Introduction
Paragangliomas (also known as extra-adrenal
pheochromocytomas) are rare tumors that arise from extra-adrenal
chromaffin cells (1,2). Paragangliomas originate from paraganglia
at a number of anatomical sites, including the head, neck, thorax
and abdomen. Retroperitoneal paraganglioma represents between 21.5
and 87% of all paragangliomas (3,4).
Paragangliomas are characterized by secretions of excessive
catecholamines, including epinephrine, norepinephrine and dopamine,
which may lead to clinical symptoms, including episodic
hypertension, tachycardia and diaphoresis. However, between 40 and
50% of paragangliomas are non-functional and potentially functional
(5,6).
If functional and potentially functional retroperitoneal
paragangliomas are misdiagnosed prior to surgery, intraoperative
compression of the tumor may cause a sudden release of
catecholamines, leading to disastrous consequences.
Since retroperitoneal paragangliomas are rare, the
behavior and treatment outcomes of this type of tumor remain
unclear. In the present study, a review of resected retroperitoneal
paragangliomas over a period of 16 years was conducted, in addition
to a review of the relevant literature.
Patients and methods
Patients
The present retrospective study was approved by the
Institutional Review Broad of the First Affiliated Hospital of
Wenzhou Medical University (Wenzhou, China). All patients provided
written informed consent prior to inclusion in the present study.
The present study included 34 patients with retroperitoneal
paragangliomas, who underwent resection at the First Affiliated
Hospital of Wenzhou Medical University by experienced surgeons
between December 1999 and December 2015. All paragangliomas were
diagnosed using pathological examinations.
Patient information, including demographics,
clinical symptoms and signs, tumor functional status, surgical
procedure, intraoperative results, tumor pathology, radiological
results and postoperative survival time, was extracted from
hospital records. Functional tumors were defined as those tumors
which exhibited increased urine or serum catecholamine levels,
attributable to the presence of the tumor. Malignant tumors were
defined as those associated with identified lymph node metastases
or distant metastases. Clinical characteristics of the 34 patients
with retroperitoneal paragangliomas are presented in Table I. All patients were followed up via
telephone and hospital visits at least every 6 months until they
succumbed or the endpoint date was reached (May 2016). The median
follow-up time was 67 months (range, 6–188 months).
 | Table I.Clinical characteristics of 34
patients with retroperitoneal paragangliomas. |
Table I.
Clinical characteristics of 34
patients with retroperitoneal paragangliomas.
| Patient no. | Sex | Age, years | Date of
surgery | Symptoms and
signs | Functional
status | Location | Size, cm | Intraoperative
results | Metastasis | Resection of other
organs |
|---|
| 1 | M | 60 | 28 November,
1999 | Hypertension,
palpitation | Yes | Peri-abdominal
aorta, close to the inferior pole of the right kidney | 8×7×5 | Adhesion of upper
part of the tumor to the duodenum | No | No |
| 2 | F | 20 | 9 October,
2000 | Abdominal pain | No | Inferior to the
pancreatic head, superior to the horizontal part | 6×5×5 | Encapsulated tumor
adjacent to the superior mesenteric vein | No | No |
| 3 | M | 78 | 17 August,
2001 | Hypertension,
abdominal pain | Yes | Peri-left kidney,
posterior to the intestine | 8×7×6 | Encapsulated tumor
with clear demarcation | No | No |
| 4 | F | 54 | 4 March, 2003 | Hypertension,
umbilical discomfort | Yes | Posterior to the
inferior vena cava, inferior to the caudate lobe of the liver | 7×6×6 | Encapsulated tumor
adhesive to the right adrenal gland | No | Right adrenal
gland |
| 5 | F | 53 | 17 September,
2003 | Hypertension,
abdominal pain after urination | Yes | Right to the neck
of the urinary bladder on the bottom of the pelvic cavity | 5×3×3 | Encapsulated tumor
with clear demarcation | No | No |
| 6 | M | 49 | 8 November,
2004 | Emaciation | No | Inferior to the
caudate with clear demarcation | 6×6×5 | Encapsulated tumor
with clear demarcation | No | No |
| 7 | M | 33 | 7 February,
2005 | Abdominal mass
(imaging results) | No | Peri-abdominal
aorta on the left upper abdomen | 3×2×2 | Encapsulated tumor
with clear demarcation | Yes | Spleen |
|
|
|
|
|
|
| Peri-abdominal
aorta on clear demarcation | 9×8×8 | Encapsulated tumor
with clear demarcation |
|
|
| 8 | F | 35 | 24 May, 2005 | Abdominal mass
(imaging results) | Yes | Posterior to the
juncture between the inferior vena cava and right renal vein | 4×3×3 | Encapsulated tumor
adjacent to surrounding vessels | No | No |
| 9 | F | 42 | 10 August,
2005 | Abdominal mass
(palpation identified) | No | Inferior to the
pancreatic head, anterior to the abdominal aorta | 12×8×8 | Encapsulated tumor
