Introduction
Colorectal cancer (CRC) is a prominent cause of
mortality worldwide (1). It is
surgically curable in early stages, when it is localized or limited
to loco-regional invasion; however, the metastatic stages are
associated with a high mortality rate (2). According to GLOBOCAN estimates (1), there were 1,360,602 new cases of CRC
worldwide in 2012, making it the third most common type of cancer
worldwide and accounting for 9.7% of all cases of cancer that were
not non-melanoma skin cancer (2). The
majority of CRCs are adenocarcinomas. When these are diagnosed
early, the prognosis is good: The 5-year relative survival rate is
91% for localized cancer and 70% for cancer with loco-regional
invasion. However, the 5-year survival rate is ~11% in metastatic
cases, which includes ~25% of patients at the point of diagnosis
(3) The rate of CRC mortality has
decreased over the last 20 years due to advances in disease
management, including earlier diagnosis and improved treatment
modalities. However, a lack of reliable methods for the early
detection of CRC is impacting patient prognoses (4).
At present, carcinoembryonic antigen (CEA), a
<200-kilodalton (kDa) glycoprotein that acts to mediate cell
adhesion in cancer, is used in the initial assessment and
monitoring of CRC (5). It was first
identified in the blood serum of patients with CRC (6). However, 30% of cases of CRC recurrence
do not produce CEA, irrespective of the amount associated with the
original disease. Furthermore, it is estimated that 44% of patients
with normal CEA levels prior to surgery have an increased CEA level
in disease recurrence (7). The
diagnostic value of CEA also varies according to the site. The
sensitivity of CEA for the detection of hepatic and retroperitoneal
metastases is greater than for the detection of lung metastases;
sensitivity is also improved in cases of multiple recurrences
compared with a single recurrence. A number of potential diagnostic
and prognostic biomarkers for CRC have been evaluated thus far
(Table I), including cytokines,
chemokines and enzymes (8–14).
 | Table I.Examples of biomarkers for colorectal
cancer. |
Table I.
Examples of biomarkers for colorectal
cancer.
| Author (year) | Marker | Sample type | (Refs.) |
|---|
| Yörüker et
al (2016) | DNA integrity | Serum/plasma |
(8) |
| Fan et al
(2014) | α-2-HS glycol
protein | Serum |
(9) |
| Newton et al
(2012) | Carcinoembryonic
antigen | Serum | (10) |
| Wang et al
(2012) | MicroRNA-21 | Serum/plasma | (11) |
| Cohen et al
(2009) | Circulating tumor
cells | Peripheral
blood | (12) |
| Taback et al
(2006) | Microsatellite
instability | Serum/plasma | (13) |
| Bazan et al
(2006) | KRas mutations | Serum/plasma | (14) |
CRC and biomarkers
Blood serum biomarkers are of interest for the
screening and monitoring of disease as they do not require invasive
procedures and may facilitate rapid detection (8). CRC biomarkers in the blood include
circulating tumor cells, DNA, RNA and proteins (8). In Table I,
a number of serum biomarkers that may be used for the early
detection or monitoring of CRC are summarized.
Circulating tumor cells in CRC have been validated
as prognostic markers for advanced disease and are associated with
a poor overall survival time (15).
The US Food and Drug Administration has validated them as
monitoring markers, as they may predict response to treatment
(16). Circulating DNA is also
considered as a promising type of prognostic marker. For example,
the detection of a Kirsten rat sarcoma mutation preoperatively, or
its persistence subsequent to surgery, is associated with a poor
prognosis (17).
These biomarkers are accessible with simple and
rapid methods throughout the fluctuating course of the disease. A
number of studies (15,17) have demonstrated the utility of these
biomarkers in prognosis and monitoring, and as theranostic markers,
and recommend their routine use in the near future. However,
according to consensus-based recommendations from the American
Society of Clinical Oncology (18),
only CEA levels should be monitored preoperatively to assist in
staging and surgical planning. There is currently no biomarker for
the early detection of CRC, with negative consequences for patient
prognosis (10).
E-cadherin and cancer
Similar to CEA, one of the putative protein
biomarkers for the prediction of tumor progression, E-cadherin, is
an adhesion molecule. The loss of cell adhesion is one of the
mechanisms underlying cancer invasion and progression (19).
E cadherin belongs to the ‘classical’ or type-I
cadherin subfamily. A total of 16 molecules of ~120 kDa each have
been identified in this subfamily. Four subclasses exist:
Non-neuronal epithelial (E-), placental (P-), neuronal (N-) and
retinal (R-) cadherins. E-cadherin is encoded by the CDH1 gene; it
is a calcium-dependent transmembrane glycoprotein that is localized
to adherens junctions at the basolateral surface of epithelial
cells, and is involved in cell-cell interactions, including in
cancer (20).
