Introduction
Human gastric adenocarcinoma (AGS), one of the most
common types of malignant neoplasm, is derived from the glandular
epithelium of the gastric mucosa (1,2). Although
chemotherapy is the primary treatment for gastric cancer, the toxic
side effects and low efficacy of existing chemotherapeutic drugs
remain a matter of concern (3).
Therefore, studies to identify other potential anticancer agents
and novel therapeutic approaches, including natural compounds, are
important (4). For example, the
plants used in traditional medicine are comparatively safe and
cost-effective; their potential as alternatives to existing
chemotherapeutic drugs should be investigated.
Withania somnifera (winter cherry) and active
constituents derived from this plant have been used extensively in
the traditional medicine of south Asia (5). Withaferin A (WA), a bioactive
withanolide compound isolated from this plant, possibly contributes
to the therapeutic effects of W. somnifera, particularly
against cancer (6). Indeed, WA has
been demonstrated to produce antitumorigenic effects in multiple
cancer cell lines, including breast, prostate and lung cancer cells
(7–9).
Senthil et al (10) recently
reported that an extract from W. somnifera inhibits the
proliferation of AGS cells by inducing apoptosis and cell cycle
arrest. Furthermore, we have recently reported that WA efficiently
decreased pro-inflammatory processes in gastric epithelial cells,
as well as immune cells, in response to Helicobacter pylori
(11,12), which serves a pivotal role in the high
incidence of gastric cancer (2).
However, a complete understanding of the antitumor effects of WA in
gastric cancer remains to be achieved. Therefore, in the present
study, the cell proliferation, apoptosis, cell cycle regulation and
migration/invasion in AGS cells in response to WA treatment were
examined, and the potential therapeutic effects of WA in AGS were
further characterized.
Materials and methods
Cell culture and WA
AGS human gastric epithelial cells were purchased
from the Korean Cell Line Bank (Seoul, Korea) and cultured with
RPMI medium (Welgene, Inc., Daegu, Korea) containing 10% fetal
bovine serum (FBS; Corning Incorporated, Corning, NY, USA) and
penicillin/streptomycin in a 5% CO2 incubator at 37°C.
WA was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). WA was dissolved in dimethyl sulfoxide (DMSO;
Sigma-Aldrich; Merck KGaA) for use.
MTT assay
An MTT assay was performed to determine the
cytotoxicity of WA in AGS cells. Cells (1×104 cells/well) were
seeded in 200 µl complete culture medium (RPMI medium with 10% FBS
and penicillin/streptomycin) in 48-well plates and incubated
overnight at 37°C. The cells were then incubated with different
concentrations of WA (0, 0.5, 1, 2.5 or 5 µM) for 24 h. Each well
was washed with PBS twice and then 200 µl MTT (4 mg/ml) was added.
Following a 4 h incubation at 37°C, the MTT solution was removed
and 200 µl DMSO was added. The plates were agitated for 5 min to
dissolve formazan crystals. The optical density (OD) values were
determined at 570 nm using an ELISA plate reader (Epoch; BioTek
Instruments, Inc., Winooski, VT, USA). Experiments were performed
in triplicate with identical conditions.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double-staining assay
An Annexin V-FITC/PI double-staining assay was
performed to analyze cell death in WA-treated AGS cells. Cells were
seeded in complete culture medium at a density of 5×104 cells/ml
into a 60-ml dish and incubated overnight. The cells were incubated
with various concentrations of WA (0, 1, 2.5 and 5 µM) for 6 or 18
h at 37°C. The cells were stained using a FITC Annexin V apoptosis
detection kit I (BD Pharmingen, San Diego, CA, USA) according to
the manufacturer's protocol and immediately analyzed using flow
cytometry (BD FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA). The proportions of cells in four stages, including live,
early apoptosis, late apoptosis and necrotis, were calculated using
CellQuest™ Pro software (version 5.1; BD Biosciences, San Jose, CA,
USA).
Western blotting
AGS cells were harvested, washed twice with PBS and
lysed in a buffer containing 1% Nonidet-P40 supplemented with
protease inhibitors (complete Mini EDTA-free; Roche Applied
Science, Mannheim, Germany) and 2 mM dithiothreitol on ice. The
extracted protein concentration was determined using a protein
assay kit (cat no. 500-0006; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Lysates (30 µg) were separated by 10, 12 and
15% SDS-PAGE and transferred onto nitrocellulose membranes by
electroblotting at constant voltage (100 V) for 90 min. The
membranes were blocked with 5% skimmed milk at room temperature for
1 h and incubated overnight with primary antibodies against cleaved
caspase-3 (1:1,000; cat. no. 9664; rabbit), caspase-7 (1:1,000;
cat. no. 8438; rabbit) and caspase-9 (1:1,000; cat. no. 7237;
rabbit), B-cell lymphoma 2 (Bcl-2; 1:1,000; cat. no. 2872; rabbit),
cleaved poly (ADP-ribose polymerase) (PARP) (1:1,000; cat. no.
