Introduction
An irreversible non-enzymatic reaction between
carbohydrates and proteins produce advanced glycation end products
(AGEs) (1,2). The accumulation of AGEs increases with
age (3,4) and is typically higher in patients with
diabetes mellitus (DM) (5). AGEs have
been revealed to be a risk factor for complications of DM (6–9) and an
important toxicity moiety for neuronal cells in Alzheimer's disease
(10–13). Previous studies have demonstrated that
cancer malignancy can be promoted by AGEs (14–16).
Furthermore, the migration of oral cancer cells has been revealed
to be increased by the receptor for AGEs (17). In a clinical setting, patients with DM
exhibit a higher rate of metastasis of oral cancer and a lower
cancer-associated survival rate (18). A strong association appears to exist
between AGEs and oral cancer; however, the underlying mechanism of
the involvement of AGEs in oral cancer remains to be
elucidated.
Antioxidant responsive element is regulated by
nuclear factor-erythroid 2-related factor 2 (Nrf-2), which in turn
regulates the expression of antioxidant genes (19–22).
Reactive oxygen species degradation (23,24),
anti-inflammatory responses (25–28), and
neuroprotection (29) are regulated
by Nrf-2 through the downstream antioxidant genes heme oxygenase 1
(HO-1) and NAD(P)H dehydrogenase quinone 1 (30–33). In
addition, Nrf-2 regulates the apoptotic response via tumor protein
p53 regulation (34). Furthermore, in
oral cancer cells, Nrf-2 and HO-1 upregulation appear to be
associated with apoptosis (35).
A previous study by our group demonstrated that AGEs
regulate cell migration via extracellular signal-regulated kinase
(ERK) phosphorylation (17).
Therefore, it was hypothesized that AGEs regulate Nrf-2 and
downstream signaling pathways through ERK phosphorylation. The
expression of various apoptosis-associated proteins, including
Nrf-2, HO-1, p53, Bcl-2 associated × apoptosis regulator (Bax) and
apoptosis regulator Bcl-x (Bcl-xl), in SAS oral cancer cells
following treatment with AGEs was analyzed through western blot
analysis, in order to investigate the role and underlying mechanism
of AGEs in oral cancer.
Materials and methods
Reagents
Phenylmethylsulfonyl fluoride, bovine serum albumin
(BSA), DL-glyceraldehyde, resveratrol and PD98059 were purchased
from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum
(FBS), penicillin, streptomycin and Hank's Balanced Salt Solution
were purchased from Invitrogen (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). GAPDH was purchased from Chemicon International,
Inc. (Temecula, CA, USA). ERK, phosphorylated (p)-ERK, Nrf-2, HO-1,
p53, Bax and Bcl-xl were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Nitrocellulose membranes were purchased
from Pall Corporation (Port Washington, NY, USA). The enhanced
chemiluminescence (ECL) Immobilon western chemiluminescent HRP
substrate kit was purchased from EMD Millipore (Billerica, MA,
USA).
Preparation of AGEs
AGEs were prepared by incubation with BSA (pH 7.4)
in PBS with 20 mM DL-glyceraldehyde at 37°C for 1 week. The product
was dialyzed using dialysis membranes (cat. no. MWCO 6000; Orange
Scientific, Braine-l'Alleud, Belgium) in PBS at 4°C for 2 h, and
the cycle was repeated five times. The product was then
concentrated at 4°C using Amicon Ultra-15 centrifugal filter units
(EMD Millipore) and centrifuged at 830 × g for 30 min prior to
storage at −80°C as described in a previous study (36).
Cell culture and treatment
The oral cancer cell line SAS (Japanese Collection
of Research Bioresources Cell Bank, Osaka, Japan) was cultured in
an atmosphere of 5% CO2 at 37°C. The culture was
maintained in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS, 100 U/ml penicillin, 2 mM L-glutamine
and 100 µg/ml streptomycin. Cells were cultured in serum-free DMEM
for 24 h prior to treatment.
Western blot analysis
Total protein (30 µg) was resolved using SDS-PAGE on
a 10% gel and transferred to nitrocellulose membranes (Pall
Corporation). The membranes were blocked using non-fat milk and
incubated overnight at 4°C with primary antibodies directed against
the following proteins: p-ERK, ERK, Nrf-2, HO-1, p53, Bax, Bcl-xl
(all dilution, 1:1,000) and GAPDH (dilution, 1:40,000). Primary
antibodies were removed and the membranes were washed using PBS
with Tween-20 (PBST) buffer three times for 30 min at room
temperature. The membranes were subsequently incubated for 45 min
at room temperature with the following secondary antibodies:
Horseradish peroxidase-conjugated anti-mouse (cat. no. AP124P;
Chemicon International, Inc.), anti-rabbit (cat. no. AP132P; Merck
Millipore) and anti-goat (cat. no. 605-4313; Rockland
Immunochemicals Inc., Limerick, PA, USA) (all dilutions, 1:4,000).
The secondary antibodies were removed and the membranes were washed
using PBST buffer twice for 30 min. Protein bands were detected
using Millipore ECL. The density of the protein bands was
quantified using Image J software version 1.4 (National Institutes
of Health, Bethesda, MA, USA) following normalization with GAPDH.
All data are presented as the mean ± standard deviation from
experiments performed in triplicate.