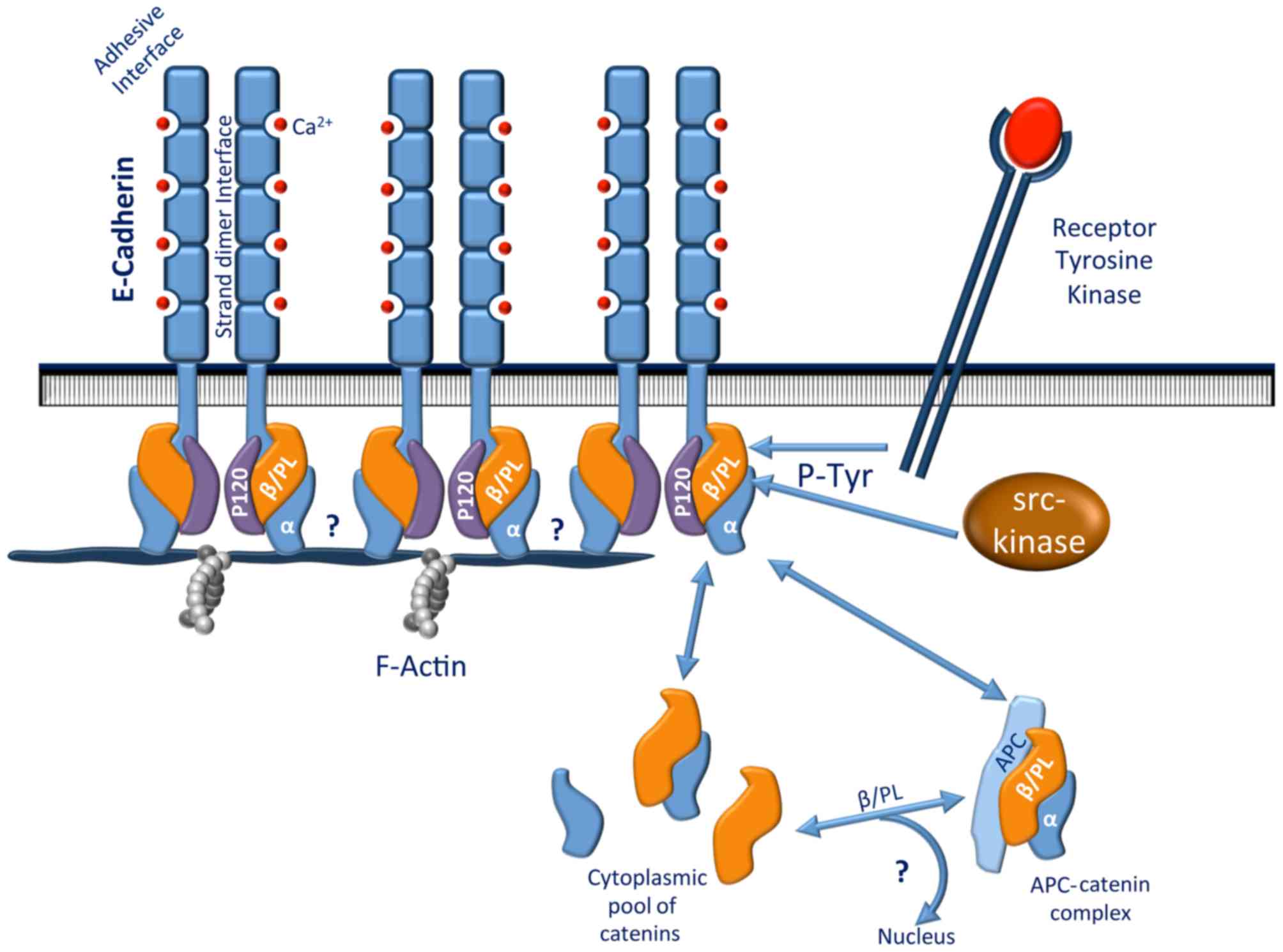
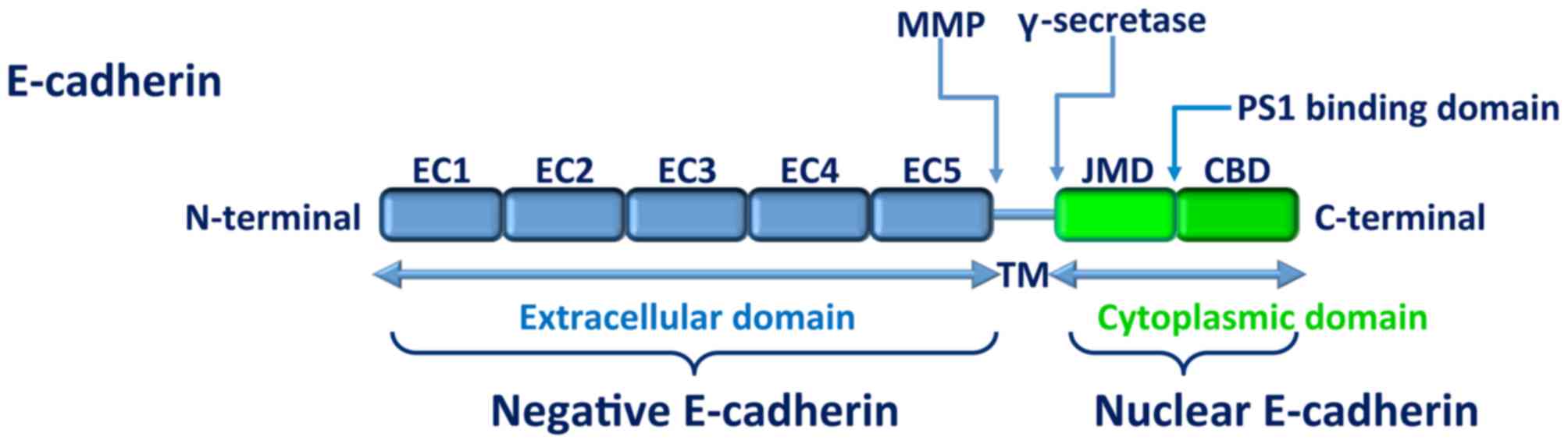
The E-cadherin molecule is composed of a cytoplasmic
domain, a single-pass transmembrane domain and an extracellular
domain that consists of five cadherin-motif tandem repeat
subdomains that have putative calcium binding sites (21). The cytoplasmic domain interacts with
catenin molecules, including β-catenin or plakoglobin (also called
γ-catenin), to mediate its binding to actin filaments of the
cytoskeleton (22). Either β-catenin
or plakoglobin may bind α-catenin, giving rise to two distinct
cadherin-catenin complexes. α-catenin then links these two
complexes to actin filaments. This anchorage has been speculated to
be regulated by tyrosine phosphorylation (23) (Fig. 1).
β-catenin may also bind to the cytoplasmic domain of the epidermal
growth factor receptor (EGFR) (24).
E-cadherin downregulation is associated with certain
malignant characteristics, including tumor progression, loss of
differentiation, invasion and metastasis (25,26).
E-cadherin may be inactivated in cancer by mechanisms including
mutations, epigenetic silencing, and increased endocytosis and
proteolysis (22). Studies on the
loss of heterozygosity in chromosome 16q21-22 have linked
E-cadherin downregulation to gastric, prostate, hepatocellular and
esophageal carcinomas (27). Despite
this, E-cadherin mutations are rare in carcinomas of the bladder,
colon, endometrium, lung, esophagus, ovary and thyroid, and in
intra-hepatic cholangiocarcinoma (28).
Promoter hypermethylation is an important epigenetic
event associated with the loss of E-cadherin expression (29). Several suppressors of E-cadherin
transcription have been associated with the progression of multiple
cancer types. For example, increased SNAI1 expression, as is common
in ductal breast carcinoma, is strongly associated with reduced
E-cadherin gene expression (30).
Other mechanisms may disturb normal E-cadherin
function under pathological conditions. E-cadherin is removed from
the plasma membrane by endocytosis and recycled to the sites of
novel cell-cell contacts. Abnormal activation of proto-oncogenes,
including Src and EGFR, results in increased phosphorylation of
tyrosine residues in the cytoplasmic domain of E-cadherin (Fig. 2), leading to the recruitment of the
E3-ubiquitin-protein ligase Hakai and subsequently enhancing the
endocytosis and ubiquitin-dependent degradation of E-cadherin
(31).
