Introduction
Melanoma's incidence rates are rising worldwide
(1). Melanoma is the third most
common type of cancer metastasizing to the brain, subsequent to
lung and breast cancer (2). The
behavior of cutaneous melanoma is notoriously unpredictable, and
the 5-year survival rate deteriorates as the cancer stage
progresses (3,4). Morbidity and mortality are mainly
associated with metastatic disease, therefore, when metastasis is
clinically evident, the prognosis is extremely poor. In total, 90%
of melanoma patients with ≥3 sites of metastasis succumb to disease
within 1 year (3,4). Brain metastases are particularly
important in the context of malignant melanoma, since 44% of
patients with metastatic melanoma will develop symptomatic brain
metastasis (5–7). Intracranial manifestations account for
20–54% of mortalities in patients with melanoma (5–7).
Metastases to the breast from malignant melanoma or from other
extra-mammary tumors are rare and represent ~1.3–2.7% of cases
(8,9).
Melanoma, lymphoma and lung cancers are the most commonly reported
tumors metastasizing to the breast (8–10). In the
present study, the case of a premenopausal woman with a history of
malignant spindle cell melanoma of the left maxillary alveolus
extending to the left hard palate is reported. At 1 year after the
initial diagnosis, the patient presented with neurological deficits
and a palpable breast mass. The diagnostic and instrumental
procedures revealed metastases to the breast, brain and other
organs. The patient underwent a right parietal craniotomy and
excision of the metastatic melanoma, and planned to start
whole-brain radiation and potential immunotherapy (ipilimumab).
However, the patient developed multiple metastases and did not
receive radiation therapy. The patient was subsequently referred to
the palliative care team.
Case report
The present study reports the case of a 50-year-old
premenopausal woman with a negative family history of cancer. The
patient had diabetes mellitus type 2 and was receiving oral
hypoglycemic medication. In September 2014, the patient presented
to King Faisal Specialist Hospital (Riyadh, Saudi Arabia) with a
swelling of the left maxillary alveolus of 6-month duration, in
addition to increased size and intermittent bleeding. Clinically,
there was a 3×3-cm irregular, firm and non-tender lesion at the
left maxillary alveolus. The lesion extended to the hard palate and
buccal mucosa, alongside a large left cervical lymph node.
Preoperative computed tomography (CT) scan of the chest, abdomen
and pelvis, as well as bone scan and whole-body fluorodeoxyglucose
(FDG)-positron emission tomography (PET)/CT scan, revealed a lesion
extending to the hard palate and buccal mucosa, which was eroding
the maxillary alveolar process. In addition, there was an enlarged
necrotic, level-II, left lymph node measuring 3.6 cm, with no
evidence of distant or bone metastasis. The patient was diagnosed
with a malignant spindle cell melanoma of the left maxillary
alveolus extending to the left hard palate. The patient underwent
left inferior maxillectomy with obturator and left extended
supraomohyoid neck dissection (level I–IV), followed by an adjuvant
course of radiation therapy (48 Gy in 20 fractions), which was
completed in December 2014. Final histology revealed an
undifferentiated malignant melanoma (spindle-cell type). The tumor
was 4 cm in size and 1.5 cm in thickness. The tumor invaded the
submucosa and exhibited prominent lympho-vascular invasion. The
bone surrounding margins were free from the tumor. One lymph node
was positive for metastatic disease from a total of 62 lymph nodes
harvested, with no extra-nodal extension. Molecular
characterization by DNA sequencing revealed no mutation in the
codon 600 of BRAF (BRAF wild type). No mutation was detected in the
C-KIT gene at exons 8, 9, 11 or 17. DNA was extracted manually from
a tissue sample. Genetic examinations were performed by polymerase
chain reaction using exon 15 specific primers of the BRAF gene. The
amplified sequences were then determined using the BigDye
terminator sequencing kit and analyzed on an ABI 3730 XL automated
sequencer (both supplied by Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) from the two strands (mutation
nomenclature was based on GeneBank accession number NM_004333.4).
Immunohistochemical staining was performed using the primary
antibodies listed in Table I and the
secondary antibody for immunostaining was Ultraview universal DAB
detection kit (no. 760–500; Ventana Medical Systems, Inc., Tucson,
AZ, USA) according to the manufacturers protocol. Immunopositivity
was demonstrated for S-100 protein, melan A, human melanoma
black-45 and microphthalmia-associated transcription factor.