5625; rabbit), cyclin B1 (1:1,000; cat. no. 4138; rabbit) (all Cell
Signaling Technology, Inc., Danvers, MA, USA), and β-actin
(1:1,000; cat. no. sc130656; rabbit; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). Following incubation with secondary
horseradish peroxidase-conjugated goat anti-rabbit (1:4,000; cat.
no. sc2301; Santa Cruz Biotechnology, Inc.) or goat anti-mouse IgG
(1:2,000; cat. no. sc2031; Santa Cruz Biotechnology, Inc.)
antibodies for 2 h at room temperature. Proteins were detected with
SuperSignal™ West Pico chemiluminescent substrate
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Bands were
visualized by exposing the blots onto CP-BU film (Agfa HealthCare
NV, Mortsel, Belgium).
Cell cycle analysis
To perform cell cycle analysis, AGS cells were
seeded in complete culture medium at a density of 5×105 cells/ml in
6-well plates and incubated overnight at 37°C. The cells were then
treated with 0, 1, 2.5 or 5 µM WA for 12 h at 37°C. Following the
incubation, cells were harvested with trypsin and fixed with 50%
ethanol overnight at −20°C. The cells were washed with ice-cold PBS
and incubated in PBS containing 50 µg/ml PI and 100 µg/ml RNase A
solution (both from Sigma-Aldrich; Merck KGaA) for 30 min at 37°C
in the dark, followed by flow cytometric analysis (BD FACSCalibur;
BD Biosciences). Cell cycle distribution (G1/G0, S and G2/M) was
determined using CellQuest™ Pro software.
In vitro wound healing assay
An in vitro wound healing assay of AGS cells
was performed at various time points, as described previously
(13–15). Briefly, cells were plated in 6-well
plates and grown to 100% confluency. Following the creation of a
wound by scraping with a pipette tip, the cells were washed with
PBS, treated with 0, 10, 100 or 500 µM WA in complete culture
medium, and subsequently incubated at 37°C for 0, 24, 48 or 56 h.
Cells were imaged at each time point with an IX51 widefield
microscope (Olympus Corporation, Tokyo, Japan) at ×5 magnification
with an Orca ER charge-coupled device camera (Hamamatsu Photonics
K.K., Hamamatsu, Japan).
Boyden chamber assay (invasion
assay)
The Boyden chamber technique was used to evaluate
the invasion activity of AGS cells. The cells were seeded in
complete culture medium at a density of 1×105 cells/ml in a 60 ml
dish, incubated overnight at 37°C and subsequently treated with 0,
1, 2.5 or 5 µM WA for 24 h at 37°C. Briefly, 27 µl RPMI medium
containing 10% FBS was added to the lower chambers. A
collagen-coated membrane with 8 µm pores (Neuro Probe, Inc.,
Gaithersburg, MD, USA) was placed on top of the lower chambers. AGS
cells (1.8×106 cells/well) were added to the upper chambers and
incubated for 6 h at 37°C. The cells remaining on the upper side of
the membrane were carefully removed using a cotton swab and stained
using the Diff-Quick staining kit (Siemens Healthcare GmbH,
Erlangen, Germany). The stained cells were counted manually at ×100
magnification using a light microscope.
Statistical analysis
All experiments were performed at least in
triplicate. Results were expressed as the mean ± standard error of
the mean. All statistical analyses (including a paired student's
t-test and a one-way analysis of variance with a Bonferroni post
hoc test) were performed using GraphPad Prism (version 5.00;
GraphPad Software, Inc., La Jolla, CA, USA). P<0.01 was
considered to indicate a statistically significant difference.
Results
Treatment with WA results in a
dose-dependent inhibition of AGS cell growth
The viability of AGS cells treated with WA was
determined using the MTT assay. Cells (1×104 cells/well in a
48-well plate) were exposed to various concentrations (0, 0.5, 1,
2.5, and 5.0 µM) of WA for 24 h. The proportion of viable cells was
significantly decreased at the lowest concentration of WA treatment
(0.5 µM, P<0.01; Fig. 1). The
half-maximal inhibitory concentration of WA in AGS cells at 24 h
was 0.75±0.03 µM. At concentrations >1 µM, a more significant
decrease in cell viability was observed (P<0.001; Fig. 1). The data indicate that WA exerted a
significant inhibitory effect on AGS cell proliferation.