Statistical analysis
Student's t-tests were conducted using GraphPad
Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
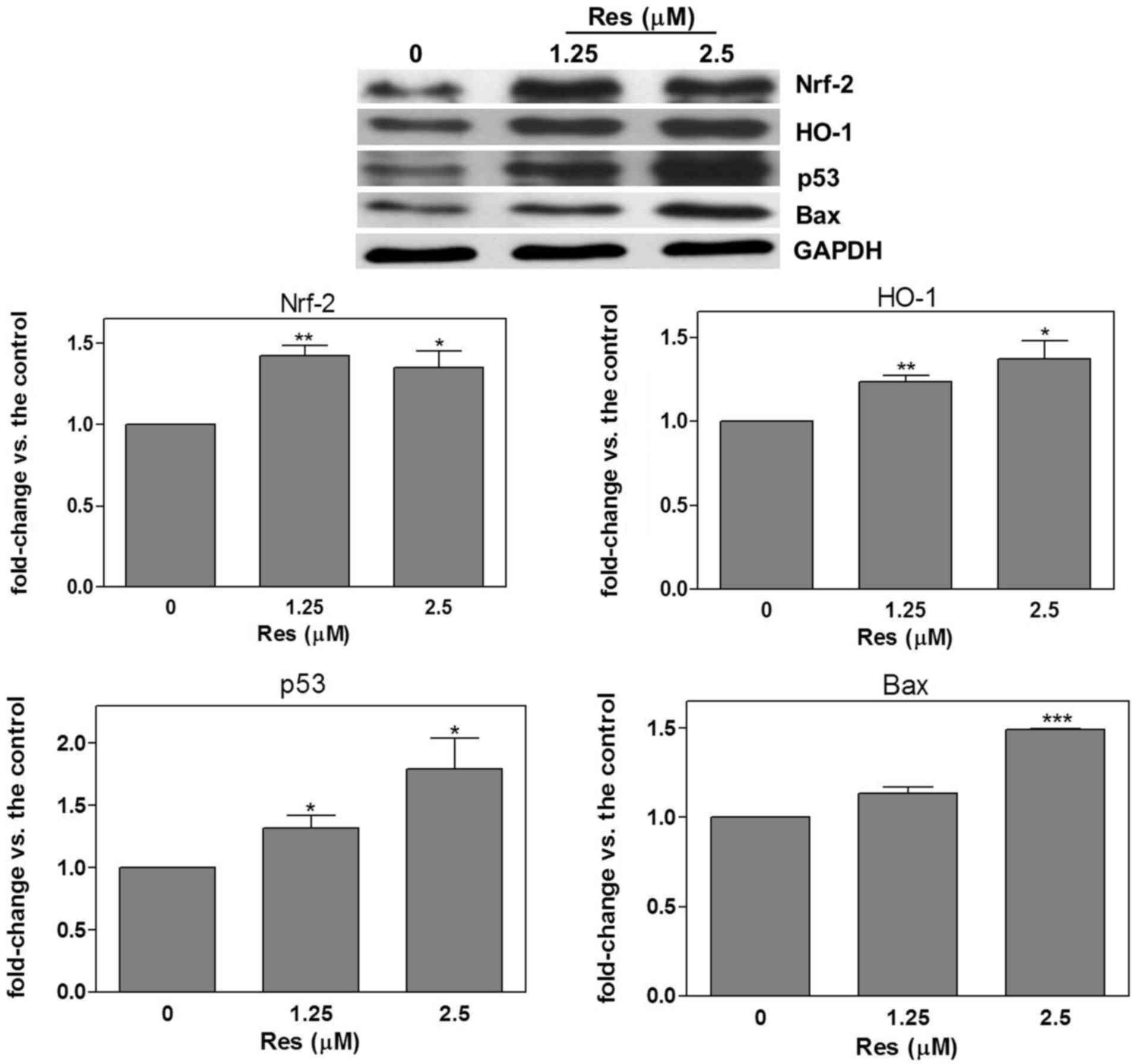
Pathways associated with apoptosis are
regulated by resveratrol
SAS cells were treated with resveratrol (1.25 and
2.5 µM) for 24 h, and Nrf-2, HO-1, p53 and Bax protein expression
was detected using western blot analysis. The results demonstrated
that, compared with the untreated control group, resveratrol
significantly increased Nrf-2 (1.25 µM, 1.42±0.06, P=0.002; 2.5 µM,
1.35±0.1, P=0.03), HO-1 (1.25 µM, 1.24±0.04, P=0.003; 2.5 µM,
1.37±0.11, P=0.03), p53 (1.25 µM, 1.32±0.1, P=0.04; 2.5 µM,
1.79±0.24, P=0.03) and Bax (2.5 µM, 1.49±0.03, P<0.0001)
expression (Fig. 1).
Regulation of apoptosis-associated
pathways by AGEs
SAS cells were treated with AGEs (200 and 400 µg/ml)
or BSA (400 µg/ml; negative control) for 24 h. Western blot
analysis was used to detect Nrf-2, HO-1, p53 and Bax protein
expression. The results revealed that compared with the untreated
control group, treatment with 400 µg/ml AGEs significantly
decreased Nrf-2 (0.64±0.05; P=0.002), p53 (0.72±0.06; P=0.008) and
Bax (0.7±0.03; P=0.0005) expression (Fig.
2).