no local metastasis | No | No |
| 10 | F | 29 | 12 January,
2006 | Abdominal mass
(palpation identified) | No |
Retroperitoneallarge occupation on the
left | 23×15×12 | Adhesion to the
superior occupation on the left | No | Portion of blood
vessels |
| 11 | F | 36 | 25 May, 2006 | Abdominal pain | Yes | Inferior to the
caudate lobe of the liver, posterior to the inferior vena cava | 4×3×3 | Encapsulated tumor
with clear demarcation | No | No |
| 12 | M | 63 | 17 July, 2006 | Hypertension,
palpitation | Yes | Inferior to the
pancreas and duodenum, anterior to the abdominal aorta | 3×3×2 | Encapsulated tumor
with clear demarcation | No | No |
| 13 | M | 45 | 25 April, 2007 | Abdominal mass
(palpation identified) | Yes | Retroperitoneal in
the left middle abdomen | 10×8×8 | Encapsulated tumor
with clear demarcation | No | No |
| 14 | M | 50 | 2 August, 2007 | Abdominal mass
(imaging results) | No | Retroperitoneal in
the left upper abdomen | 14×12×10 | Encapsulated tumor
with infiltration into the pancreatic tail, left renal vessels, and
diaphragmatic crus | No | Pancreatic body and
tail, spleen, left kidney |
| 15 | M | 45 | 15 October,
2007 | Blood urine | Yes | In the left
adrenal, posterior to the inferior vena cava | 20×12×5 | Adhesion to the
spleen | No | Spleen |
|
|
|
|
|
|
|
| 5×4×4 | Encapsulated tumor
with clear demarcation | No |
|
| 16 | M | 61 | 4 July, 2008 | Abdominal mass
(imaging results) | Yes | Inferior to the
pancreas, anterior to the left kidney | 10×9×8 | Encapsulated tumor
with clear demarcation | No | No |
| 17 | M | 59 | 21 July, 2008 | Hypertension,
abdominal pain, diabetes | Yes | Peri-abdominal
aorta | 6×5×4 | Encapsulated tumor
with clear demarcation | No | Radical gastrectomy
and D3 lymph node dissection |
| 18 | M | 75 | 25 February,
2009 | Hypertension,
abdominal mass (imaging results) | Yes | Posterosuperior to
the pancreas, anterior to the left adrenal gland | 6×6×4 | Encapsulated tumor
with clear demarcation | No | No |
| 19 | F | 75 | 15 March, 2009 | Abdominal mass
(palpation identified) | No | Posterior to the
right mesentery, anterior to the psoas major | 15×15×10 | Tumor surface
adhesion to the appendix | No | Appendix |
| 20 | F | 48 | 30 November,
2009 | Abdominal mass
(imaging results) | Yes | Peri-abdominal
aorta, posterior to the right renal vein and inferior vena
cava | 5×5×3 | Encapsulate tumor
adhesive to the abdominal aorta | No | No |
| 21 | F | 52 | 19 May, 2011 | Abdominal mass
(imaging results) | No | Peri-abdominal
aorta, anterior to the right renal vein and inferior | 7×7×7 | Encapsulated tumor
with clear demarcation, adjacent to the | No | No |
| 22 | M | 65 | 28 September,
2011 | Hypertension, chest
pain and tightness | Yes | Peri-abdominal
aorta | 4×4×2 | Tumor surround the
abdominal aorta | No | No |
| 23 | F | 39 | 10 October,
2011 | Abdominal pain | No | Peri-abdominal
aorta, inferior to the left renal vein, medial to the left ovarian
vein | 5×5×3 | Tumor with unclear
demarcation | No | No |
| 24 | F | 65 | 7 December,
2011 | Abdominal pain | No | Peri-abdominal
aorta | 25×20×20 | Adhesion to the
pancreas, colon, and kidney, surrounding the renal vessels, rich
blood supply with engorged vessels | No | Left kidney and
spleen |
| 25 | M | 70 | 25 April, 2012 | Abdominal pain,
palpitation, unconsciousness | Yes | Between the
abdominal aorta and inferior vena cava | 5×5×5 | Tumor with
cleardemarcation, adjacent to the duodenum anteriorly and to the
pancreas posteriorly | No | No |
| 26 | F | 59 | 29 May, 2012 | Abdominal mass
(imaging results), hypertension | No | Peri-abdominal
aorta, inferior to the left renal vein, medial to the left ovarian
vein | 11×9×8 | Encapsulated tumor
compressing the left renal vein, and adjacent to the left ureter
and reproductive veins | No | No |
| 27 | F | 57 | 12 June, 2012 | Abdominal mass
(imaging results), hypertension | Yes | Anteromedial to the
left kidney | 8×6×6 | Encapsulated tumor
with clear demarcation | No | No |
| 28 | M | 56 | 6 August, 2012 | Abdominal bloating,
nausea | Yes | Peri-abdominal
aorta, posterior to the duodenum and pancreatic head | 6×5×5 | Tumor with clear
demarcation, compressing the abdominal aorta and inferior vena
cava | No | No |
| 29 | F | 51 | 23 August,
2012 | Hypertension | Yes | Between
peri-abdominal aorta and lower middle pole of kidney | 4×3×3 | Encapsulated tumor
with clear demarcation | No | No |
| 30 | M | 61 | 20 November,
2012 | Abdominal mass
(imaging results) | Yes | Anterior to
abdominal aorta and inferior vena cava, posterosuperior to the
horizontal part of duodenum | 6×5 | Encapsulated tumor
with clear demarcation | No | No |
| 31 | M | 60 | 23 September,
2013 | Abdominal mass
(palpation identified) | No | Left posterior to
the abdominal aorta and superior mesenteric artery | 7×5 | Encapsulated tumor
with clear demarcation, mobilizable | No | No |
| 32 | F | 44 | 7 November,
2013 | Abdominal mass