WA inhibits the viability of AGS cells
by inducing apoptosis
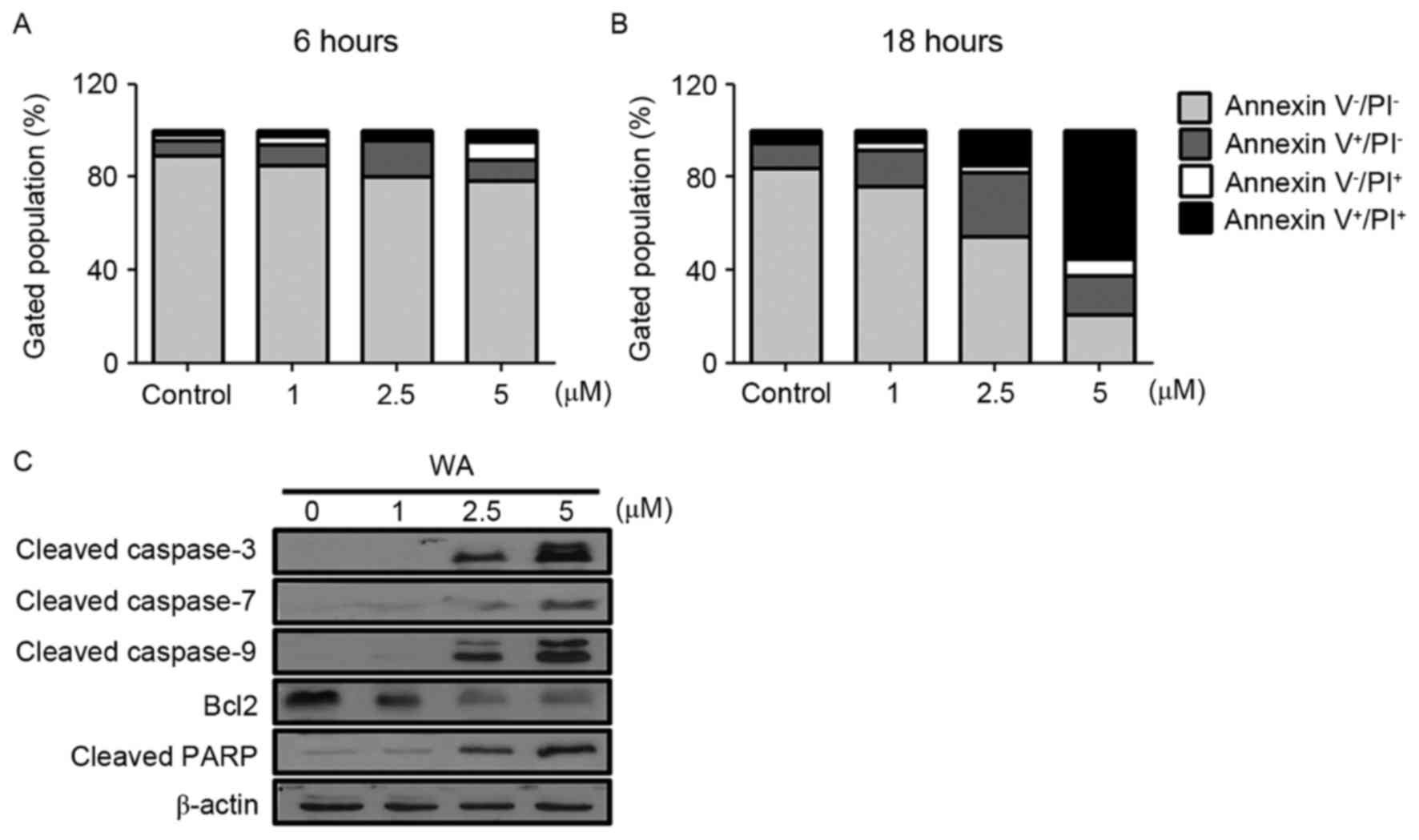
To determine whether the decreased viability was due
to apoptosis in WA-treated AGS cells, an Annexin V-FITC/PI
double-staining assay was performed for the identification of early
and late apoptotic cells. As demonstrated in Fig. 2A (6 h) and B (18 h), an increase in
the frequency of Annexin V-positive cells was observed with
increasing concentration of WA (at 6 and 18 h time points),
compared with the control (Fig. 2A and
B). The rate of apoptosis, including early (Annexin
V+/PI−) and late (Annexin
V+/PI+) stages, for each WA dose was
increased compared with untreated cells (Fig. 2A and B). The proportion of early and
late apoptotic cells increased in a WA dose-dependent manner
(Fig. 2A and B). To understand the
apoptotic events in WA-treated AGS cells, cleaved caspase-3, −7 and
−9, and PARP were examined as pro-apoptotic markers, and Bcl-2 was
examined as an anti-apoptotic marker. Cleaved caspase-3, −7 and −9,
and PARP proteins expression was most evident at 2.5 and 5 µM WA,
whereas Bcl-2 was decreased (Fig.