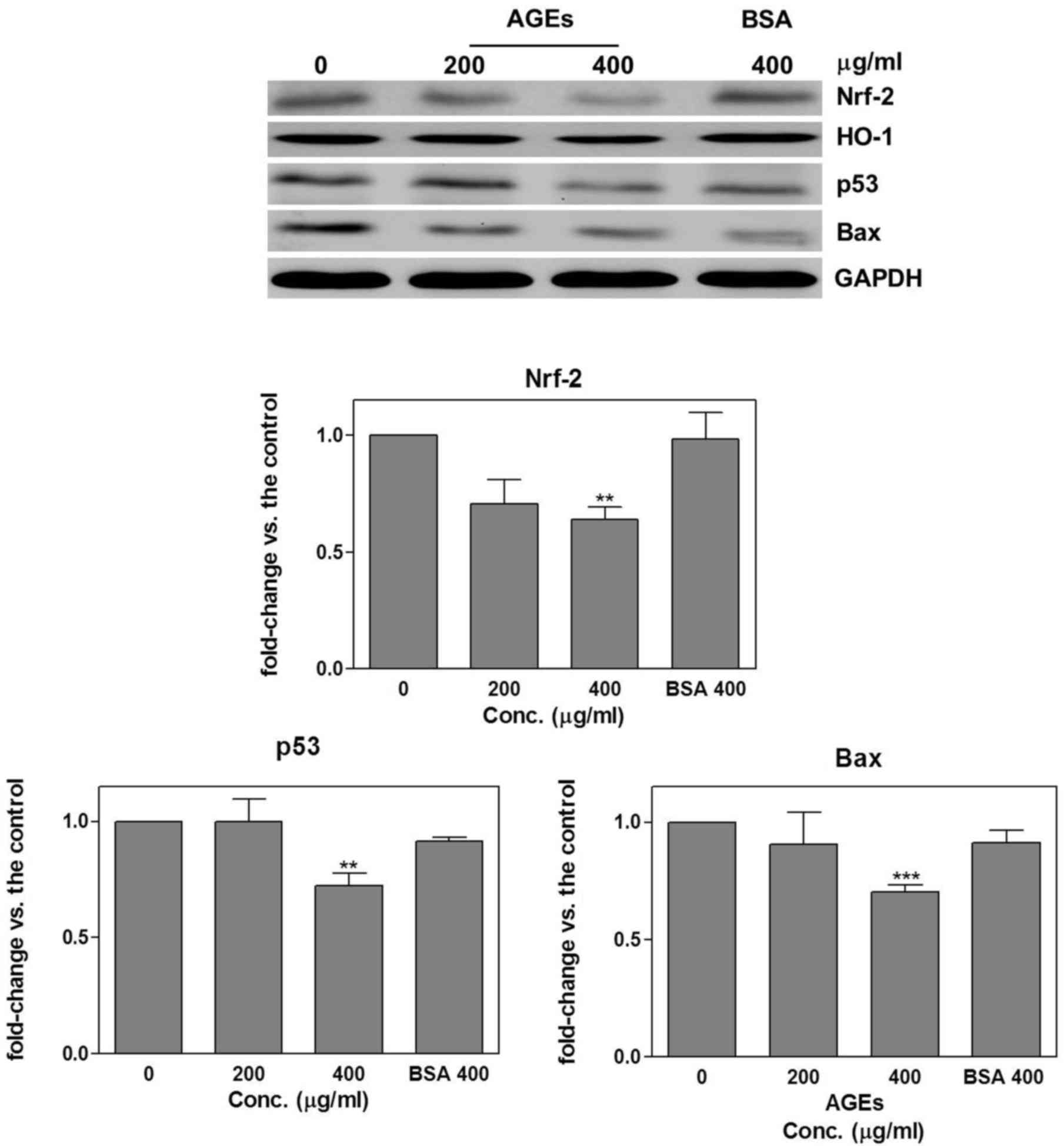 | Figure 2.Regulation of apoptosis-associated
pathways by AGEs. SAS cells were treated with AGEs (200 and 400
µg/ml) or BSA (400 µg/ml; negative control) for 24 h. Western blot
analysis was then performed to detect Nrf-2, HO-1, p53 and Bax
protein expression. Treatment with AGEs resulted in a significant
decrease in Nrf-2, p53 and Bax expression compared with the
untreated control group. Results are presented as the mean ±
standard deviation. **P<0.001; ***P<0.0001 all in comparison
to the control. AGEs, advanced glycation end products; BSA, bovine
serum albumin; Nrf-2, nuclear factor-erythroid 2-related factor 2;
p53, tumor protein p53; HO-1, heme oxygenase 1; Bax, Bcl-2
associated × apoptosis regulator; Res, resveratrol; conc.,
concentration. |
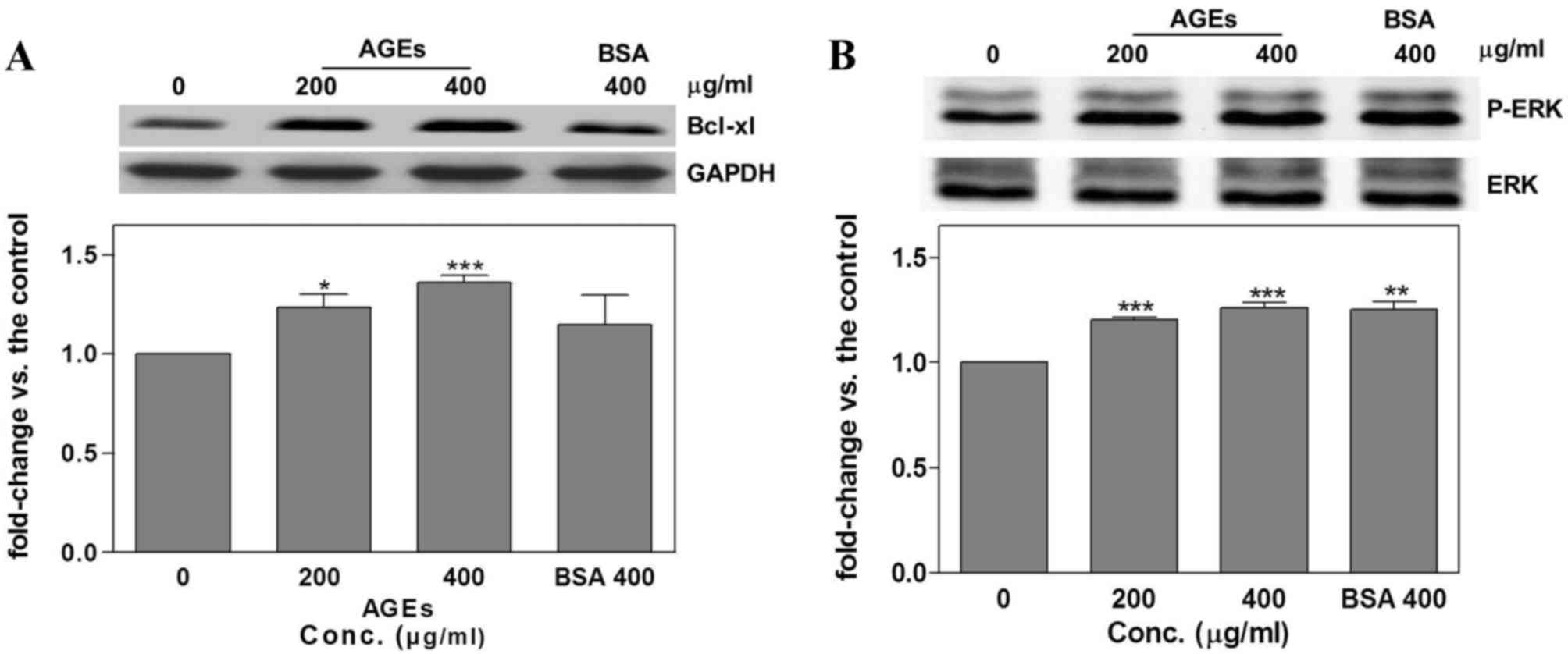
AGEs regulation of