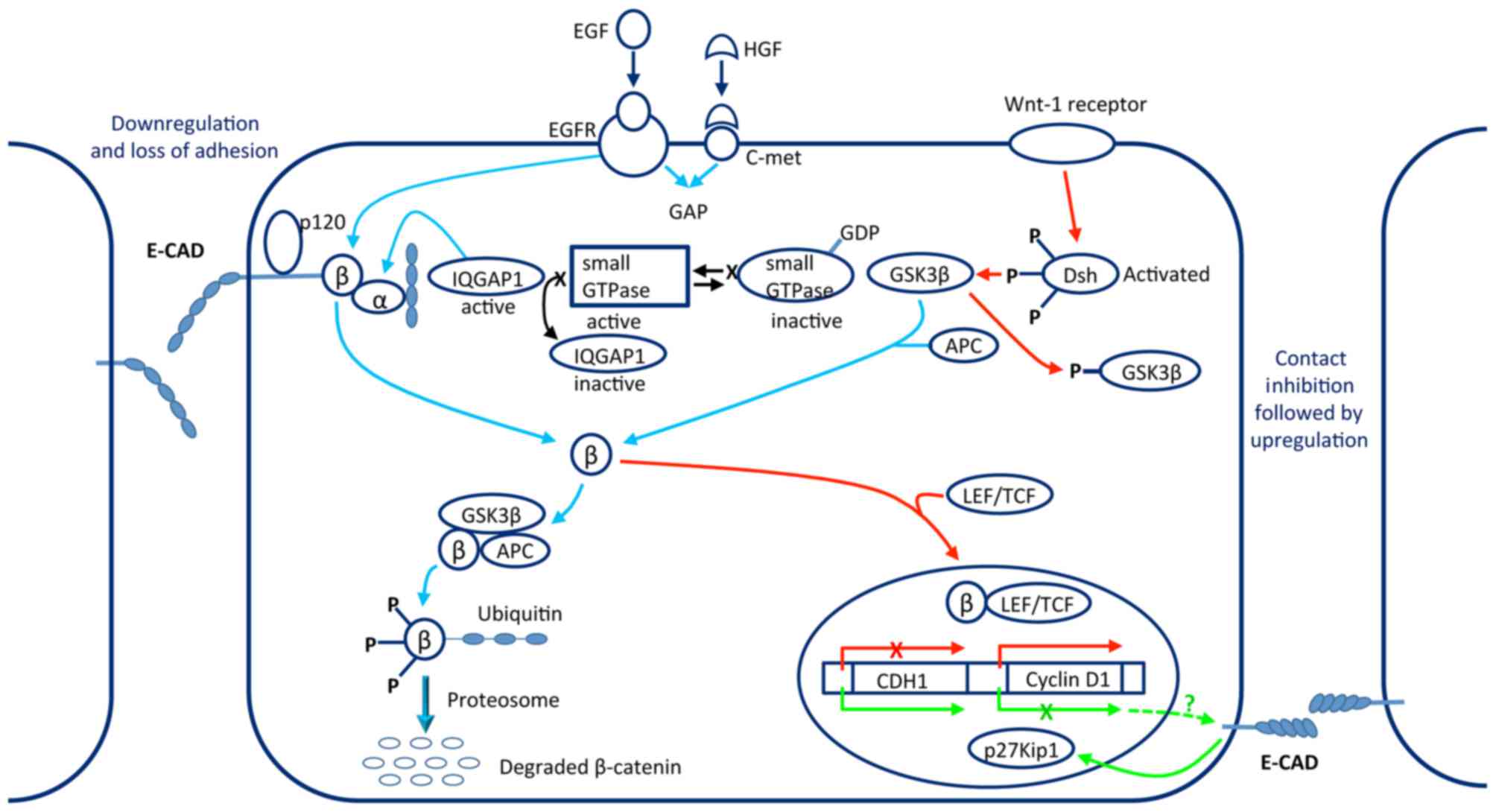 | Figure 2.Operating mode of the
E-cadherin-catenin complex in tumor metastasis. Stimulation of EGFR
and c-Met receptors led to the translocation of ß-catenin into the
cytosolic pool where it can be degraded. If the Wnt1 pathway is
activated at the same time as the other two pathways, the
degradation of ß-catenin is inhibited and it is translocated to the
nucleus to combine with LEF/TCF, causing transcription of the
cyclin D1 gene and downregulation of the CDH1 gene. Reversal by way
of contact inhibition is poorly understood. E-CAD, E-cadherin; EGF,
epidermal growth factor; EGFR, epidermal growth factor receptor;
HGF, hepatocyte growth factor; c-Met, hepatocyte growth factor
receptor; GAP, GTPase activating protein; β, β-catenin; α,
α-catenin; IQGAP1, IQ motif-containing GTPase activating protein 1;
GDP, guanosine diphosphate; GSK3β, glycogen synthase kinase 3β;
APC, adenomatosis polyposis coli Wnt signaling pathway regulator;
P, phosphorylation group; Dsh, disheveled; LEF/TCF, lymphoid
enhancer factor/T-cell factor family of transcription factors;
CDH1, cadherin 1 (E-cadherin gene); p27Kip1, cyclin-dependent
kinase inhibitor 1B. |
Matrix metalloproteinases (MMPs; Fig. 3), including stromelysin-1, matrilysin,
MMP9 and MT1-MMP, cleave E-cadherin ectodomains close to the plasma
membrane (32). Several other
proteases, including serine protease kallikrein-7, may be involved.
Pancreatic adenocarcinomas frequently overexpress kallikrein-7,
which facilitates tumor cell invasiveness via the cleavage and
release of soluble E-cadherin (33).