2C). This result suggests that WA-treated AGS cells undergo
cell death via activation of caspases, PARP cleavage and the loss
of Bcl-2, which is associated with apoptosis.
WA leads to G2/M cell cycle arrest in
AGS cells
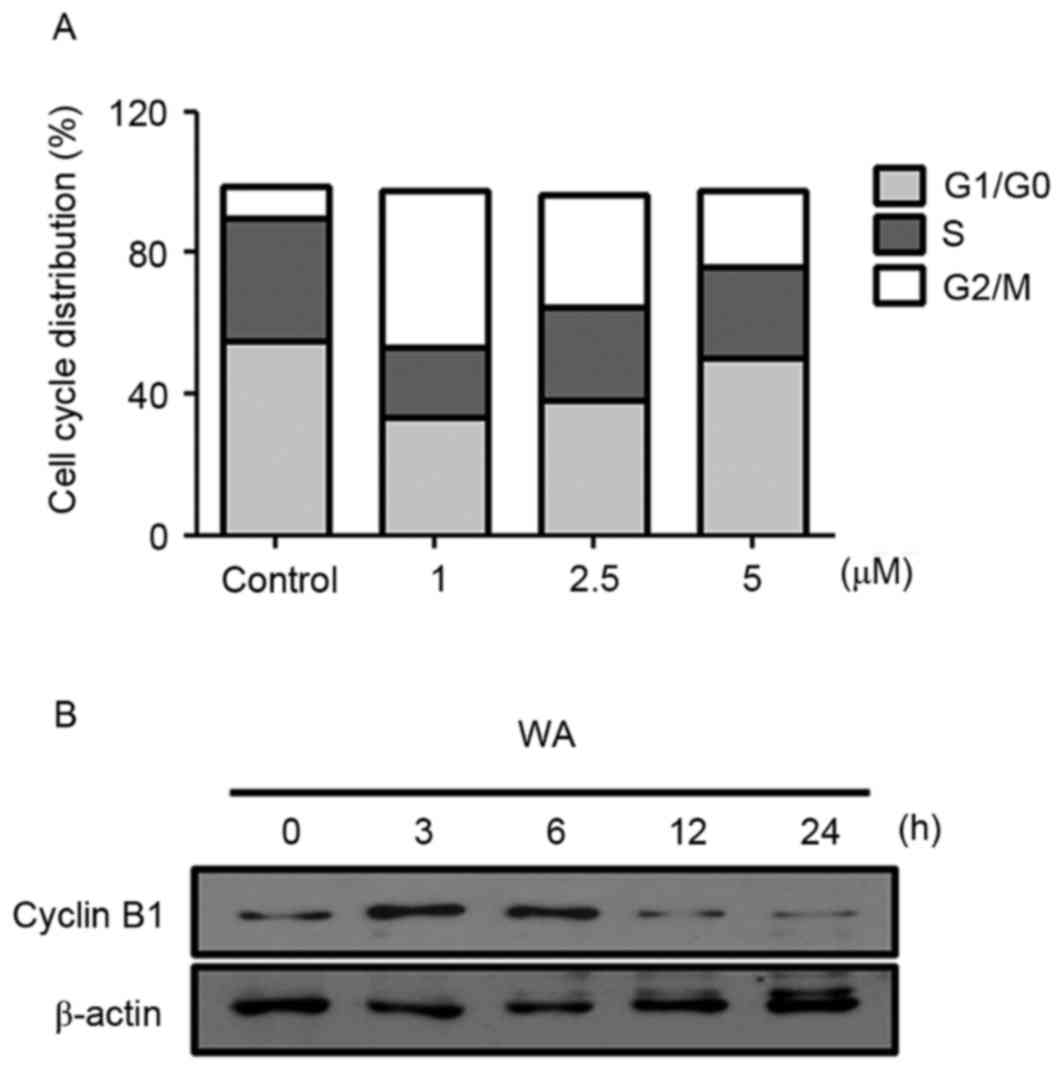
Cell cycle arrest is a key mechanism involved in the
induction of cell growth inhibition (16). To test whether WA affects the cell
cycle progression of AGS cells, cell cycle distribution analysis
was performed with PI staining. AGS cells were treated with 0, 1,
2.5 or 5 µM WA for 12 h. Compared with the negative control
(9.02%), WA treatment resulted in an increase in the proportion of
G2/M cells to 43.96, 31.67 and 21.82% in cells treated with 1, 2.5
and 5 µM of WA for 12 h, respectively (Fig. 3A). This phase shift was most marked at
lower concentrations of WA treatment. WA-treated cells exhibited
lower proportions of G1/G0 cells (33.5% at 1 µM, 38.04% at 2.5 µM
and 50.08% at 5 µM) compared with untreated cells (55.05%; Fig. 3A). Similar to the effects on the
proportion of cells in G1/G0 phase, WA treatment also resulted in a
decrease in the proportion of cells in S phase (Fig. 3A). These results suggest that WA leads
specifically to a G2/M phase cell cycle arrest, accounting for the
inhibition of proliferation in AGS cells. Moreover, an evident
decrease in the expression of Cyclin B1 at 2.5 µM WA treatment for
12 or 24 h was identified (Fig. 3B),
which may cause the inhibition of G2/M progression (17). These data suggest that WA has an
inhibitory effect on cell cycle progression, thereby resulting in
the reduction of proliferation of AGS cells.