apoptosis-associated signaling pathways via ERK
phosphorylation
Following the treatment of SAS cells with AGEs or
BSA for 24 h, Bcl-xl and p-ERK were detected using western blot
analysis. Treatment with AGEs was associated with a significant
increase in Bcl-xl (200 µg/ml, 1.23±0.07, P=0.03; 400 µg/ml,
1.36±0.04; P=0.0006; Fig. 3A) and ERK
phosphorylation (200 µg/ml, 1.2±0.01, P<0.00001; 400 µg/ml,
1.26±0.02, P=0.0004; Fig. 3B)
compared with the untreated control groups. However, treatment with
400 µg/ml BSA also significantly increased p-ERK compared with the
control group (1.25±0.04; P=0.003; Fig.
3B). A pretreatment of 10 µM PD98059 for 1 h was used on the
cells to inhibit the phosphorylation of ERK (Fig. 4A). Pretreatment with PD98059 blocked
the effect of AGEs on HO-1, Bcl-xl and Bax expression (Fig. 4B). Furthermore, compared with the
untreated control group, pretreatment with PD98059 prior to
treatment with AGEs significantly increased the expression of Nrf-2
(200 µg/ml, 1.27±0.04, P=0.003; 400 µg/ml, 1.24±0.03, P=0.001) and
p53 (200 µg/ml, 1.43±0.06, P=0.002; 400 µg/ml, 1.39±0.08, P=0.01)
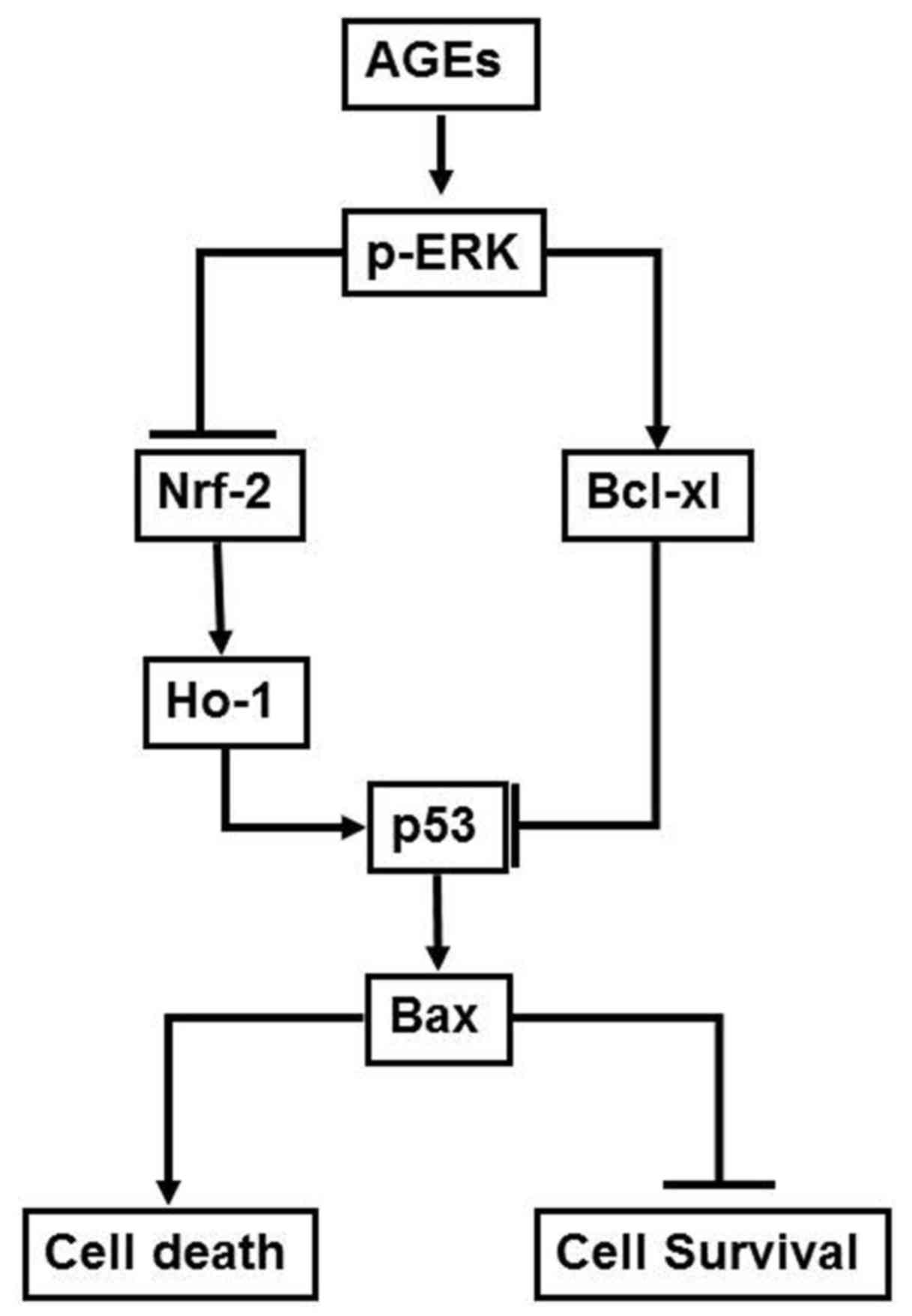
(Fig. 4B). The mechanism by which
AGEs were observed to influence the survival rate of oral cancer
cells in the present study is presented in Fig. 5.
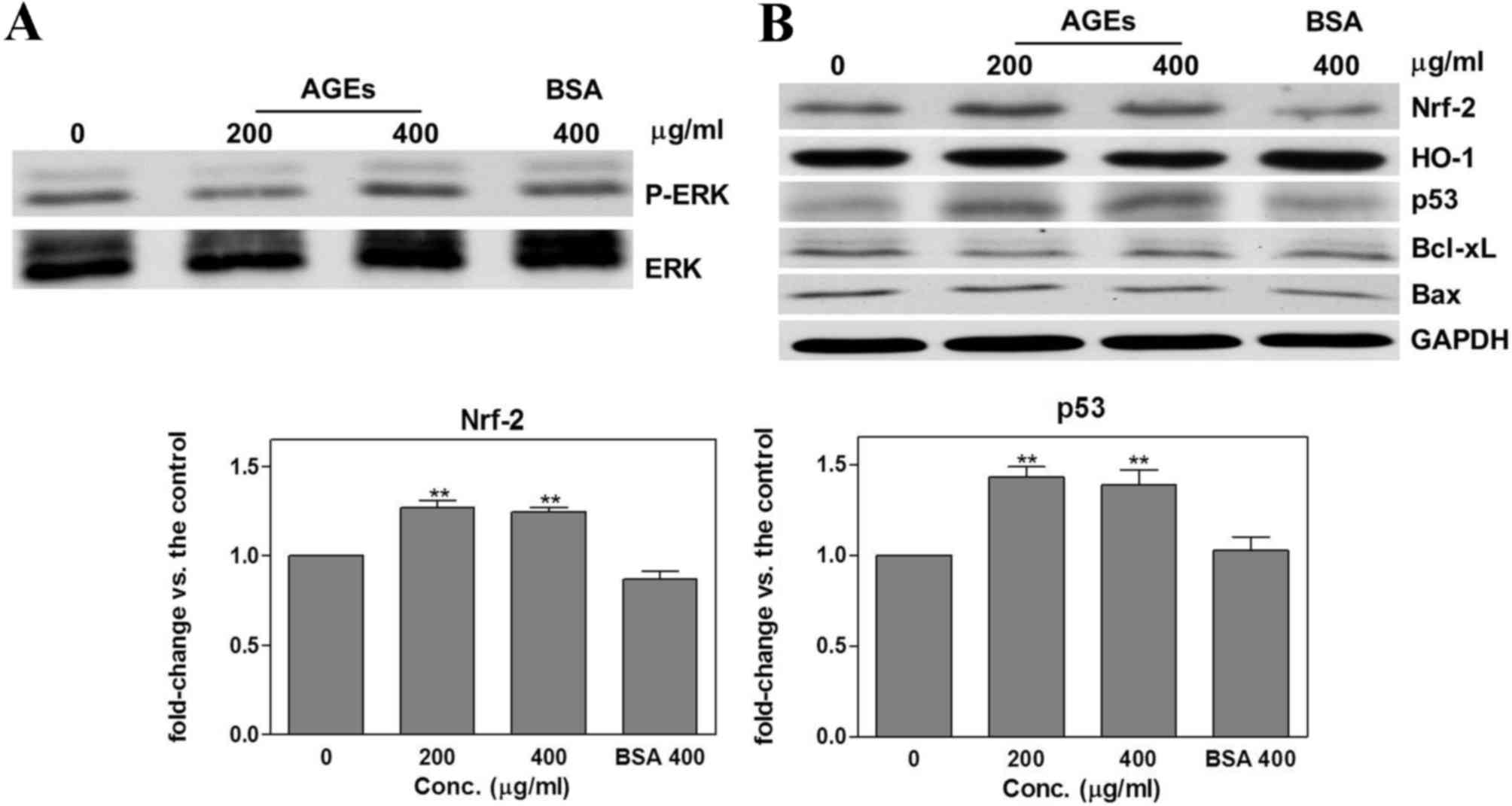 | Figure 4.Effects of AGEs following PD98059
pretreatment. Pretreating cells with 10 µM PD98059 for 1 h resulted
in (A) a reduction in the expression of p-ERK and (B) inhibition of
the effects of AGEs on HO-1, Bcl-xl and Bax expression, and
significant increase in Nrf-2 and p53 expression compared with the
untreated control group. Results are presented as the mean ±
standard deviation. **P<0.001 all in comparison to control.