However, staining for cluster of differentiation 34, p63 and smooth
muscle antigen was negative. Cytogenetic testing for Ewing sarcoma
RNA binding protein 1 (22q12) rearrangement was not detected by
interphase fluorescence in situ hybridization. Paraffin
embedded tissue for Ewing sarcoma tumors are dewaxed with xylene
and treated with citric acid in boiling temperature. Treatment was
performed using a medical microwave on maximum temperature for 24
min in 6 min intervals. Detection of EWSR1 rearrangement was
analyzed using Vysis LSI EWSR1 (22q12) Dual Color, Break Apart FISH
Rearrangement Probe (20 µl, 350 ng/µl) provided by Abbott
Pharmaceutical Co. Ltd., (Probe cat. no. 3N59.20; Lake Bluff, IL,
USA) according to manufacturer's protocol. Examination of slide is
performed using a fluorescent microscope and 100 cells from each
field were analyzed. Postoperatively, the patient recovered well
and was subjected to regular follow-up.
 | Table I.Details of the antibodies used in the
immunohistochemical staining. |
Table I.
Details of the antibodies used in the
immunohistochemical staining.
| Target protein | Cat. no. | Supplier | Dilution, µg/ml | Duration, min | Temperature, °C |
|---|
| S-100 | 790–2523 | Ventana Medical
Systems, Inc., Tucson, AZ, USA | ~10.00 | 24 | 37 |
| Melan-A | 790–2990 | Ventana Medical
Systems, Inc. | ~3.40 | 24 | 37 |
| MITF | 790–4367 | Ventana Medical
Systems, Inc. | ~6.70 | 16 | 36 |
| CD34 | 790–2927 | Ventana Medical
Systems, Inc. | ~0.80 | 24 | 37 |
| p63 | 790–4509 | Ventana Medical
Systems, Inc. | ~0.14 | 24 | 37 |
| SMA | 760–2833 | Ventana Medical
Systems, Inc. | ~0.02 | 24 | 37 |
| HMB45 | 790–4366 | Ventana Medical