WA reduces the migration and invasion
activity of AGS cells in vitro
The migratory and invasive abilities of AGS cells
treated with WA were evaluated using an in vitro wound
healing assay and a Boyden chamber assay. WA treatment tended to
inhibit the migration of cells towards the wound at earlier time
points (between 24 and 48 h) and the difference compared with the
extent of cell migration observed in the untreated cells was
statistically significant at 56 h (P<0.01, n=3; Fig. 4A and B). In the Boyden chamber assay,
it was identified that AGS cells treated with 1, 2.5 or 5 µM WA
exhibited a significant decrease in invasive ability compared with
untreated cells at 24 h (Fig. 5A and
B), suggesting that WA has an inhibitory effect on the
migratory and invasive capabilities of AGS cells.
Discussion
The aim of the present study was to assess the
effects of WA on human AGS cells. WA, isolated from W.
somnifera, has been ascribed therapeutic properties and its
antitumor effects against various types of cancer have been
reported (6). Previous studies have
demonstrated that WA inhibited the growth of cancer cells,
including cutaneous melanoma (18),
ovarian cancer (19), prostate cancer
(8) and leukemia (4) cells. Multiple molecular mechanisms
underlying the antitumor activity of WA have been reported.
Reactive oxygen species serve a role in the pro-apoptotic effect of
WA in various types of cancer, including leukemia (20) and renal cancer (21). Proteasome inhibition, the induction of
endoplasmic reticulum stress, downregulation of Akt
serine/threonine kinase phosphorylation and the downregulation of
Janus kinase/signal transducer and activator of transcription 3
signaling are also suggested to contribute to cancer cell apoptosis
following WA treatment (4,22,23).
Transcription factors, including nuclear factor-κB (NF-κB) and
mitogen-activated protein kinases, contribute to WA-mediated
induction of apoptosis of leukemia (4,24),
glioblastoma (25) and breast cancer
(26). The antitumor effects of WA on
human AGS were not previously studied, although W. somnifera
leaf and root extract have been assessed for their effect on AGS
cells (10). In the present study, it
was identified that apoptosis was significantly increased in AGS
cells treated with WA. As expected, WA treatment of AGS cells
upregulated the expression of the initiator (caspase-9) and
effector (capase-3 and −7) caspases, which cleave PARP protein. In
addition, WA-treated cells exhibited relatively decreased Bcl-2
levels, which promotes gastric cancer cell survival. Indeed, the
downregulation of Bcl-2 has been previously associated with the
mechanisms underlying the anti-proliferative effect of WA in a
variety of cultured cell types (18,21).
Apoptosis induced by WA may be associated with cell
cycle arrest at different phases in cancer cell lines. For example,
WA was demonstrated to cause the arrest of cell cycle at the sub-G1
phase in renal carcinoma cells (21),
and in the G2/M phase in leukemia (27), uveal melanoma cells (28), breast cancer cells (29) and ovarian carcinoma cells (19). The results of the present study
indicated that WA treatment caused G2/M cell cycle arrest in AGS
cells, thus delaying the progression of the cells toward mitosis.
This G2/M phase shift was more pronounced at lower WA
concentrations, whereas apoptotic events were increased at higher
WA concentration (Figs. 2 and
3) WA-treated AGS cells also
exhibited depleted cyclin B1, which is required to promote G2/M
transition (Fig. 3B). The alteration
in cyclin B1 has been associated with the induction of cell cycle
arrest in the presence of WA; WA was previously demonstrated to
decrease the activity of cyclin B1-dependent kinase 1 (Cdk1) and
complex formation between cyclin B1 and Cdk1 in cancer cells
(30,31). In human breast cancer and ovarian
carcinoma cells, WA treatment resulted in the downregulation of
cell division cycle 25°C, which activates cyclin B1-Cdk1 complexes
(19,31). These data suggest that apoptosis
induced by WA may be initiated by G2/M phase cell cycle arrest due
to the change in the levels of cyclin B1.