AGEs, advanced glycation end products; Bcl-xl, apoptosis regulator
Bcl-x; Nrf-2, nuclear factor-erythroid 2-related factor 2; p53,
tumor protein p53; HO-1, heme oxygenase 1; Bax, Bcl-2 associated ×
apoptosis regulator; ERK, extracellular signal-regulated kinase;
p-, phosphorylated; Res, resveratrol; conc., concentration. |
Discussion
An association has been demonstrated between oral
cancer and DM (37–39); however, the mechanism underlying this
association remains to be elucidated. A previous study by our group
revealed that AGEs regulate oral cancer cell migration via the ERK
signaling pathway (17). The results
demonstrated the mechanism by which AGEs regulate p53 via ERK and
downstream Nrf-2 and Bcl-xl. To the best of our knowledge, this is
the first study to elucidate the mechanism of action of AGEs in
oral cancer cells.
Previous studies have demonstrated the
antitumorigenic effects of resveratrol (40–42). In
oral cancer, resveratrol suppresses cell growth, DNA synthesis,
migration and invasion, and increases cell apoptosis (43–45). A
previous study reported that Nrf-2 regulates the p53 signaling
pathway, leading to an apoptotic response (34). A study by Lee et al (35) suggested that the apoptosis of oral
cancer cells is regulated by Nrf-2 and downstream HO-1 and p53.
These results support the contention that Nrf-2 enhances cell
apoptosis via the p53 signaling pathway. The results of the present
study demonstrated that resveratrol significantly increased Nrf-2,
HO-1, p53 and Bax protein expression, suggesting that resveratrol
induces apoptosis through Nrf-2, HO-1, p53 and Bax signaling
pathways. The results of the current study demonstrated that, in
contrast to resveratrol, AGEs significantly decrease Nrf-2, HO-1,
p53 and Bax, and increase Bcl-xl protein expression. This indicates
that AGEs modulate oral cancer survival via regulation of the
expression of Nrf-2 and Bcl-xl.
In the present study, ERK phosphorylation was
significantly upregulated by AGEs and the pretreatment of SAS cells
with PD98059 to suppress ERK activation inhibited the effects of
AGEs on p-ERK, HO-1, Bcl-xl and Bax expression, whereas Nrf-2 and
p53 expression significantly increased. Treatment with AGEs
significantly increased Bcl-xl, and decreased p53 protein
expression. A previous study by Chipuk et al (46) suggested that an interaction between
Bcl-xl and p53 inhibits the activation of Bax. Li et al
(47) reported that Bcl-xl inhibits
p53 resulting in an anti-apoptotic effect. These results suggest
that AGEs regulate p53 via ERK phosphorylation to inhibit Nrf-2 and
activate Bcl-xl. In addition, AGEs and BSA increased ERK
phosphorylation in the current study. However, AGEs decreased Nrf-2
and p53 protein expression. The pretreatment of SAS cells with
PD98059 increased Nrf-2 and p53 expression. The results of the
present study suggest that treatment with AGEs or BSA differ
regarding their effects on oral cancer cells (17).
The results of the current study suggest that AGEs
decrease Nrf-2 and p53 expression and increase Bcl-xl expression
via ERK phosphorylation. To the best of our knowledge, this is the
first study to demonstrate that AGEs regulate the expression of
Nrf-2 and Bcl-xl, which subsequently influences p53 expression via
ERK phosphorylation. In conclusion, the results of the present
study suggest a mechanism by which AGEs influence the survival rate
of oral cancer cells. This mechanism involves AGEs increasing ERK
phosphorylation, which stimulates downstream Nrf-2 inhibition and
Bcl-xl upregulation. This subsequently suppresses p53 and Bax
expression, the effects of which manifest as a change in the
survival rate of oral cancer cells. These findings explain the
increase in oral cancer invasiveness and decrease in the survival
rate of patients with DM, who typically have higher levels of AGEs.
In addition, the results of the current study indicate that the
accumulation of AGEs due to aging or DM promotes the progression of
oral cancer.
Acknowledgements
The present study was supported by the National
Science Council of Taiwan (grant no. 100-2314-B-309-002-MY3).
Glossary
Abbreviation
Abbreviations:
|
AGEs
|
advanced glycation end products
|
References
|
1
|
Kasper M and Funk RH: Age-related changes
in cells and tissues due to advanced glycation end products (AGEs).
Arch Gerontol Geriatr. 32:233–243. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramasamy R, Vannucci SJ, Yan SS, Herold K,
Yan SF and Schmidt AM: Advanced glycation end products and RAGE: A
common thread in aging, diabetes, neurodegeneration, and
inflammation. Glycobiology. 15:16R–28R. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luth HJ, Ogunlade V, Kuhla B,
Kientsch-Engel R, Stahl P, Webster J, Arendt T and Münch G: Age-
and stage-dependent accumulation of advanced glycation end products
in intracellular deposits in normal and Alzheimer's disease brains.