Another example of E-cadherin disturbance contributing to tumor
malignancy is peritoneal metastasis in advanced epithelial ovarian
cancer, where calpain-mediated E-cadherin fragmentation appears to
promote intraperitoneal cancer progression (34). In the human colon cancer cell line
HT-29, syndecan-2, a cell-surface heparan sulfate proteoglycan
induces extracellular shedding of E-cadherin and supports the
acquisition of a fibroblast-like morphology by regulating MMP-7
expression (35).
Post-translational modifications of E-cadherin have
also been described. The O-mannosylation of E-cadherin is crucial
for its adhesive functions in homeostasis. Carvalho et al
(36) demonstrated that E-cadherin
underwent a decrease in O-mannosylation in gastric carcinoma that
resulted in the impairment of its function through interference
with its cell membrane localization and, subsequently, with the
assembly and competence of adherens junctions.
Recently, Petrova et al (37) demonstrated that the metastasis of an
E-cadherin-expressing mammary cell from the mammary gland to the
lung is dependent on reduced E-cadherin adhesive function. An
activating monoclonal antibody against E-cadherin that induced a
high-adhesive state significantly reduced the number of cells that
metastasized to the lung. Thus, stimulating the activity of
E-cadherin on the cell surface inhibits metastatic progression,
suggesting that the downregulation of adhesion in these tumor cells
contributes to their metastatic potential.
E-cadherin and colon cancer
Recent studies have identified that the loss of
expression of E-cadherin and colon cancer invasiveness are
associated (38); however, this is
rarely attributed to E-cadherin gene mutation (39). The mechanism for downregulation is
more frequently post-translational modifications, as suggested by
Kitadai et al (40).
A number of studies have identified E-cadherin as a
good biomarker for CRC prognosis. In the earliest study, tissues
samples were examined. Invasion and metastasis were revealed to be
associated with the reduction of α-catenin alone or with reduction
of α-catenin and E-cadherin co-expression, not purely with
E-cadherin expression alone (41).
More recent studies were performed on sera samples. In a study by
Velikova et al (42), soluble
E-cadherin concentrations in patients with CRC were not
significantly increased compared with those of the control group.
In another study conducted with 36 patients with CRC, E-cadherin
was demonstrated to be a good marker of CRC. However, it lacked the
required specificity to predict tumor progression; concentrations
of E-cadherin were higher for patients with CRC as well as for
patients with benign tumors (43). In
another study, Weiss et al (44) compared soluble E-cadherin levels in 59
patients with CRC to the levels in patients with other conditions,
including colorectal adenomas, inflammatory bowel disease or
familial adenomatous polyposis. There was a significant elevation
of soluble E-cadherin levels in patients with advanced CRC (stages
III and IV). This study suggested a potential application for
soluble E-cadherin as a diagnostic marker for monitoring disease in
patients with CEA-negative tumors (44). The largest study was conducted on 186
patients with CRC; a preoperatively elevated soluble E-cadherin
level was associated with a worse prognosis (38). The study demonstrated that E-cadherin
was a metastasis prediction marker and a pre-therapeutic prognostic
marker for patients with CRC and hepatic metastases. All these data
confirmed that soluble E-cadherin levels increase with advancing
tumor stage (38,41–44).
E-cadherin serves a crucial role in cell-cell
adhesion and maintaining epithelial morphology. Loss of E-cadherin
leads to the loss of epithelial differentiation and the acquisition
of a motile and invasive phenotype (45). A recent study (46) enrolled patients with signet ring cell
carcinoma, a rare type of colorectal adenocarcinoma with a worse
prognosis than classical colorectal adenocarcinoma. The study
demonstrated that patients with a loss of tumor E-cadherin
expression had a lower survival time. Loss of E-cadherin expression
was a significant, independent predictor for poor prognosis. The
implications of E-cadherin in CRC progression has also been
recently demonstrated (47): The
expression of E-cadherin in 108 patients with CRC metastasis was
lower than that in normal adjacent tissues, and was associated with
tumor differentiation, invasion depth, lymph node metastasis and
tumor stage. Furthermore, expression of E-cadherin prolonged the
survival time of mice with patient-derived CRC xenografts (47).
As a consequence of this association, E-cadherin is
a potential target for anticancer treatment. Chen et al
(48) illustrated this; dimethoxy
curcumin inhibited cell growth and enhanced E-cadherin expression
in two CRC cell lines (HT-29 and SW480). In a CRC liver metastasis
mouse model, the combination of a vascular disrupting agent with
the anti-angiogenic drug sunitinib increased treatment efficacy; it
reduced the number of viable tumor cells and prolonged animal
survival time. E-cadherin staining was lower for the treated cells
than for controls. The surviving tumor cells underwent a
redistribution of E-cadherin from the cell junctions to the
cytoplasm and nucleus (49)
exhibiting epithelial-mesenchymal transition (EMT) and contributing
to therapy resistance. Regorafenib (Stivarga®) targets
protein tyrosine phosphatase and is approved for the
pharmacotherapy of CRC metastasis. Regorafenib also targets EMT; it
directly activates SH2-domain-containing phosphatase 1 (SHP-1) to
inhibit EMT. SHP-1 expression is positively correlated with
E-cadherin expression, and is significantly correlated with the
overall survival time of patients with CRC (50).