Systems, Inc. | ~0.50 | 24 | 37 |
In June 2015, the patient presented to King Faisal
Specialist Hospital with left-sided hemiparesis and slurred speech
of sudden onset, which was associated with ipsilateral jerky
movements. On examination, the vital signs were stable, and the
patient was fully conscious. No pallor, jaundice, skin rashes or
cutaneous lesions were detected. Both pupils (measuring 2 mm) were
equally reactive. Left-sided weakness was detected with a muscle
strength of 3 out of 5, while no cranial or sensory deficit was
noticed. A hard mass of 6×6 cm was observed at the upper outer
quadrant of the left breast, which was not attached to the
underlying structures. Neither skin or nipple alterations, nor
palpable lymph nodes in the axilla, were identified. The right
breast and axilla were normal. The rest of the examination was
unremarkable.
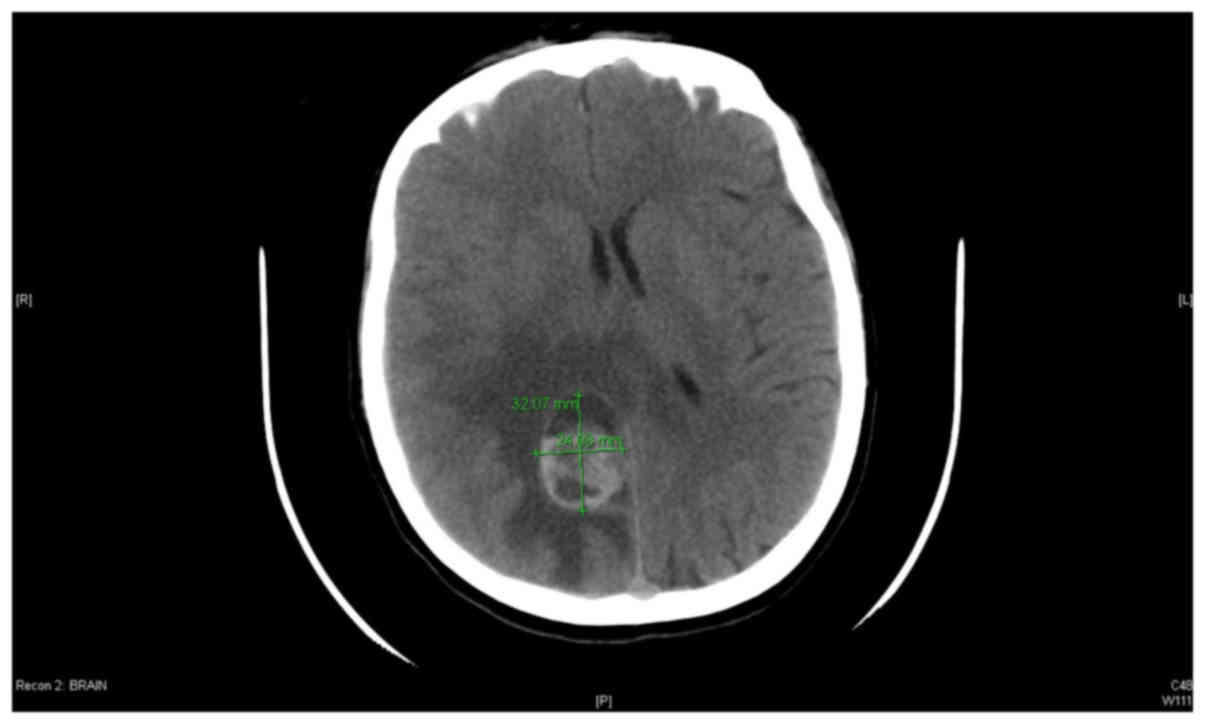
CT scan of the brain revealed a heterogonous complex
mass with a cystic component, which was located at the left
parasagittal posterior parietal region with surrounding edema. The
size of the lesion was 2.4×3.2 cm, and the edema extended into the
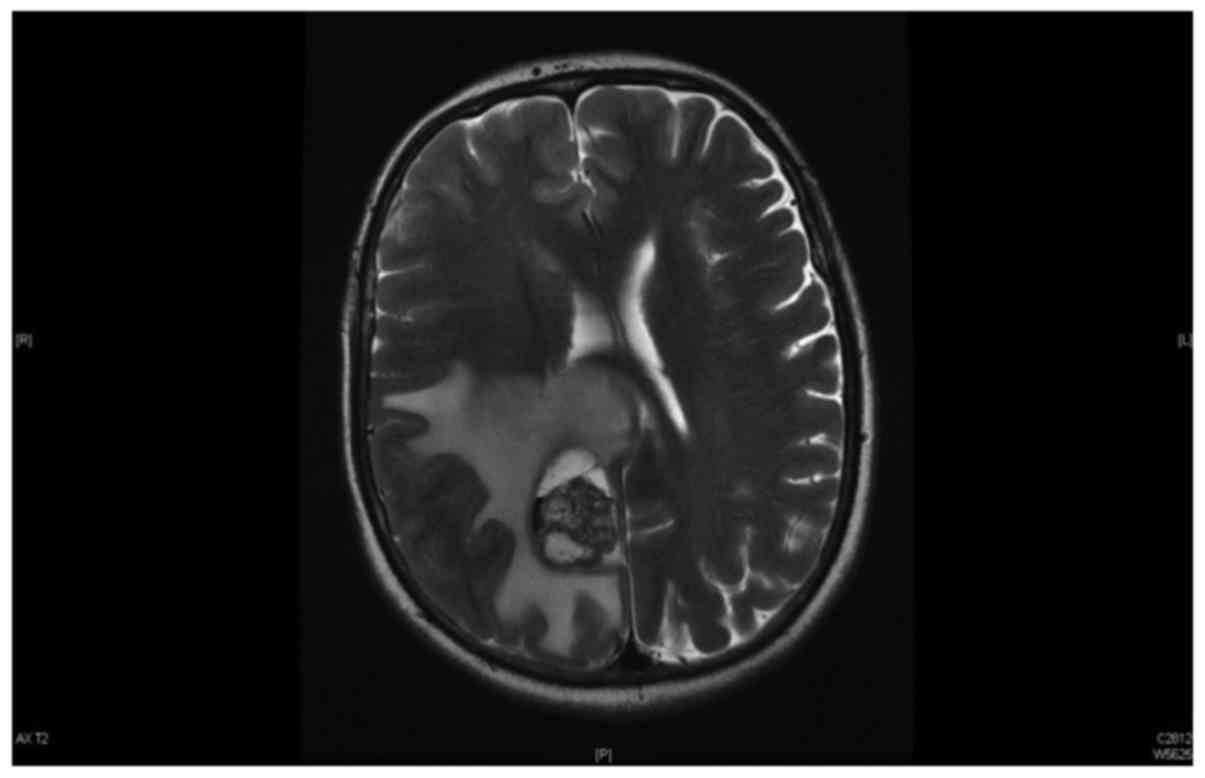
corpus callosum and the contralateral side (Fig. 1). Magnetic resonance imaging (MRI) of
the brain revealed a space-occupying lesion with massive
peritumoral edema, which was compatible with the findings of the
brain CT scan (Fig. 2). Whole-body
FDG-PET/CT scan did not reveal evidence of local recurrence in the
left maxilla. However, the lesion in the right cerebral parietal
lobe close to the middle line was FDG-avid. There were two newly
identified nodules in the left breast compatible with metastasis.