Cereb Cortex. 15:211–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Munch G, Thome J, Foley P, Schinzel R and
Riederer P: Advanced glycation endproducts in ageing and
Alzheimer's disease. Brain Res Brain Res Rev. 23:134–143. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thorpe SR and Baynes JW: Role of the
Maillard reaction in diabetes mellitus and diseases of aging. Drugs
Aging. 9:69–77. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmidt AM, Yan SD, Yan SF and Stern DM:
The biology of the receptor for advanced glycation end products and
its ligands. Biochim Biophys Acta. 1498:99–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sato T, Iwaki M, Shimogaito N, Wu X,
Yamagishi S and Takeuchi M: TAGE (toxic AGEs) theory in diabetic
complications. Curr Mol Med. 6:351–358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Basta G, Schmidt AM and De Caterina R:
Advanced glycation end products and vascular inflammation:
Implications for accelerated atherosclerosis in diabetes.
Cardiovasc Res. 63:582–592. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thornalley PJ: Glycation free adduct
accumulation in renal disease: The new AGE. Pediatr Nephrol.
20:1515–1522. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeuchi M, Kikuchi S, Sasaki N, Suzuki T,
Watai T, Iwaki M, Bucala R and Yamagishi S: Involvement of advanced
glycation end-products (AGEs) in Alzheimer's disease. Curr
Alzheimer Res. 1:39–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato T, Shimogaito N, Wu X, Kikuchi S,
Yamagishi S and Takeuchi M: Toxic advanced glycation end products
(TAGE) theory in Alzheimer's disease. Am J Alzheimers Dis Other
Demen. 21:197–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takeuchi M, Bucala R, Suzuki T, Ohkubo T,
Yamazaki M, Koike T, Kameda Y and Makita Z: Neurotoxicity of
advanced glycation end-products for cultured cortical neurons. J
Neuropathol Exp Neurol. 59:1094–1105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choei H, Sasaki N, Takeuchi M, Yoshida T,
Ukai W, Yamagishi S, Kikuchi S and Saito T: Glyceraldehyde-derived
advanced glycation end products in Alzheimer's disease. Acta
Neuropathol. 108:189–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhawal UK, Ozaki Y, Nishimura M, Sugiyama
M, Sasahira T, Nomura Y, Sato F, Fujimoto K, Sasaki N, Ikeda MA, et
al: Association of expression of receptor for advanced glycation
end products and invasive activity of oral squamous cell carcinoma.
Oncology. 69:246–255. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai Q, Li BY, Gao HQ, Zhang JH, Wang JF,
Yu F, Yin M and Zhang Z: Grape seed procyanidin b2 inhibits human
aortic smooth muscle cell proliferation and migration induced by
advanced glycation end products. Biosci Biotechnol Biochem.
75:1692–1697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takino J, Yamagishi S and Takeuchi M:
Cancer malignancy is enhanced by glyceraldehyde-derived advanced
glycation end-products. J Oncol. 2010:7398522010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ko SY, Ko HA, Shieh TM, Chang WC, Chen HI,
Chang SS and Lin IH: Cell migration is regulated by AGE-RAGE
interaction in human oral cancer cells in vitro. PLoS One.
9:e1105422014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu CH, Wu TY, Li CC, Lui MT, Chang KW and
Kao SY: Impact of diabetes mellitus on the prognosis of patients
with oral squamous cell carcinoma: A retrospective cohort study.
Ann Surg Oncol. 17:2175–2183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Motohashi H, Katsuoka F, Engel JD and
Yamamoto M: Small Maf proteins serve as transcriptional cofactors
for keratinocyte differentiation in the Keap1-Nrf2 regulatory
pathway. Proc Natl Acad Sci USA. 101:6379–6384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishii T, Itoh K, Takahashi S, Sato H,
Yanagawa T, Katoh Y, Bannai S and Yamamoto M: Transcription factor
Nrf2 coordinately regulates a group of oxidative stress-inducible
genes in macrophages. J Biol Chem. 275:16023–16029. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishii T, Itoh K and Yamamoto M: Roles of
Nrf2 in activation of antioxidant enzyme genes via antioxidant
responsive elements. Methods Enzymol. 348:182–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsieh CY, Hsiao HY, Wu WY, Liu CA, Tsai
YC, Chao YJ, Wang DL and Hsieh HJ: Regulation of shear-induced
nuclear translocation of the Nrf2 transcription factor in
endothelial cells. J Biomed Sci. 16:122009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mann GE, Rowlands DJ, Li FY, de Winter P
and Siow RC: Activation of endothelial nitric oxide synthase by
dietary isoflavones: Role of NO in Nrf2-mediated antioxidant gene
expression. Cardiovasc Res. 75:261–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Itoh K, Mochizuki M, Ishii Y, Ishii T,
Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K and Yamamoto
M: Transcription factor Nrf2 regulates inflammation by mediating
the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2). Mol Cell
Biol. 24:36–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen XL, Dodd G, Thomas S, Zhang X,
Wasserman MA, Rovin BH and Kunsch C: Activation of Nrf2/ARE pathway
protects endothelial cells from oxidant injury and inhibits
inflammatory gene expression. Am J Physiol Heart Circ Physiol.