Furthermore, by upregulating E-cadherin expression,
CRC risk may be reduced. E-cadherin expression was increased in
patients supplemented with calcium and vitamin D (51). Calcium and vitamin D may thus be
chemopreventive agents against CRC.
Conclusion
E-cadherin protein expression is associated with CRC
tumor progression. The detection of soluble E-cadherin may allow
the early detection of CRC and the monitoring of tumor progression.
However, current tests lack sensitivity and specificity. Studies on
larger cohorts may be illuminating in this area. Another
possibility is to compare the levels of E-cadherin with those of
other key molecules in CRC progression. A combination of key
molecule levels may be more effective than one molecule alone.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Faivre J, Lepage C and Viguier J: Cancer
colorectal: Du diagnostic au dépistage. Gastroentérologie Clin
Biol. 33:660–671. 2009. View Article : Google Scholar
|
|
3
|
Institut National Du Cancer (INCA), . Les
traitements du cancer du côlon, collection Guides patients Cancer
info. INCA. 2010, http://www.e-cancer.fr/Patients-et-proches/Les-cancers/Cancer-du-colon/Points-cles
|
|
4
|
Rawson JB and Bapat B: Epigenetic
biomarkers in colorectal cancer diagnostics. Expert Rev Mol Diagn.
12:499–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hatakeyama K, Wakabayashi-Nakao K, Ohshima
K, Sakura N, Yamaguchi K and Mochizuki T: Novel protein isoforms of
carcinoembryonic antigen are secreted from pancreatic, gastric and
colorectal cancer cells. BMC Res Notes. 6:3812013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gold P and Freedman SO: Specific
carcinoembryonic antigens of the human digestive system. J Exp Med.
122:467–481. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bast RC Jr, Ravdin P, Hayes DF, Bates S,
Fritsche H Jr, Jessup JM, Kemeny N, Locker GY, Mennel RG and
Somerfield MR: American Society of Clinical Oncology Tumor Markers
Expert Panel: 2000, Update of recommendations for the use of tumor
markers in breast and colorectal cancer: Clinical practice
guidelines of the American society of clinical oncology. J Clin
Oncol. 19:1865–1878. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yörüker EE, Holdenrieder S and Gezer U:
Blood-based biomarkers for diagnosis, prognosis and treatment of
colorectal cancer. Clin Chim Acta. 455:26–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fan F, Samuel S, Evans KW, Lu J, Xia L,
Zhou Y, Sceusi E, Tozzi F, Ye XC, Mani SA and Ellis LM:
Overexpression of Snail induces epithelial-mesenchymal transition
and a cancer stem cell-like phenotype in human colorectal cancer
cells. Cancer Med. 1:5–16. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Newton KF, Newman W and Hill J: Review of
biomarkers in colorectal cancer. Colorectal Dis. 14:3–17. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang B and Zhang Q: The expression and
clinical significance of circulating microRNA-21 in serum of five
solid tumors. J Cancer Res Clin Oncol. 138:1659–1666. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen SJ, Punt CJA, Iannotti N, Saidman
BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller
MC, et al: Prognostic significance of circulating tumor cells in
patients with metastatic colorectal cancer. Ann Oncol.
20:1223–1229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taback B, Saha S and Hoon DS: Comparative
analysis of mesenteric and peripheral blood circulating tumor DNA
in colorectal cancer patients. Ann N Y Acad Sci. 1075:197–203.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bazan V, Bruno L, Augello C, Agnese V,
Calò V, Corsale S, Gargano G, Terrasi M, Schirò V, Di Fede G, et
al: Molecular detection of TP53, Ki-Ras and p16INK4A promoter
methylation in plasma of patients with colorectal cancer and its
association with prognosis. Results of a 3-year GOIM (Gruppo
Oncologico dell'Italia Meridionale) prospective study. Ann Oncol.
17(Suppl 7): vii84–vii90. 2006.PubMed/NCBI
|
|
15
|
Rahbari NN, Aigner M, Thorlund K, Mollberg
N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M and Weitz
J: Meta-analysis shows that detection of circulating tumor cells
indicates poor prognosis in patients with colorectal cancer.