In addition, CT scan of the chest, abdomen and pelvis revealed
development of a new, enhanced, soft-tissue mass in the left upper
outer breast. The mass had a necrotic center, measured 5.6×4.7×7.1
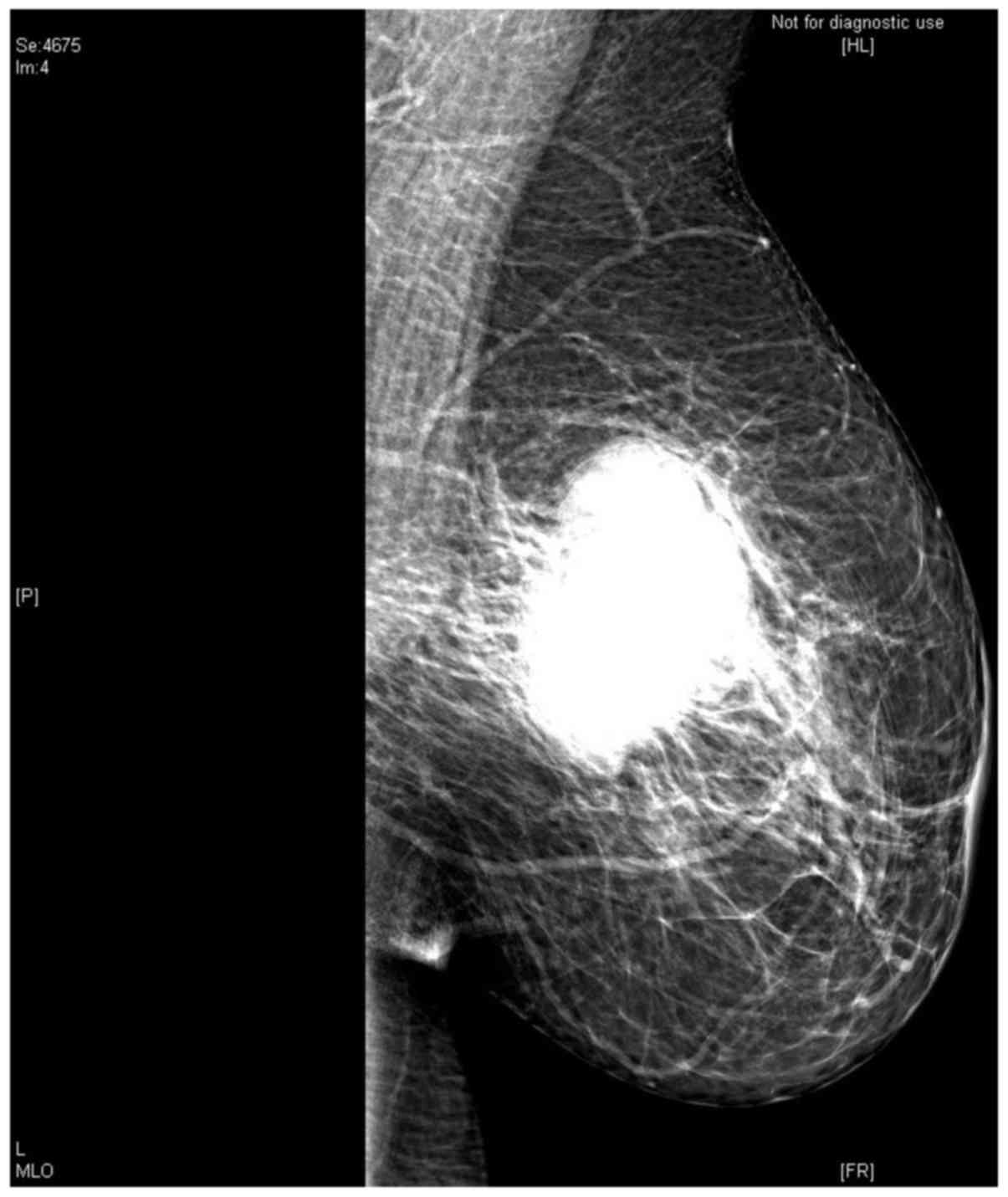
cm and did not present visceral metastasis. Mammogram examination
identified a macrolobulated mass in the left breast measuring 5×5
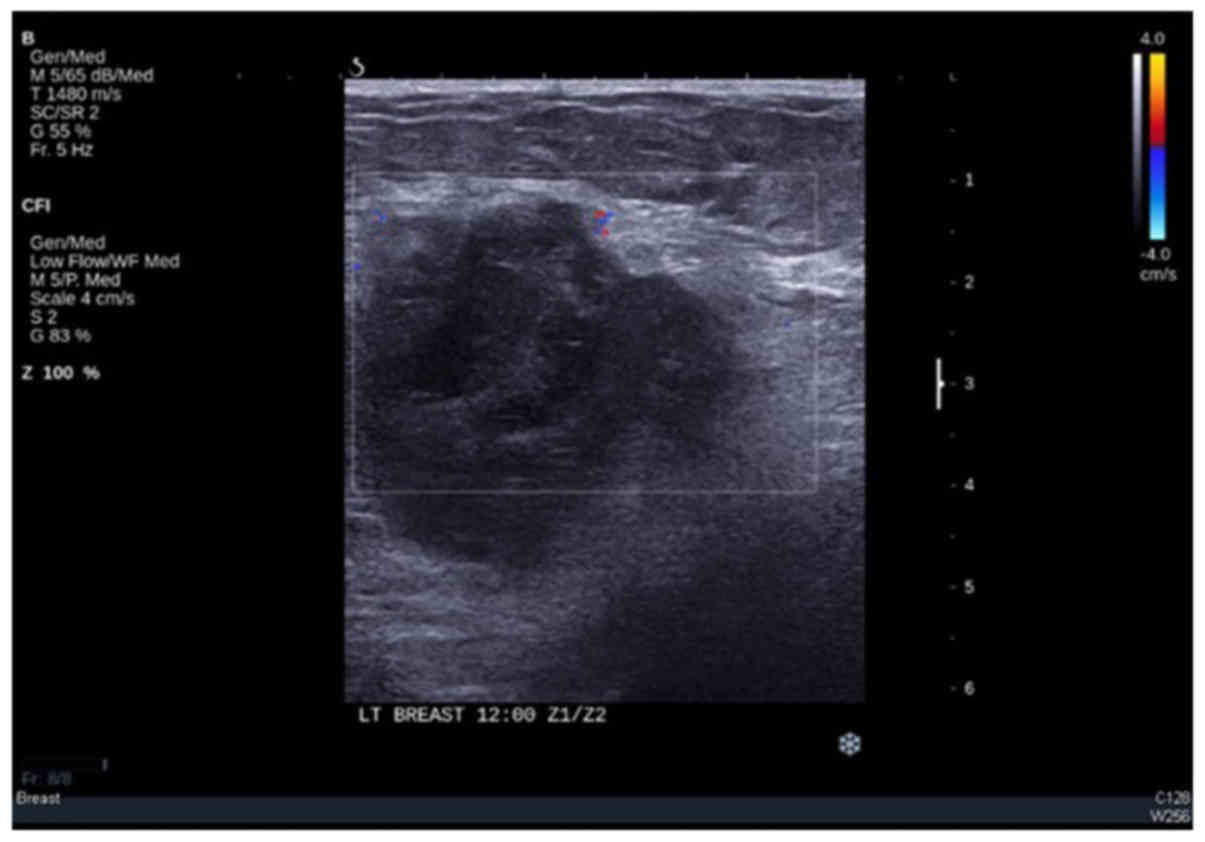
cm (Fig. 3). Ultrasound examination
of the left breast revealed a nonvascular heterogeneous, irregular,
macrolobulated mass measuring 5.3×3 cm (Fig. 4). Several reactive-looking axillary
lymph nodes were noted bilaterally. Biopsy of the left breast mass
was positive for metastatic malignant melanoma compatible with the
primary site.
The patient received anti-seizure medication. Since
the brain lesion was symptomatic, relatively large and associated
with ample edema, neurosurgical excision followed by immunotherapy
and radiation was considered. Radiosurgery could be considered as
an alternative option. Thus, the patient underwent right parietal
craniotomy and excision of the metastatic melanoma. The final
histopathology confirmed the diagnosis of metastatic malignant
melanoma. Postoperative CT scan of the brain demonstrated no
residual disease. The patient was discharged on October 29, 2015,
in good condition, and planned to start whole-brain radiation and
potential immunotherapy with the anti-cytotoxic
T-lymphocyte-associated antigen 4 (CTLA-4)-blocking agent
ipilimumab.
However, 4 weeks later, the patient presented to
King Faisal Specialist Hospital through the Emergency Department
complaining of headache, right shoulder painful swelling and
shortness of breath on mild exertion. On examination, the patient
was in respiratory distress, and a tender mass was identified in
the right shoulder. The CT scan revealed the left breast mass with