(imaging results) | No | Left anterior to
the abdominal aorta, posterior to the left vessel of kidney | 12×6 | Encapsulated tumor
with clear demarcation, adjacent to left vessel of kidney and
superior mesenteric vein | No | No |
| 33 | F | 58 | 5 February,
2015 | Abdominal pain | Yes | Anterior to the
left kidney, left lateral to the abdominal aorta | 6×5 | Encapsulated tumor
with clear demarcation | No | No |
| 34 | M | 69 | 21 December,
2015 | Abdominal mass
(imaging results) | No | Posterosuperior to
the body of pancreas | 3.5×3 | Encapsulated tumor
with clear demarcation | No | No |
Immunohistochemistry
Tissue sections (thickness, 4 µm) were obtained from
formalin-fixed and paraffin-embedded tissue blocks from the
retroperitoneal paraganglioma samples. Sections were washed in
xylene at room temperature to remove the paraffin, rehydrated with
serial dilutions of alcohol, followed by washing with PBS.
Endogenous peroxidase activity was blocked with 3%
H2O2 at room temperature for 10 min. Antigen
retrieval was performed by treating the slide in citrate buffer pH
6.0 (OriGene Technologies, Beijing, China) in a microwave for 15
min. Sections were incubated in 5% normal goat serum at room
temperature for 20 min to block non-specific protein-binding sites.
Sections were subsequently incubated with primary antibodies
against chromogranin A (Cg-A) (1:100; cat. no. ZM-0076), S-100
protein (1:100; cat. no. ZM-0224), Ki-67 (1:100; cat. no. ZM-0165),
vimentin (1:100; cat. no. ZM-0260), heat-shock protein (HSP)-90
(1:100; cat. no. TA326371) and insulin growth factor (IGF)-2
(1:100; cat. no. EIA-1076; all from OriGene Technologies) overnight
at 4°C. Subsequently, the primary antibody was washed off and
sections were incubated with biotin-conjugated goat anti-rabbit
secondary antibodies (1:400; cat. no. 111-065-144; Jackson
ImmunoResearch, Inc., West Grove, PA, USA) for 30 min at 37°C.
Sections were incubated with streptavidin-horseradish peroxidase
for 30 min at 37°C. Subsequently, 3,3-diaminobenzidine substrate
was applied to the sections and sections were counterstained with
hematoxylin. Sections in which primary antibodies were omitted were
used as a negative control. Sections were observed using a light
microscope at magnifications of ×50-400.
The immunohistochemical scoring for HSP-90 was
evaluated using a scoring system, according to the proportion of
stained cells and the intensity of the immunoreactivity as
previously described by Boltze et al (7). The proportion of stained cells was
scored as follows: 0, no staining; 1, ≤10% stained cells; 2, 11–50%
stained cells; 3, 51–80% stained cells; and 4, >80% stained
cells. The intensity of immunoreactivity was scored as follows: 0,
no staining; 1, weak staining; 2, moderate staining; and 3, strong
staining. The final immunoreactive score was determined by
multiplying the intensity score by the score for the proportion of
positively stained cells. The minimum score was 0 and the maximum
score was 12. The negative, moderate and positive immunoreactivity
of HSP-90 was defined by a final score of ≤2, 3–6 and >6,
respectively. The immunohistochemical scoring for IGF-2 was
evaluated according to the intensity of the immunoreactivity as
follows: 0, no staining; 1, weak staining; 2, moderate staining;
and 3, strong staining. For Ki-67, all immunostained nuclei of
tumor cells were counted as positive, regardless of staining
intensity. The proportion of Ki-67-positive cells was determined by
the number of Ki-67-positive cells relative to the total number of
tumor cells. For Cg-A, S-100 and vimentin, the immunostained cells
were counted as positive, regardless of staining intensity.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). The rank sum
test was used to evaluate ranked data of imaging evaluation
results. The survival time was calculated as the period between the
day of surgery and the disease-associated mortality or last known
follow-up. The Kaplan-Meier estimator method was used to calculate
the survival rate. Log-rank tests were used to compare differences
between the survival rates. P<0.05 (two-tailed t-test) indicated
a statistically significant difference.
Results
Clinical characteristics
Table I summarizes the
clinical characteristics of 34 patients with retroperitoneal
paragangliomas who underwent resection at the First Affiliated
Hospital, Wenzhou Medical University. The median age of these
patients was 55 years (range, 20–78 years), and 17 patients were
male and 17 patients were female. The most common type of
presenting symptom was abdominal mass (50%), followed by
hypertension (32%) and abdominal pain (24%). Additional symptoms,
including palpitation, umbilical discomfort, emaciation, chest pain
and nausea, accounted for ~25% of all symptoms. None of the
patients had first-degree relatives or family members with
previously or subsequently developed paragangliomas.