Gastroenterology. 138:1714–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abdallah EA, Fanelli MF, Buim ME, Netto MC
Machado, Junior JL Gasparini, Souza E, Silva V, Dettino AL, Mingues
NB, Romero JV, Ocea LM, et al: Thymidylate synthase expression in
circulating tumor cells: A new tool to predict 5-fluorouracil
resistance in metastatic colorectal cancer patients. Int J Cancer.
137:1397–1405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lecomte T, Berger A, Zinzindohoué F,
Micard S, Landi B, Blons H, Beaune P, Cugnenc PH and Laurent-Puig
P: Detection of free-circulating tumor-associated DNA in plasma of
colorectal cancer patients and its association with prognosis. Int
J Cancer. 100:542–548. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Locker GY, Hamilton S, Harris J, Jessup
JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF and Bast RC Jr:
ASCO: ASCO 2006 update of recommendations for the use of tumor
markers in gastrointestinal cancer. J Clin Oncol. 24:5313–5327.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Behrens J: The role of cell adhesion
molecules in cancer invasion and metastasis. Breast Cancer Res
Treat. 24:175–184. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shiozaki H, Oka H, Inoue M, Tamura S and
Monden M: E-cadherin mediated adhesion system in cancer cells.
Cancer. 77:1605–1613. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagar B, Overduin M, Ikura M and Rini JM:
Structural basis of calcium-induced E-cadherin rigidification and
dimerization. Nature. 380:360–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Roya F and Berxb G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aberle H, Schwartz H and Kemler R:
Cadherin-catenin complex: Protein interactions and their
implications for cadherin function. J Cell Biochem. 61:514–523.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shapiro L, Fannon AM, Kwong PD, Thompson
A, Lehmann MS, Grübel G, Legrand JF, Als-Nielsen J, Colman DR and
Hendrickson WA: Structural basis of cell-cell adhesion by
cadherins. Nature. 374:327–337. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu DS, Hoefnagel SJM, Fisher OM,
Krishnadath KK, Montgomery KG, Busuttil RA, Colebatch AJ, Read M,
Duong CP, Phillips WA and Clemons NJ: Novel metastatic models of
esophageal adenocarcinoma derived from FLO-1 cells highlight the
importance of E-cadherin in cancer metastasis. Oncotarget.
7:83342–83358. 2016.PubMed/NCBI
|
|
27
|
Strathdee G: Epigenetic versus genetic
alterations in the inactivation of E-cadherin. Semin Cancer Biol.
12:373–379. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vécsey-Semjén B, Becker KF, Sinski A,
Blennow E, Vietor I, Zatloukal K, Beug H, Wagner E and Huber LA:
Novel colon cancer cell lines leading to better understanding of
the diversity of respective primary cancers. Oncogene.
21:4646–4662. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanazawa T, Watanabe T, Kazama S, Tada T,
Koketsu S and Nagawa H: Poorly differentiated adenocarcinoma and
mucinous carcinoma of the colon and rectum show higher rates of
loss of heterozygosity and loss of E-cadherin expression due to
methylation of promoter region. Int J Cancer. 102:225–229. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng CW, Wu PE, Yu JC, Huang CS, Yue CT,
Wu CW and Shen CY: Mechanisms of inactivation of E-cadherin in
breast carcinoma: Modification of the two-hit hypothesis of tumor
suppressor gene. Oncogene. 20:3814–3823. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen Y, Hirsch DS, Sasiela CA and Wu WJ:
Cdc42 Regulates E-cadherin ubiquitination and degradation through
an epidermal growth factor receptor to Src-mediated pathway. J Biol
Chem. 283:5127–5137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Symowicz J, Adley BP, Gleason KJ, Johnson
JJ, Ghosh S, Fishman DA, Hudson LG and Stack MS: Engagement of
collagen-binding integrins promotes matrix
metalloproteinase-9-dependent E-cadherin ectodomain shedding in
ovarian carcinoma cells. Cancer Res. 67:2030–2039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Johnson SK, Ramani VC, Hennings L and Haun
RS: Kallikrein 7 enhances pancreatic cancer cell invasion by
shedding E-cadherin. Cancer. 109:1811–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Trillsch F, Kuerti S, Eulenburg C, Burandt
E, Woelber L, Prieske K, Eylmann K, Oliveira-Ferrer L,
Milde-Langosch K and Mahner S: E-cadherin fragments as potential
mediators for peritoneal metastasis in advanced epithelial ovarian
cancer. Br J Cancer. 114:213–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jang B, Jung H, Chung H, Moon BI and Oh
ES: Syndecan-2 enhances E-cadherin shedding and fibroblast-like
morphological changes by inducing MMP-7 expression in colon cancer
cells. Biochem Biophys Res Commun. 477:47–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carvalho S, Oliveira T, Bartels MF,
Miyoshi E, Pierce M, Taniguchi N, Carneiro F, Seruca R, Reis CA,
Strahl S and Pinho SS: O-mannosylation and N-glycosylation: Two
coordinated mechanisms regulating the tumour suppressor functions
of E-cadherin in cancer. Oncotarget. 7:65231–652446.