Invasion and metastasis are hallmarks of cancer
development. WA has been reported to inhibit the metastatic
behavior of human cancer cells (32).
In the in vitro setting of the present study, AGS cell
migration and invasion were markedly inhibited by WA. Vimentin and
matrix metalloproteinases (MMPs) may be potential target molecules
for WA in AGS cells, although these were not examined in the
present study. Vimentin, the level of which is associated with cell
metastasis and motility in patients with gastric cancer (33), was expressed at a high level in the
metastatic gastric cancer cell line TMC-1, and at a low level in
the non-invasive gastric cancer cell line SC-M1 (34). WA directly binds to the cysteine
residues of vimentin to modify the domain structure of vimentin
(35), resulting in the impairment of
the function of this protein in gastric cancer (33). In addition to vimentin, MMPs are
associated with gastric cancer invasion and metastasis, and are
used as markers to assess the progression of gastric cancer
(36). In gastric cancer cells, MMP-2
and MMP-9 serve important roles in cancer infiltration, and MMP-2
and MMP-9 expression was demonstrated to be associated with the
progression of the tumor (37). WA
blocks MMP-9 activity in metastatic cancer cell lines (38), suggesting a possible explanation for
WA-induced inhibition of metastasis in AGS cells. Further
investigation is warranted, however, to clarify the role of
vimentin and MMPs in the pharmacological activity of WA in AGS
cells.
The inflammatory response is one of the major
factors promoting the initiation and progression of gastric cancer
(39). In particular, H.
pylori infection may induce gastric cancer by enhancing the
inflammatory response (40). In
addition, upregulation of interleukin 8, vascular endothelial
growth factor, angiogenin, urokinase-type plasminogen activator and
MMP-9 genes by H. pylori was reported to regulate the
metastasis and the invasion of gastric cancer cells (41,42).
Recently, our group reported that WA inhibited the activation of
NF-κB and pro-inflammatory cytokine production in gastric
epithelial and immune cells infected with H. pylori
(11,12). Therefore, there is evidence to suggest
that the anti-inflammatory effects of WA may hinder the progression
of gastric adenocarcinogenesis.
To the best of our knowledge, the results of the
present study demonstrate for the first time that WA inhibits human
AGS cell growth by the induction of G2/M cell cycle arrest and
apoptosis. WA also suppressed AGS cell migration and invasion.
These results provide support for the development of WA as a novel
therapeutic strategy against gastric cancer.
Acknowledgements
The present study was supported by the Program for
Basic Research in Science and Engineering, funded by the National
Research Foundation of Korea in the Ministry of Science,
Information and Communications Technology and Future Planning of
Korea (grant no. NRF-2015R1A2A2A01002360) and a grant from the
Korea Research Institute of Bioscience and Biotechnology (grant no.
KGM4241642).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fock KM: Review article: The epidemiology
and prevention of gastric cancer. Aliment Pharmacol Ther.
40:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dai ZJ, Gao J, Ji ZZ, Wang XJ, Ren HT, Liu
XX, Wu WY, Kang HF and Guan HT: Matrine induces apoptosis in
gastric carcinoma cells via alteration of Fas/FasL and activation
of caspase-3. J Ethnopharmacol. 123:91–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oh JH, Lee TJ, Kim SH, Choi YH, Lee SH,
Lee JM, Kim YH, Park JW and Kwon TK: Induction of apoptosis by
withaferin A in human leukemia U937 cells through down-regulation
of Akt phosphorylation. Apoptosis. 13:1494–1504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mirjalili MH, Moyano E, Bonfill M, Cusido
RM and Palazón J: Steroidal lactones from Withania somnifera, an
ancient plant for novel medicine. Molecules. 14:2373–2393. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palliyaguru DL, Singh SV and Kensler TW:
Withania somnifera: From prevention to treatment of cancer. Mol
Nutr Food Res. 60:1342–1353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hahm ER and Singh SV: Withaferin A-induced
apoptosis in human breast cancer cells is associated with
suppression of inhibitor of apoptosis family protein expression.