290:H1862–H1870. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mochizuki M, Ishii Y, Itoh K, Iizuka T,
Morishima Y, Kimura T, Kiwamoto T, Matsuno Y, Hegab AE, Nomura A,
et al: Role of 15-deoxy delta(12,14) prostaglandin J2 and Nrf2
pathways in protection against acute lung injury. Am J Respir Crit
Care Med. 171:1260–1266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harada N, Kanayama M, Maruyama A, Yoshida
A, Tazumi K, Hosoya T, Mimura J, Toki T, Maher JM, Yamamoto M and
Itoh K: Nrf2 regulates ferroportin 1-mediated iron efflux and
counteracts lipopolysaccharide-induced ferroportin 1 mRNA
suppression in macrophages. Arch Biochem Biophys. 508:101–109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bell KF, Al-Mubarak B, Fowler JH, Baxter
PS, Gupta K, Tsujita T, Chowdhry S, Patani R, Chandran S, Horsburgh
K, et al: Mild oxidative stress activates Nrf2 in astrocytes, which
contributes to neuroprotective ischemic preconditioning. Proc Natl
Acad Sci USA. 108:E1–E2; author reply E3-4. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Riley RJ and Workman P: DT-diaphorase and
cancer chemotherapy. Biochem Pharmacol. 43:1657–1669. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Clark JE, Foresti R, Green CJ and
Motterlini R: Dynamics of haem oxygenase-1 expression and bilirubin
production in cellular protection against oxidative stress. Biochem
J 348 Pt. 3:615–619. 2000. View Article : Google Scholar
|
|
32
|
Jyrkkanen HK, Kansanen E, Inkala M, Kivelä
AM, Hurttila H, Heinonen SE, Goldsteins G, Jauhiainen S, Tiainen S,
Makkonen H, et al: Nrf2 regulates antioxidant gene expression
evoked by oxidized phospholipids in endothelial cells and murine
arteries in vivo. Circ Res. 103:e1–e9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nioi P, McMahon M, Itoh K, Yamamoto M and
Hayes JD: Identification of a novel Nrf2-regulated antioxidant
response element (ARE) in the mouse NAD(P)H: Quinone oxidoreductase
1 gene: Reassessment of the ARE consensus sequence. Biochem J.
374:337–348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
You A, Nam CW, Wakabayashi N, Yamamoto M,
Kensler TW and Kwak MK: Transcription factor Nrf2 maintains the
basal expression of Mdm2: An implication of the regulation of p53
signaling by Nrf2. Arch Biochem Biophys. 507:356–364. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee YM, Auh QS, Lee DW, Kim JY, Jung HJ,
Lee SH and Kim EC: Involvement of Nrf2-mediated upregulation of
heme oxygenase-1 in mollugin-induced growth inhibition and
apoptosis in human oral cancer cells. Biomed Res Int.
2013:2106042013.PubMed/NCBI
|
|
36
|
Ko SY, Lin YP, Lin YS and Chang SS:
Advanced glycation end products enhance amyloid precursor protein
expression by inducing reactive oxygen species. Free Radic Biol
Med. 49:474–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vairaktaris E, Spyridonidou S, Goutzanis
L, Vylliotis A, Lazaris A, Donta I, Perrea D, Yapijakis C and
Patsouris E: Diabetes and oral oncogenesis. Anticancer Res.
27:4185–4193. 2007.PubMed/NCBI
|
|
38
|
Girtan M, Zurac S, Stăniceanu F, Bastian
A, Popp C, Nichita L, Laba E and Forna N: Oral epithelial
hyperplasia in diabetes mellitus. Rom J Intern Med. 47:201–203.
2009.PubMed/NCBI
|
|
39
|
Vairaktaris E, Kalokerinos G, Goutzanis L,
Yapijakis C, Derka S, Vassiliou S, Spyridonidou S, Vylliotis A,
Nkenke E, Lazaris A and Patsouris E: Diabetes enhances cell
proliferation but not Bax/Bcl-2-mediated apoptosis during oral
oncogenesis. Int J Oral Maxillofac Surg. 37:60–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ulrich S, Loitsch SM, Rau O, von Knethen
A, Brüne B, Schubert-Zsilavecz M and Stein JM: Peroxisome
proliferator-activated receptor gamma as a molecular target of
reveratrol-induced modulation of polyamine metabolism. Cancer Res.
66:7348–7354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Frazzi R, Valli R, Tamagnini I, Casali B,
Latruffe N and Merli F: Resveratrolmediated apoptosis of hodgkin
lymphoma cells invovles SIRT1 inhibition and FOXO3a
hyperacetylation. Int J Cancer. 132:1013–1021. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang Q, Wang B, Zang W, Wang X, Liu Z, Li
W and Jia J: Resveratrol inhibits the growth of gastric cancer by
inducing G1 phase arrest and senescence in a Sirt1-dependent
manner. PLoS One. 8:e706272013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Elattar TM and Virji AS: The effect of red
wine and its components on growth and proliferation of human oral
squamous carcinoma cells. Anticancer Res. 19:5407–5414.
1999.PubMed/NCBI
|
|
44
|
Shan Z, Yang G, Xiang W, Pei-jun W and Bin
Z: Effects of resveratrol on oral squamous cell carcinoma (OSCC)
cells in vitro. J Cancer Res Clin Oncol. 140:371–374. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim SH, Kim HJ, Lee MH, Yu SK, Kim CS,
Kook JK, Chun HS, Park E, Lee SY, Kim SG, et al: Resveratrol
induces apoptosis of KB human oral cancer cells. J Korean Soc Appl
Biol Chem. 54:966–971. 2011. View Article : Google Scholar
|
|
46
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
Bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li G, Xie N, Yao Y, Zhang Y, Guo J, Feng
Y, Lv F, Xiao RP and Cao CM: Identification of PI3K regulatory
subunit p55γ as a novel inhibitor of vascular smooth muscle cell
proliferation and neointimal formation. Cardiovasc Res. 105:75–85.
2014. View Article : Google Scholar : PubMed/NCBI
|