Radiological results
As presented in Fig.
1, all the patients underwent CT scans, which revealed
retroperitoneal soft tissue masses, including homogeneous masses in
12 cases and inhomogeneous masses with cystic changes in 22 cases.
Of the 34 patients, 20 patients exhibited strong enhancement with
an increase in the maximum CT value >30 Hounsfield units (HU). A
total of 10 cases exhibited mild to moderate enhancement which
primarily occurred in the arterial phase (Fig. 1B, C, E, F and H). Thick and tortuous
arteries and veins were observed in 4 cases, inside or at the
periphery of the tumor (Fig. 1E, F,
H-J). The structures of the tumors and surrounding tissues were
clearly observed on preoperative CT scans of all patients.
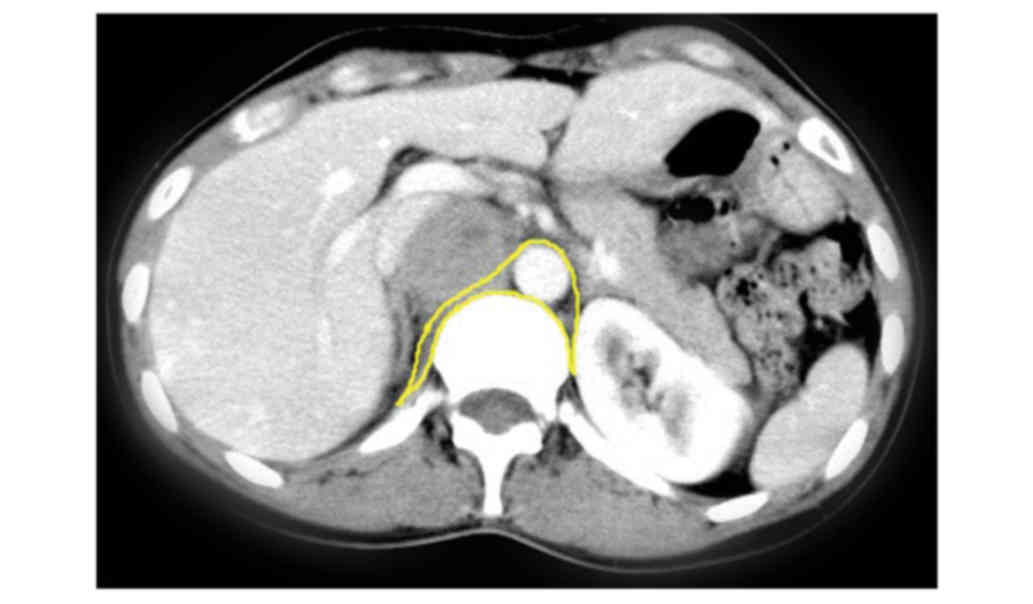
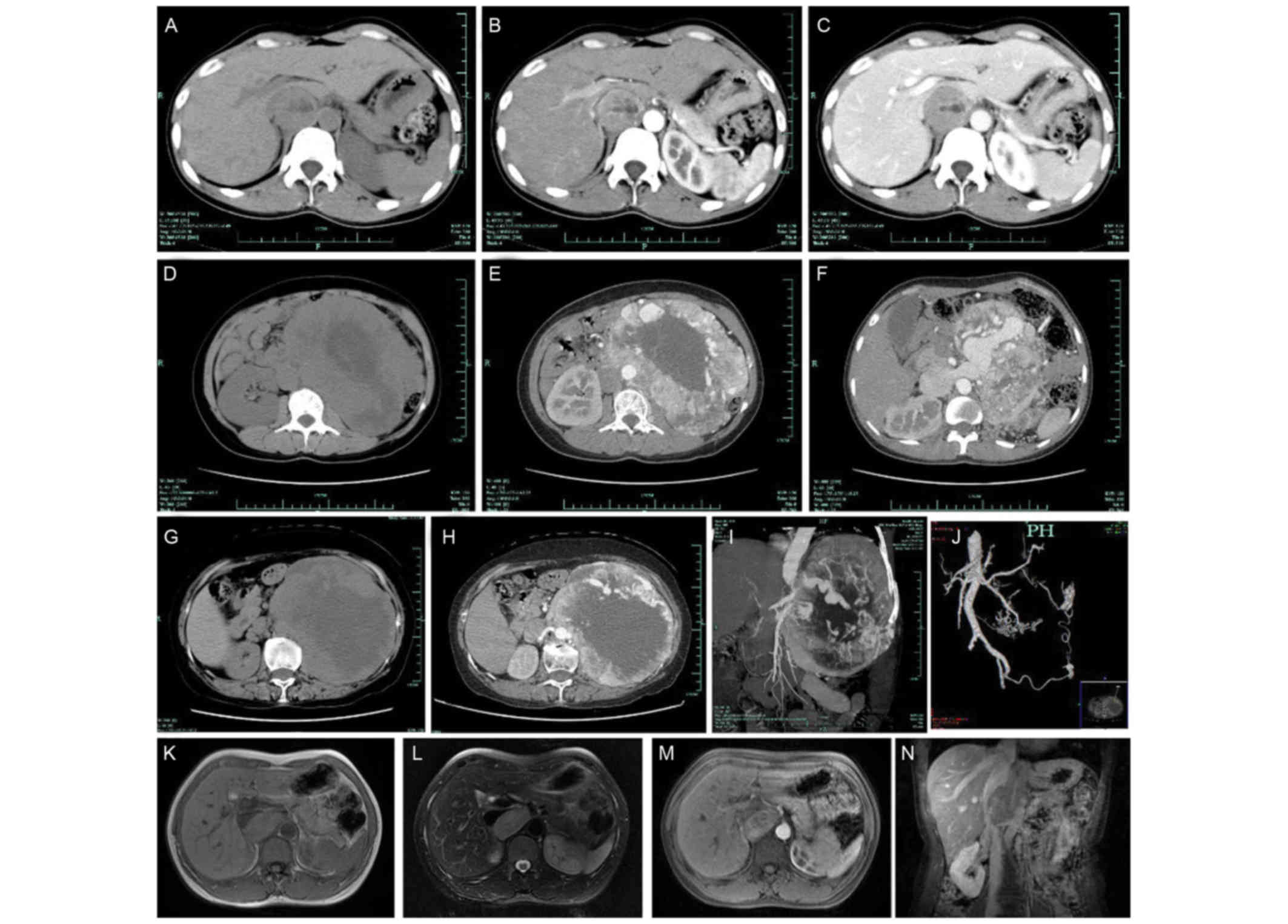 | Figure 1.Representative radiological images of
retroperitoneal paragangliomas. (A) CT image of patient no. 11; (B)
CT image of patient no. 11; and (C) CT image of patient no. 11
which demonstrate a round demarcated soft tissue mass with cystic
degeneration. The inferior vena cava was depressed by the mass and
migrated laterally. The parenchyma of the mass exhibited
enhancement, primarily in the arterial phase. (D) CT image of
patient no. 10; (E) CT image of patient no. 10; and (F) CT image of
patient no. 10, revealing a large oval retroperitoneal mass with
cystic degeneration. The parenchyma of the mass exhibited
enhancement, and thick tortuous arteries and veins were observed
inside the tumor. The juncture point where the tumor vein joined
the inferior vena cava was observed. (G) CT image of patient no.
24; (H) CT image of patient no. 24; (I) CT image of patient no. 24;
and (J) CT image of patient no. 24, demonstrating a high oval
retroperitoneal cystic mass on the left. The parenchyma of the mass