2016.PubMed/NCBI
|
|
37
|
Petrova YI, Schecterson L and Gumbiner BM:
Roles for E-cadherin cell surface regulation in cancer. Mol Biol
Cell. 27:3233–3244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okugawa Y, Toiyama Y, Inoue Y, Iwata T,
Fujikawa H, Saigusa S, Konishi N, Tanaka K, Uchida K and Kusunoki
M: Clinical significance of serum soluble E-cadherin in colorectal
carcinoma1. J Surg Res. 175:e67–e73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Efstathiou JA, Liu D, Wheeler JM, Kim HC,
Beck NE, Ilyas M, Karayiannakis AJ, Mortensen NJ, Kmiot W, Playford
RJ, et al: Mutated epithelial cadherin is associated with increased
tumorigenicity and loss of adhesion and of responsiveness to the
motogenic trefoil factor 2 in colon carcinoma cells. Proc Natl Acad
Sci. 96:pp. 2316–2321. 1999; View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kitadai Y, Bucana CD, Ellis LM, Anzai H,
Tahara E and Fidler IJ: In situ mRNA hybridization technique for
analysis of metastases related genes in human colon carcinoma.
cells. 147:1238–1247. 1995.
|
|
41
|
Gofuku J, Shiozaki H, Tsujinaka T, Inoue
M, Tamura S, Doki Y, Matsui S, Tsukita S, Kikkawa N and Monden M:
Expression of E-cadherin and alpha-catenin in patients with
colorectal carcinoma. Correlation with cancer invasion and
metastasis. Am J Clin Pathol. 111:29–37. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Velikova G, Banks RE, Gearing A, Hemingway
I, Forbes MA, Preston SR, Hall NR, Jones M, Wyatt J, Miller K, et
al: Serum concentrations of soluble adhesion molecules in patients
with colorectal cancer. Br J Cancer. 77:1857–1863. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wilmanns C, Grossmann J, Steinhauer S,
Manthey G, Weinhold B, Schmitt-Gräff A and von Specht BU: Soluble
serum E-cadherin as a marker of tumour progression in colorectal
cancer patients. Clin Exp Metastasis. 21:75–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weiss JV, Klein-Scory S, Kübler S,
Reinacher-Schick A, Stricker I, Schmiegel W and Schwarte-Waldhoff
I: Soluble E-cadherin as a serum biomarker candidate: Elevated
levels in patients with late-stage colorectal carcinoma and FAP.
Int J Cancer. 128:1384–1392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cao H, Xu E, Liu H, Wan L and Lai M:
Epithelial-mesenchymal transition in colorectal cancer metastasis:
A system review. Pathol Res Pract. 211:557–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang R, Ma X, Li Y, He Y, Huang D, Cai S
and Peng J: The characteristics and prognostic effect of E-cadherin
expression in colorectal signet ring cell carcinoma. PLOS One.
11:e01605272016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gao M, Zhang X, Li D, He P, Tian W and
Zeng B: Expression analysis and clinical significance of eIF4E,
VEGF-C, E-cadherin and MMP-2 in colorectal adenocarcinoma.
Oncotarget. 7:85502–85514. 2016.PubMed/NCBI
|
|
48
|
Chen D, Dai F, Chen Z, Wang S, Cheng X,
Sheng Q, Lin J and Chen W: Dimethoxy curcumin induces apoptosis by
suppressing survivin and inhibits invasion by enhancing E-cadherin
in colon cancer cells. Med Sci Monit. 22:3215–3222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nguyen L, Fifis T and Christophi C:
Vascular disruptive agent OXi4503 and anti-angiogenic agent
Sunitinib combination treatment prolong survival of mice with CRC
liver metastasis. BMC Cancer. 16:5332016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fan LC, Teng HW, Shiau CW, Tai WT, Hung
MH, Yang SH, Jiang JK and Chen KF: Regorafenib (Stivarga)
pharmacologically targets epithelial-mesenchymal transition in
colorectal cancer. Oncotarget. 7:64136–64147. 2016.PubMed/NCBI
|
|
51
|
Liu S, Barry EL, Baron JA, Rutherford RE,
Seabrook ME and Bostick RM: Effects of supplemental calcium and
vitamin D on the APC/β-catenin pathway in the normal colorectal
mucosa of colorectal adenoma patients. Mol Carcinog. 56:412–424.
2017. View Article : Google Scholar : PubMed/NCBI
|