skin thickening, presence of new left axillary lymph nodes,
enlargement of the mediastinal lymph nodes, development of
pericardial effusion and bilateral pleural effusion. Further
examination, including MRI of the brain, revealed residual enhanced
lesion at the surgical resection bed of the right parietal lobe,
and extra-axial enhanced lesion at the right Meckel's cave
extending to the right precrural cistern, which was consistent with
metastasis. Whole-body PET/CT scan demonstrated increased uptake in
the brain, left breast, axilla and right shoulder, as well as
presence of new mediastinal lymph nodes, bilateral pleural effusion
and pericardial effusion. Ultrasound-guided fine needle aspiration
of the right shoulder mass was positive for metastatic melanoma.
Although radiotherapy and immunotherapy were planned previously,
due to the short period of relapse, widespread metastasis and
notable deterioration in performance status, on December 2015, the
multidisciplinary team at King Faisal Specialist Hospital
(including the oncologist, surgeon and palliative care physician)
and the patient's family agreed that further active therapy was
likely to be futile. Therefore, the patient was transferred to the
palliative care team for pain control and end of life care.
Discussion
Primary oral malignant melanoma is rare,
representing 0.2–8.0% of all melanoma cases (11). The predominant location for this type
of cancer is the hard palate and maxillary alveolus (12). It frequently exhibits a markedly
aggressive behavior, with rapid growth, high propensity to
metastasize and poor prognosis, which is worse than that of
cutaneous melanoma (11–13). Independent risk factors that determine
the outcome include undifferentiated tumor cell morphology,
vascular and neural invasion, tumor necrosis, tumor thickness, and
cervical lymph node metastasis (14).