exhibited enhancement, and thick tortuous arteries and veins were
observed inside the tumor. The tumor arteries originated from the
spleen artery, left renal artery, abdominal aortic artery and left
internal iliac artery. (K) MRI image of patient no. 20; (L) MRI
image of patient no. 20; (M) MRI image of patient no. 20; and (N)
MRI image of patient no. 20, demonstrating an oval soft tissue mass
posterior to the inferior vena cava. The mass exhibited equal
intensities on T1WI and T2WI, and cystic degeneration was observed
inside the mass. The mass exhibited an enhancement. The vena cava
arched and became thin due to the tumor compression. CT, computed
tomography; MRI, magnetic resonance imaging; T1WI, T1-weighted
image; T2WI, T2-weighted image. |
Masses were correctly localized to be
retroperitoneal in 32 cases and were incorrectly localized to be
intra-abdominal in 2 cases (Table
II). All tumors were diagnosed using contrast-enhanced CT.
Between November 1999 and December 2009, 3 cases were correctly
diagnosed as retroperitoneal paragangliomas, 9 cases were diagnosed
as retroperitoneal tumors (without diagnosis of a specific tumor)
and 8 cases were misdiagnosed, as fibrosarcoma (n=2), stromal tumor
(n=2), lymph node metastasis (n=1), leiomyoma (n=1), vascular tumor
(n=1) and neurofibroma (n=1). Between January 2010 and December
2015, 8 cases were correctly diagnosed as retroperitoneal
paragangliomas, 4 cases were diagnosed as retroperitoneal tumors
(without diagnosis of a specific tumor) and 2 cases were
misdiagnosed, as teratoma (n=1) and small intestinal lymphoma
(n=1). The CT diagnostic accuracy between2000 and 2015 was
significantly increased compared with that between 1999 and 2009
(P=0.014, z=−2.454).
 | Table II.Image diagnosis by contrast-enhanced
CT between 1999 and 2009, and between 2010 and 2015. |
Table II.
