38–45. 2014.
|
|
43
|
Chatterjea A, Meijer G, van Blitterswijk C
and de Boer J: Clinical application of human mesenchymal stromal
cells for bone tissue engineering. Stem Cells Int. 215–625.
2010.
|
|
44
|
Salem HK and Thiemermann C: Mesenchymal
stromal cells: Current understanding and clinical status. Stem
Cells. 28:585–596. 2010.PubMed/NCBI
|
|
45
|
Chen Y, Shao JZ, Xiang LX, Dong XJ and
Zhang GR: Mesenchymal stem cells: A promising candidate in
regenerative medicine. Int J Biochem Cell Biol. 40:815–820. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lda S Meirelles, Fontes AM, Covas DT and
Caplan AI: Mechanisms involved in the therapeutic properties of
mesenchymal stem cells. Cytokine Growth Factor Rev. 20:419–427.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hocking AM and Gibran NS: Mesenchymal stem
cells: Paracrine signaling and differentiation during cutaneous
wound repair. Exp Cell Res. 316:2213–2219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kinnaird T, Stabile E, Burnett MS, Lee CW,
Barr S, Fuchs S and Epstein SE: Marrow-derived stromal cells
express genes encoding a broad spectrum of arteriogenic cytokines
and promote in vitro and in vivo arteriogenesis through paracrine
mechanisms. Circ Res. 94:678–685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rehman J, Traktuev D, Li J, Merfeld-Clauss
S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV
and March KL: Secretion of angiogenic and antiapoptotic factors by
human adipose stromal cells. Circulation. 109:1292–1298. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Takahashi M, Li TS, Suzuki R, Kobayashi T,
Ito H, Ikeda Y, Matsuzaki M and Hamano K: Cytokines produced by
bone marrow cells can contribute to functional improvement of the
infarcted heart by protecting cardiomyocytes from ischemic injury.
Am J Physiol Heart Circ Physiol. 291:H8862006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li W, Ma G, Brazile B, Li N, Dai W, Butler
JR, Claude AA, Wertheim JA, Liao J and Wang B: Investigating the
potential of amnion-based scaffolds as a barrier membrane for
guided bone regeneration. Langmuir. 31:8642–8653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ducheyne P, De Meester P and Aernoudt E:
Influence of a functional dynamic loading on bone ingrowth into
surface pores of orthopedic implants. J Biomed Mater Res.
11:811–838. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Heck D, Nakajima I, Kelly P and Chao E:
The effect of load alteration on the biological and biomechanical
performance of a titanium fiber-metal segmental prosthesis. J Bone
Joint Surg Am. 68:118–126. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gutta R, Baker RA, Bartolucci AA and Louis
PJ: Barrier membranes used for ridge augmentation: Is there an
optimal pore size? J Oral Maxillofac Surg. 67:1218–1225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Amano Y, Ota M, Sekiguchi K, Shibukawa Y
and Yamada S: Evaluation of a poly-l-lactic acid membrane and
membrane fixing pin for guided tissue regeneration on bone defects
in dogs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
97:155–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
von Arx T, Cochran DL, Hermann JS, Schenk
RK and Buser D: Lateral ridge augmentation using different bone
fillers and barrier membrane application. A histologic and
histomorphometric pilot study in the canine mandible. Clin Oral
Implants Res. 12:260–269. 2001. View Article : Google Scholar : PubMed/NCBI
|