Cancer Lett. 334:101–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roy RV, Suman S, Das TP, Luevano JE and
Damodaran C: Withaferin A, a steroidal lactone from Withania
somnifera, induces mitotic catastrophe and growth arrest in
prostate cancer cells. J Nat Prod. 76:1909–1915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai Y, Sheng ZY, Chen Y and Bai C: Effect
of Withaferin A on A549 cellular proliferation and apoptosis in
non-small cell lung cancer. Asian Pac J Cancer Prev. 15:1711–1714.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Senthil K, Jayakodi M,
Thirugnanasambantham P, Lee SC, Duraisamy P, Purushotham PM,
Rajasekaran K, Charles S Nancy, Roy I Mariam, Nagappan AK, et al:
Transcriptome analysis reveals in vitro cultured Withania somnifera
leaf and root tissues as a promising source for targeted
withanolide biosynthesis. BMC Genomics. 16:142015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim G, Kim TH, Kang MJ, Choi JA, Pack DY,
Lee IR, Kim MG, Han SS, Kim BY, Oh SM, et al: Inhibitory effect of
withaferin A on Helicobacter pylori-induced IL8 production and NFκB
activation in gastric epithelial cells. Mol Med Rep. 13:967–972.
2016.PubMed/NCBI
|
|
12
|
Kim JE, Lee JY, Kang MJ, Jeong YJ, Choi
JA, Oh SM, Lee KB and Park JH: Withaferin A inhibits helicobacter
pylori-induced production of IL-1β in dendritic cells by regulating
NF-κB and NLRP3 inflammasome activation. Immune Netw. 15:269–277.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fejzo MS, Anderson L, Chen HW, Anghel A,
Zhuo J, Anchoori R, Roden R and Slamon DJ: ADRM1-amplified
metastasis gene in gastric cancer. Genes Chromosomes Cancer. Jun
6–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin SH and Shih YW: Antitumor effects of
the flavone chalcone: Inhibition of invasion and migration through
the FAK/JNK signaling pathway in human gastric adenocarcinoma AGS
cells. Mol Cell Biochem. 391:47–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pal AD, Basak NP, Banerjee AS and Banerjee
S: Epstein-Barr virus latent membrane protein-2A alters
mitochondrial dynamics promoting cellular migration mediated by
Notch signaling pathway. Carcinogenesis. 35:1592–1601. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
King KL and Cidlowski JA: Cell cycle
regulation and apoptosis. Annu Rev Physiol. 60:601–617. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stark GR and Taylor WR: Control of the
G2/M transition. Mol Biotechnol. 32:227–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mayola E, Gallerne C, Esposti DD, Martel
C, Pervaiz S, Larue L, Debuire B, Lemoine A, Brenner C and Lemaire
C: Withaferin A induces apoptosis in human melanoma cells through
generation of reactive oxygen species and down-regulation of Bcl-2.
Apoptosis. 16:1014–1027. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Samadi AK, Roby KF, Timmermann B
and Cohen MS: Inhibition of cell growth and induction of apoptosis
in ovarian carcinoma cell lines CaOV3 and SKOV3 by natural
withanolide Withaferin A. Gynecol Oncol. 124:606–612. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malik F, Kumar A, Bhushan S, Khan S,
Bhatia A, Suri KA, Qazi GN and Singh J: Reactive oxygen species
generation and mitochondrial dysfunction in the apoptotic cell
death of human myeloid leukemia HL-60 cells by a dietary compound
withaferin A with concomitant protection by N-acetyl cysteine.
Apoptosis. 12:2115–2133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang ES, Choi MJ, Kim JH, Choi KS and Kwon
TK: Withaferin A enhances radiation-induced apoptosis in Caki cells
through induction of reactive oxygen species, Bcl-2 downregulation
and Akt inhibition. Chem Biol Interact. 190:9–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Um HJ, Min KJ, Kim DE and Kwon TK:
Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of
human renal carcinoma Caki cells. Biochem Biophys Res Commun.
427:24–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi MJ, Park EJ, Min KJ, Park JW and Kwon
TK: Endoplasmic reticulum stress mediates withaferin A-induced
apoptosis in human renal carcinoma cells. Toxicol In Vitro.
25:692–698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mandal C, Dutta A, Mallick A, Chandra S,
Misra L, Sangwan RS and Mandal C: Withaferin A induces apoptosis by
activating p38 mitogen-activated protein kinase signaling cascade
in leukemic cells of lymphoid and myeloid origin through
mitochondrial death cascade. Apoptosis. 13:1450–1464. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grogan PT, Sleder KD, Samadi AK, Zhang H,
Timmermann BN and Cohen MS: Cytotoxicity of withaferin A in
glioblastomas involves induction of an oxidative stress-mediated
heat shock response while altering Akt/mTOR and MAPK signaling
pathways. Invest New Drugs. 31:545–557. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hahm ER, Lee J and Singh SV: Role of
mitogen-activated protein kinases and Mcl-1 in apoptosis induction
by withaferin A in human breast cancer cells. Mol Carcinog.