Image diagnosis by contrast-enhanced
CT between 1999 and 2009, and between 2010 and 2015.
|
| Image diagnosis by
contrast-enhanced CT |
|---|
|
|
|
|---|
| Period | Correct diagnosis,
no. of cases | Unable to judge,
no. of cases | Misdiagnosis, no.
of cases (total no. of cases) | (Refs.) |
|---|
| 1999–2009 | 3 | 9 | 2 fibrosarcoma, 2
stromal tumors, 1 lymph node metastasis, 1 leiomyoma, 1 vascular
tumor and 1 neurofibroma | (8) |
| 2010–2015 | 8 | 4 | 1 teratoma and 1
small intestinal lymphoma | (2) |
A total of 7 patients underwent magnetic resonance
imaging (MRI). The parenchyma of the tumors revealed equal
intensities on T1-weighted imaging (T1WI) and T2-weighted imaging
(T2WI) (Fig. 1K and L). In all 7
cases, cystic degeneration and necrosis with short T1 and long T2
signals were observed inside the tumor. In 3 cases, an enhanced MRI
identified an enhancement in the tumor parenchyma, especially in
the arterial phase (Fig. 1M and N).
For all 7 cases, the structure of the tumors and the surrounding
tissues were clearly observed on preoperative MRI scans. Masses
were correctly localized to be retroperitoneal in all 7 cases;
however, only 2 masses were correctly diagnosed as retroperitoneal
paragangliomas. For the other 5 cases, the tumors were not
specifically diagnosed.
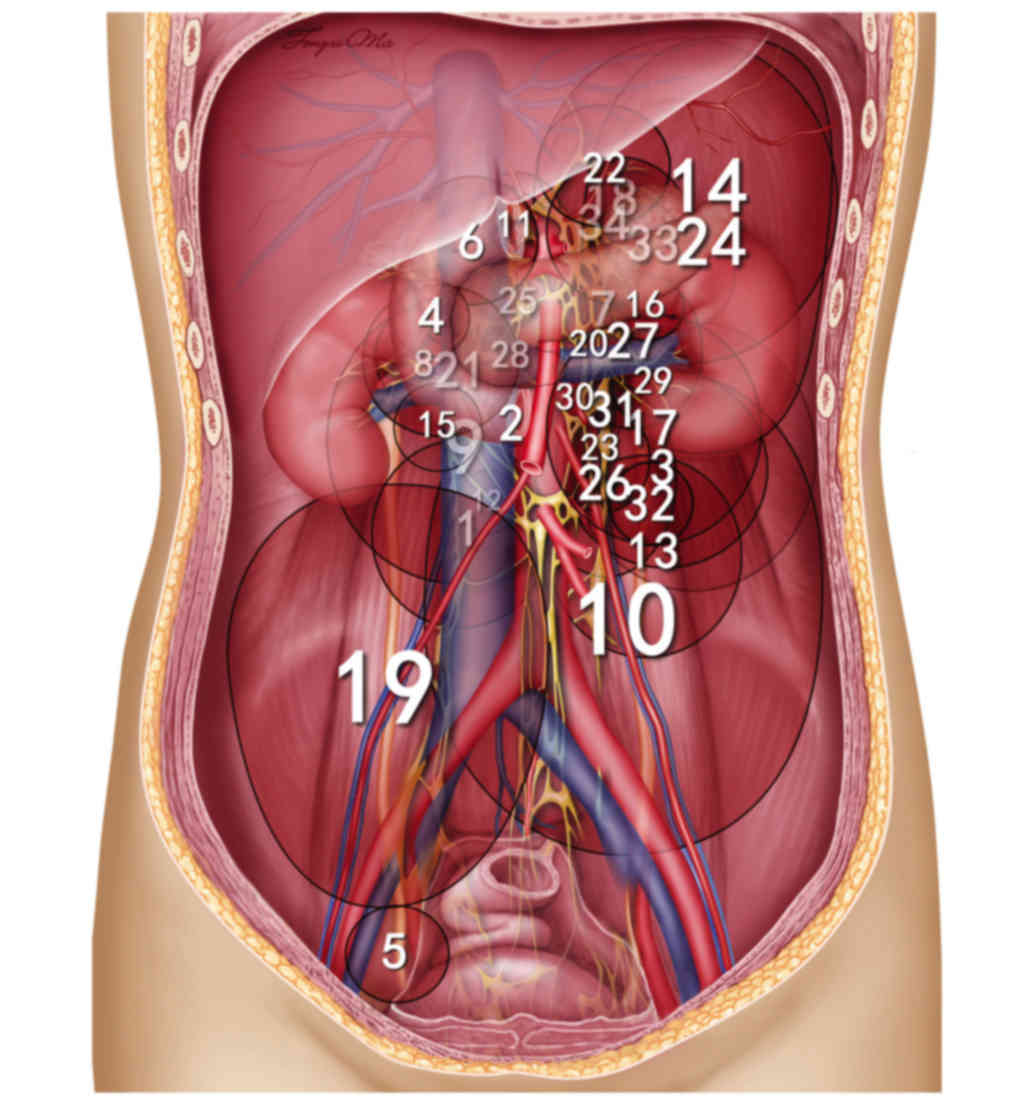
Tumor size and location
The mean maximal diameter of the 34 tumors was 8.7
cm (range, 3–25 cm). Fig. 2
summarizes the location of retroperitoneal paragangliomas in all 34
patients. A total of 33 retroperitoneal paragangliomas were located
in association with the aorta and inferior vena cava, surrounding
the adjacent renal vessels. The aforementioned tumors exhibited
increased distribution on the left side (21 on the left side vs. 12
on the right side) and the tumor was located on the bottom of the
pelvic cavity, lateral to the neck of the urinary bladder, in only
1 case. In the horizontal plane, retroperitoneal paragangliomas
were located on either side of the aorta, behind the inferior vena
cava, duodenum and pancreas (Fig.