In ~85% of cases, the melanoma will metastasize to the liver, lung,
bone or brain early in the course of the disease (15). The reported 5-year survival rate for
this cancer ranges from 12 to 16%, with a median survival rate of
18 months after the initial diagnosis (16). Surgery provides the best chance of
controlling the disease (17).
Chemotherapy and directed targeted therapy are appropriate for
patients with unresectable and metastatic disease; however, their
role as adjuvant therapies in extensive locoregional disease has
not been well defined (11).
Moreover, adjuvant radiation therapy is recommended (18,19). The
present case exhibited primary oral malignant melanoma with a
biological aggressive behavior. In addition, the patient displayed
the majority of defined risk factors for this type of cancer and
had acceptable definitive local therapy consisting of radical
surgery in combination with radiotherapy. However, no adjuvant
systemic therapy was administered, in accordance to King Faisal
Specialist Hospital and Research Centre institutional
guidelines.
Brain metastasis occurs in 10–40% of all melanoma
patients in clinical studies, and in ≤90% in autopsy (20). The brain is the first site of relapse
in 10–20% of these patients (20).
Risk factors for developing metastasis include gender (male),
presence of a head and neck or oral primary lesion, presence of
visceral metastasis, tumor thickness and ulceration of the primary
lesion (21). The prognosis of
patients with melanoma brain metastasis (MBM) is markedly poor.
Prior to the development of anti-CTLA-4/programmed cell death
protein 1 antibodies and BRAF/mitogen-activated protein kinase
(MAPK)/extracellular signal-regulated kinase inhibitors, the median
overall survival (OS) was ~6 months, with 25% of patients alive
after 1 year (22). The present case
had almost all the above risk factors, with the exception of being
a female. MBM were historically managed with surgical resection or
whole-brain radiation therapy, depending on the symptoms and the
location, number and size of tumors (23). Retrospective data from several reports
suggest that melanoma patients with limited brain metastasis
treated with surgery and/or stereotactic radiotherapy may achieve
better survival than melanoma patients with multiple metastases
(23). Temozolomide is the most
widely used systemic treatment for MBM; however clinical response
was observed only in 10% of patients (24,25). The
introduction of immune checkpoint-blockade antibodies and MAPK
inhibitors led to improved survival of metastatic melanoma
patients, with a median OS ranging between 10 and 25 months in
phase-III studies (26–28). However, patients with brain metastases
are underrepresented or excluded from the majority of clinical
trials due to historically dismal survival (29); therefore, the impact of these new
drugs on the survival of patients with MBM is not well defined.
Spagnolo et al (29) recently
reported a comprehensive systemic review aimed to analyze the
outcomes of patients with MBM treated with immune
checkpoint-blockade antibodies (ipilimumab, pembrolizumab and
nivolumab) and/or MAPK inhibitors (dabrafenib, vemurafenib and
trametinib), regardless of the study design. In total, 22 studies
were included with a total of 2,153 patients. The median OS was 7.9
months in phase I–III trials. In safety studies, the OS was 7.0
months for patients treated with immunotherapy vs. 7.9 months for
those treated with BRAF inhibitors. In safety studies, the median
OS was 4.3 and 7.7 months for patients treated with immunotherapy
and BRAF inhibitors, respectively. In that study the authors
acknowledged certain limitations in their analysis; despite these
limitations, the authors suggested that improved survival may also
be achieved in patients with MBM, and supported their inclusion in
large clinical trials (29). The
current case was unable to receive ipilimumab due to early onset of
widespread metastasis and marked rapid decline in the patient's
performance status, with subsequent early referral to a palliative
care program.
The frequency of metastatic tumors to the breast
from extra-mammary malignancy based on histological diagnosis
varies between 0.2 and 1.3% in clinical studies and between 0.2 and
6% in autopsy studies (30). The
demographics and clinical features are similar to those of primary
breast cancers, but the prognosis and management options are often
different. In 30% of patients, metastasis to the breast is the
first sign of malignancy (30–32).
Patients typically present with a rapidly growing, painless, firm
mass. In addition, diffuse skin involvement is rare, and axillary
lymph nodes are uncommon, similarly to the current patient's
presentation (30,31,33,34).
Clinical examination and imaging features of breast
metastases from melanoma usually do not allow a differential
diagnosis from breast primary tumors. The most common mammographic
appearance is a rounded mass with well-defined or slightly
irregular margins, while microcalcification is rare (30,34,35).
Ultrasound scans typically reveal a hypoechoic mass, which is
usually heterogeneous and poorly defined (35). In a review of patients presenting with
metastases to the breast from extra-mammary tumors, Toombs and
Kalisher noticed that 50% of metastases were located in the upper
outer quadrant of the breast, similar to the current case; however,
the position of a lump in the breast does not aid to distinguish
primary from secondary malignancies (31). It has been reported that patients with
breast metastases from melanoma are often young and premenopausal
(36), which supports the hypothesis
of hormonally driven progression of melanoma. Although the
influence of estrogen in the development and progression of
melanoma has been debated, the number of epidemiological studies
implicating estrogen in the etiology of melanoma has increased
(37).