53:907–916. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okamoto S, Tsujioka T, Suemori SI, Kida J,
Kondo T, Tohyama Y and Tohyama K: Withaferin A suppresses the
growth of myelodysplasia and leukemia cell lines by inhibiting cell
cycle progression. Cancer Sci. 107:1302–1314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Samadi AK, Cohen SM, Mukerji R, Chaguturu
V, Zhang X, Timmermann BN, Cohen MS and Person EA: Natural
withanolide withaferin A induces apoptosis in uveal melanoma cells
by suppression of Akt and c-MET activation. Tumour Biol.
33:1179–1189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Antony ML, Lee J, Hahm ER, Kim SH, Marcus
AI, Kumari V, Ji X, Yang Z, Vowell CL, Wipf P, et al: Growth arrest
by the antitumor steroidal lactone withaferin A in human breast
cancer cells is associated with down-regulation and covalent
binding at cysteine 303 of β-tubulin. J Biol Chem. 289:1852–1865.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Munagala R, Kausar H, Munjal C and Gupta
RC: Withaferin A induces p53-dependent apoptosis by repression of
HPV oncogenes and upregulation of tumor suppressor proteins in
human cervical cancer cells. Carcinogenesis. 32:1697–1705. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stan SD, Zeng Y and Singh SV: Ayurvedic
medicine constituent withaferin a causes G2 and M phase cell cycle
arrest in human breast cancer cells. Nutr Cancer. 60 Suppl
1:S51–S60. 2008. View Article : Google Scholar
|
|
32
|
Thaiparambil JT, Bender L, Ganesh T, Kline
E, Patel P, Liu Y, Tighiouart M, Vertino PM, Harvey RD, Garcia A
and Marcus AI: Withaferin A inhibits breast cancer invasion and
metastasis at sub-cytotoxic doses by inducing vimentin disassembly
and serine 56 phosphorylation. Int J Cancer. 129:2744–2755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fuyuhiro Y, Yashiro M, Noda S, Kashiwagi
S, Matsuoka J, Doi Y, Kato Y, Kubo N, Ohira M and Hirakawa K:
Clinical significance of vimentin-positive gastric cancer cells.
Anticancer Res. 30:5239–5243. 2010.PubMed/NCBI
|
|
34
|
Chen YR, Juan HF, Huang HC, Huang HH, Lee
YJ, Liao MY, Tseng CW, Lin LL, Chen JY, Wang MJ, et al:
Quantitative proteomic and genomic profiling reveals
metastasis-related protein expression patterns in gastric cancer
cells. J Proteome Res. 5:2727–2742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bargagna-Mohan P, Hamza A, Kim YE, Ho Y
Khuan Abby, Mor-Vaknin N, Wendschlag N, Liu J, Evans RM, Markovitz
DM, Zhan CG, et al: The tumor inhibitor and antiangiogenic agent
withaferin A targets the intermediate filament protein vimentin.
Chem Biol. 14:623–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen W, Xi H, Wei B and Chen L: The
prognostic role of matrix metalloproteinase 2 in gastric cancer: A
systematic review with meta-analysis. J Cancer Res Clin Oncol.
140:1003–1009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Parsons SL, Watson SA, Collins HM, Griffin
NR, Clarke PA and Steele RJ: Gelatinase (MMP-2 and -9) expression
in gastrointestinal malignancy. Br J Cancer. 78:1495–1502. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee DH, Lim IH, Sung EG, Kim JY, Song IH,
Park YK and Lee TJ: Withaferin A inhibits matrix
metalloproteinase-9 activity by suppressing the Akt signaling
pathway. Oncol Rep. 30:933–938. 2013.PubMed/NCBI
|
|
39
|
Rayburn ER, Ezell SJ and Zhang R:
Anti-Inflammatory agents for cancer therapy. Mol Cell Pharmacol.
1:29–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wroblewski LE, Peek RM Jr and Wilson KT:
Helicobacter pylori and gastric cancer: Factors that modulate
disease risk. Clin Microbiol Rev. 23:713–739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kitadai Y, Sasaki A, Ito M, Tanaka S, Oue
N, Yasui W, Aihara M, Imagawa K, Haruma K and Chayama K:
Helicobacter pylori infection influences expression of genes
related to angiogenesis and invasion in human gastric carcinoma
cells. Biochem Biophys Res Commun. 311:809–814. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Strowski MZ, Cramer T, Schäfer G, Jüttner
S, Walduck A, Schipani E, Kemmner W, Wessler S, Wunder C, Weber M,
et al: Helicobacter pylori stimulates host vascular endothelial
growth factor-A (vegf-A) gene expression via MEK/ERK-dependent
activation of Sp1 and Sp3. FASEB J. 18:218–220. 2004.PubMed/NCBI
|