3).
Intraoperative results
All tumors exhibited surfaces with a rich blood
supply. Of the 34 tumors, 21 (62%) tumors possessed an intact
capsulate, with clear demarcation, and were completely resected en
bloc without the removal of adjacent tissues. For the remaining 13
tumors that adhered or were close to adjacent tissues, adjacent
organ resection was required in 7 (21%) of 34 cases. Patient no. 10
exhibited a large tumor (maximal diameter, 23 cm) that adhered to
the abdominal aorta and inferior vena cava (Fig. 1D-F). A large amount of blood (~7,500
ml) was lost during the resection of the tumor for the
aforementioned patient, which required 19 units of packed red blood
cells, 1,500 ml plasma and 2,800 ml autologous blood to be
transfused. Patient no. 7, who had undergone resection of
retroperitoneal paraganglioma and splenectomy elsewhere, was
admitted to the First Affiliated Hospital of Wenzhou Medical
University exhibiting tumor recurrence and subsequently underwent
secondary resection of the tumor after 10 months; however,
mesenteric metastasis of the tumor was identified during surgery.
Furthermore, patient no. 17, diagnosed with suspected malignant
gastric cancer preoperatively, underwent a radical gastrectomy and
a D3 lymph node dissection. Postoperative pathological examinations
of this patient demonstrated that the enlarged lymph node,
preoperatively diagnosed as lymph node metastasis of gastric
cancer, was paraganglioma and that the gastric tumor was benign.
Patient no. 15 exhibited a left adrenal pheochromocytoma and a
paraganglioma adjacent to the inferior vena cava, and the two
tumors were completely removed. In addition, patient no. 24
possessed a large tumor (25×20 cm; Fig.
1G-J) and intra-abdominal bleeding occurred following the
removal of the tumor and left kidney. The aforementioned patient
underwent an exploratory laparotomy for hemostasis and
splenectomy.
Functional status
Functional tumors occurred in 20/34 patients (59%)
and of these 20 patients, 12 patients (60%) exhibited preoperative
hypertension. The remaining 8 patients (40%), with no preoperative
hypertension, exhibited a fluctuation in blood pressure during
dissection of the tumor intraoperatively, increasing to 240/150
mmHg (during the dissection of the tumor) and subsequently
decreasing to 60/40 mmHg (following the removal of the tumor).
Cardiac arrest occurred in a number of patients following the
removal of the tumor. For patient nos. 22 and 25, who were admitted
to the hospital as emergency cases due to acute coronary symptoms
caused by a sudden increase in blood pressure, no stenosis of the
coronary artery was identified using emergency coronary angiography
and retroperitoneal paraganglioma was revealed using abdominal CT.
Patients with functional tumors exhibited an increased likelihood
to present with symptoms including hypertension and palpitation,
compared with patients with non-functional tumors who exhibited an
increased likelihood to present with non-specific symptoms
including an abdominal mass. Patients with non-specific symptoms,
including patients with non-functional tumors and those without
preoperative hypertension, exhibited tumors of a markedly increased
size (average diameter, 9.9 cm) compared with patients experiencing
specific symptoms (average diameter, 6.3 cm; P=0.041).
Pathological and immunohistochemical
results
Following removal, it was identified that the
majority of tumors exhibited a capsule that was soft and
gray-yellow or gray-red. Hemorrhage, cystic degeneration and
necrosis were observed inside the tumors. Under the microscope,
tumor cells were oval or polygonal in shape and arranged in nests
or trabeculae, containing rich cytoplasm with eosinophilic fine
granules. Large nuclei were strongly stained and exhibited round or
oval nucleoli. Tumor cells with deformed, large or multiple nuclei
were observed.