Treatment for metastatic breast cancer differs
greatly from that for primary breast cancer and, due to the poor
prognosis of metastatic disease, the treatment should be
individualized. In patients with isolated metastatic disease
limited to the breast or with minimal disease burden, wide local
excision may be considered. Large bulky tumors may be palliated by
mastectomy, although this procedure should be avoided where
possible. In the past, the treatment options for metastatic
melanoma were limited. Chemotherapeutic agents and/or biological
response modifiers such as interleukin-2 (IL-2) are ineffective.
The response rate to a high dose of IL-2 has been reported to be
~7%, and long-term survival is observed only in 8% of stage-IV
patients, irrespective of the site of metastases. However, the use
of high-dose IL-2 was restricted due to critical side effects on
multiple organ systems (38).
Recently, the introduction of molecularly targeted therapy in
parallel with the development of checkpoint inhibitors has rapidly
improved the outcomes of metastatic melanoma patients (38).
In conclusion, the case reported in the present
study highlights the importance of early diagnosis and the poor
outcomes for patients with primary oral malignant melanoma, which
is a rare disease. Biologically, this type of cancer has an
aggressive behavior and poor survival. However, in the majority of
cases, the diagnosis is delayed until symptoms such as swelling,
ulceration and bleeding occur. Thus, early detection of oral
melanoma is critical. Due to the poor survival of patients with
this disease, clinical trials of novel systemic therapy using
targeted or immune modulators agents should consider enrolling
primary oral malignant melanoma patients, regardless of the
apparent extent of the disease.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
PET
|
positron emission tomography
|
|
FDG
|
fluorodeoxyglucose
|
|
MBM
|
melanoma brain metastasis
|
|
MAPK
|
mitogen-activated protein kinase
|
|
CTLA-4
|
cytotoxic T-lymphocyte-associated
antigen-4
|
|
OS
|
overall survival
|
|
IL-2
|
interleukin-2
|
References
|
1
|
Little EG and Eide M: Update on the
recurrence state of melanoma incidence. Dematol Clin. 30:355–361.
2012. View Article : Google Scholar
|
|
2
|
Patchell RA: The management of brain
metastasis. Cancer Treat Rev. 29:533–540. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balch CM, Houghton AN, Sober AJ and Soong
SJ: Cutaneous Melanoma. 4th. Quality Medical Publishing; St. Louis,
MO, USA: 2003
|
|
4
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Budman DR, Camacho E and Wittes RE: The
current causes of death in patients with malignant melanoma. Eur J
Cancer. 14:327–330. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davies MA, Liu P, McIntyre S, Kim KB,
Papadopoulos N, Hwu WJ, Hwu P and Bedikian A: Prognostic factors
for survival in melanoma patients with brain metastases. Cancer.
117:1687–1696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel JK, Didolkar MS, Pickern JW and
Moore RH: Metastatic pattern of malignant melanoma: A study of 216
autopsy cases. Am J Surg. 135:807–810. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ravdel L, Robinson WA, Lewis K and
Gonzalez R: Metastatic melanoma in the breast: A report of 27
cases. J Surg Oncol. 94:101–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al Samaraee A, Khout H, Barakat T and
Fasih T: Breast Metastasis from a melanoma. Ochsner J. 12:149–151.
2012.PubMed/NCBI
|
|
10
|
Thomson JF, Scolyer RA and Kefford RF:
Cutaneous melanoma. Lancet. 365:687–701. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rapidis AD, Apostolidis C, Vilos G and
Valsamis S: Primary malignant melanoma of the oral mucosa. J Oral
Maxillofac Surg. 61:1132–1139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen ZY, Liu W, Bao ZX, Zhou ZT and Wang
LZ: Oral melanotic macule and primary oral malignant melanoma:
Epidemiology, location involved, and clinical implications. Oral
Surg Oral Med Oral pathol Oral Radiol Endod. 112:e21–e25. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patel SG, Prasad ML, Escrig M, Singh B,
Shaha AR, Kraus DH, Boyle JO, Huvos AG, Busam K and Shah JP:
Primary mucosal malignant melanoma of the head and neck. Head Neck.
24:247–257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Keller DS, Thomay AA, Gaughan L, Olszanski
A, Wu H, Berger AC and Farma JM: Outcomes in patients with mucosal
melanomas. J Surg Oncol. 108:516–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tlholoe MM, Khammissa RA, Bouckaert M,
Altini M, Lemmer J and Feller L: Oral mucosal melanoma: Some
pathobiological considerations and illustrative report of a case.
Head Neck Pathol. 9:127–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumar K, Santhosh BS and Priya NK: Primary
oral malignant melanoma-a case report. Nig Dent J. 19:44–47.
2011.
|
|
17
|
Jayaraj SM, Hern JD, Mochloulis G and
Porter GC: Malignant melanoma arising in the frontal sinuses. J
Laryngol Otol. 111:376–378. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thompson AC, Morgan DA and Bradely PJ:
Malignant melanoma of the nasal cavity and paranasal sinuses. Clin
Otolaryngol Allied Sci. 18:34–36. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gilligan D and Selvin NJ: Radical
radiotherapy for 28 cases of mucosal melanoma in the nasal cavity
and sinuses. Br J Radiol. 64:1147–1150. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chiarion-Sileni V, Murr R, Pigozzo J,
Sarti S, Tomassi O and Romanini A: Brain metastases from malignant
melanoma. Forum (Genova). 13:170–182. 2003.PubMed/NCBI
|
|
21
|
Cohn-Cedermark G, Måsson-Brahme E,
Rutqvist LE, Larsson O, Johansson H and Ringborg U: Central nervous
system metastases of cutaneous malignant melanoma-a population
based study. Acta Oncol. 37:463–470. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Korn EL, Liu PY, Lee SJ, Chapman JA,
Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer
EA, et al: Meta-analysis of phase II cooperative group trials in
metastatic stage IV melanoma to determine progression-free survival
and overall survival benchmarks for future phase II trials. J Clin
Oncol. 26:527–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vecchio S, Spagnolo F, Merlo DF, Signori
A, Acquati M, Pronzato P and Queirolo P: The treatment of melanoma
brain metastases before the advent of targeted therapies:
Associations between therapeutic choice, clinical symptoms and
outcome with survival. Melanoma Res. 24:61–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agarwala SS, Kirkwood JM, Gore M, Dreno B,
Thatcher N, Czarnetski B, Atkins M, Buzaid A, Skarlos D and Rankin
EM: Temozolomide for treatment of brain metastases associated with
metastatic melanoma: A phase II study. J Clin Oncol. 22:2101–2107.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Margolin K, Atkins M, Thomson J, Ernstoff
S, Weber J, Flaherty L, Clark I, Weiss G, Sosman J, II Smith W, et
al: Temozolomide and whole brain radiation in melanoma metastatic
to the brain: A phase II trial of the cytokine working group. J
Cancer Res Clin Oncol. 128:214–218. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McArthur GA, Chapman PB, Robert C, Larkin
J, Haanen JB, Dummer R, Ribas A, Hogg D, Hamid O, Ascierto PA, et
al: Safety and efficacy of vemurafenib in BRAF(V600E) and
BRAF(V600K) mutation-positive melanoma (BRIM-3): Extended follow-up
of a phase 3, randomised, open-label study. Lancet Oncol.
15:323–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Long GV, Stroyakovskiy D, Gogas H,
Levchenko E, De Braud F, Larkin J, Garbe C, Jouary T, Hauschild A,
Grob JJ, et al: Dabrafenib and trametinib versus dabrafenib and
placebo for Val600 BRAF-mutant melanoma: A multicenter, double
blind, phase 3 randomised controlled trial. Lancet. 386:444–451.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Spagnolo F, Picasso V, Lambertini M,
Ottaviano V, Dozin B and Queirolo P: Survival of patients with
metastatic melanoma and brain metastases in era of MAP-kinase
inhibitors and immunologic checkpoint blockade antibodies: A
systemic review. Cancer Treat Rev. 45:38–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cabrero I Alvarado, Alvarez M Carrera,
Pérez Montiel D and Tavassoil FA: Metastases to the breast. Eur J
Surg Oncol. 29:854–855. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Toombs BD and Kalisher L: Metastatic
disease to the breast: Clinical, pathologic, and radiographic
features. AM J Roentgenol. 129:673–676. 1977. View Article : Google Scholar
|
|
32
|
Georgiannos SN, Chin J, Goode AW and
Sheaff M: Secondary neoplasms of the breast: A survey of the 20th
century. Cancer. 92:2259–2266. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amichetti M, Perani B and Boi S:
Metastases to the breast from extra-mammary malignancies. Oncology.
47:257–260. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vergier B, Trojani M, De Mascarel I,
Coindre JM and Le Treut A: Metastases to the breast: Differential
diagnosis from primary breast carcinoma. J Surg Oncol. 48:112–116.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee SH, Park JM, Kook SH, Han BK and Moon
WK: Metastatic tumors to the breast: Mammographic and
ultrasonographic findings. J Ultrasound Med. 19:257–262. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arora R and Robinson WA: Breast metastases
from malignant melanoma. J Surg Oncol. 50:27–29. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miller JG and Mac Neil S: Gender and
cutaneous melanoma. Br J Dermatol. 136:657–665. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Davey RJ, van der Westhuizen A and Bowden
NA: Metastatic melanoma treatment: Combining old and new therapies.
Crit Rev Oncol Hematol. 98:242–253. 2016. View Article : Google Scholar : PubMed/NCBI
|