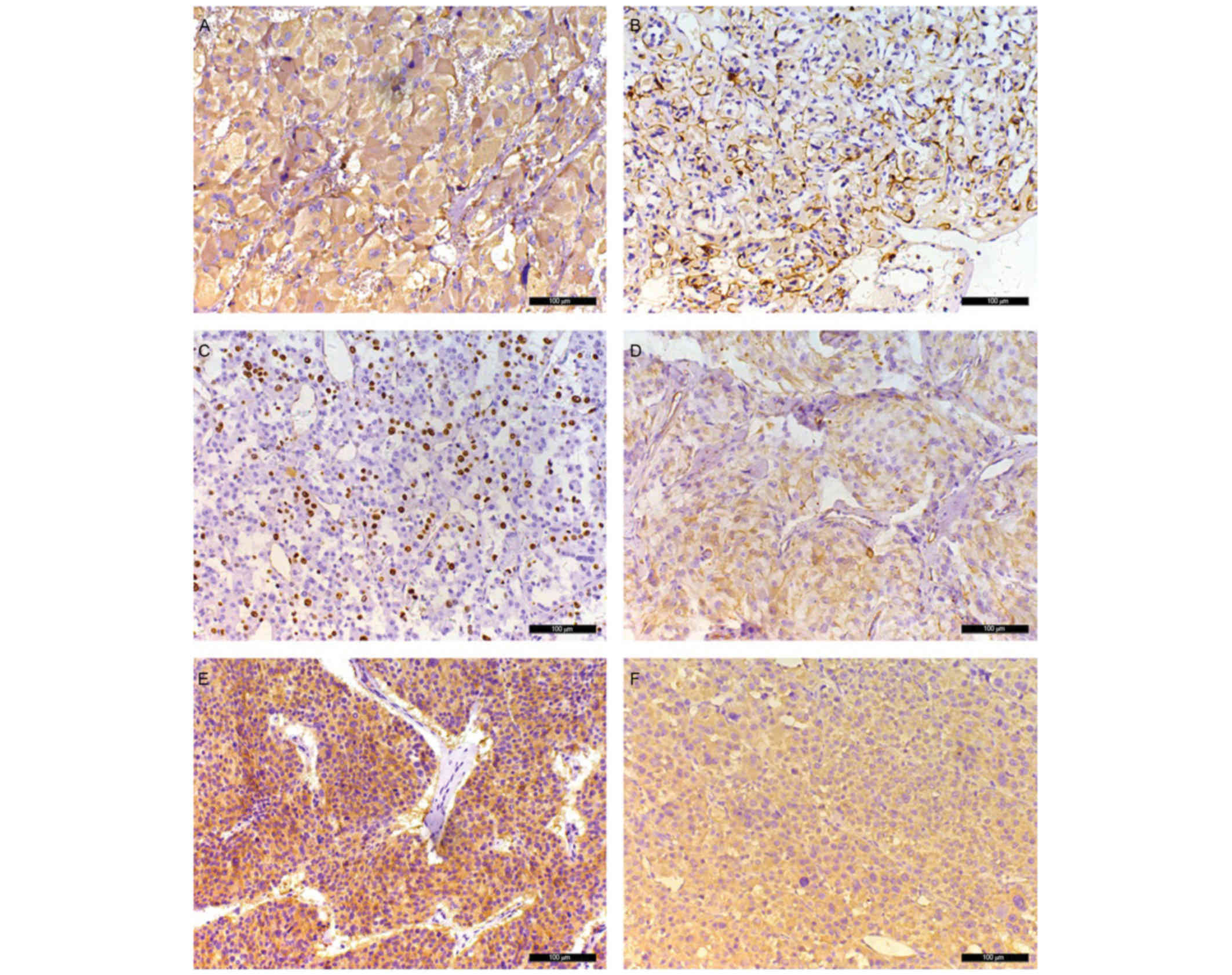
The immunochemical results are presented in Table III and Fig. 4. Of the 34 patients, immunostaining
for Cg-A, S-100, Ki-67, vimentin, HSP-90and IGF-2 was performed in
31 patient samples (Table III).
Negative immunostaining for Cg-A was identified in only 1 patient
(patient no. 7). Immunoreactivity for S-100 was observed in 27/31
(87%) patients with paraganglioma. Only 1 patient (patient no. 7)
exhibited an increased Ki-67 count (15–20%). Immunoreactivity for
vimentin was identified in 28/31 (90%) patients with paraganglioma.
Negative, moderate and positive immunoreactivity of HSP-90 was
observed in 2/31 (6.5%), 15/31 (48.5%) and 14/31 (45%) patients
with paraganglioma, respectively. Positive immunoreactivity for
IGF-2 was observed in 17/31 (55%) patients with paraganglioma,
including 9 patients with weak staining, 6 patients with moderate
staining and 2 patients with strong staining.
 | Table III.Pathological and immunohistochemical
results of 34 patients with retroperitoneal paraganglioma. |
Table III.
Pathological and immunohistochemical
results of 34 patients with retroperitoneal paraganglioma.
| Patient no. | Cg-A | S-100 | Ki67,% | Vim | HSP-90 | IGF-2 |
|---|
| 1 | ND | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND | ND |
| 3 | ND | ND | ND | ND | ND | ND |
| 4 | + | + | <1 | + | 3 | 0 |
| 5 | + | + | <1 | + | 4 | 0 |
| 6 | + | + | <1 | + | 3 | 0 |
| 7 | − | − | 15 | − | 12 | 3 |
|
| − | − | 20 | − | 12 | 3 |
| 8 | + | + | <1 | + | 6 | 0 |
| 9 | + | − | <1 | + | 3 | 0 |
| 10 | + | + | <1 | + | 3 | 0 |
| 11 | + | + | <1 | + | 8 | 2 |
| 12 | + | + | <1 | + | 8 | 0 |
| 13 | + | + | <1 | + | 4 | 0 |
| 14 | + | + | <1 | + | 3 | 0 |
| 15 | + | + | <1 | + | 2 | 2 |
|
| + | − | <1 | + | 2 | 2 |
| 16 | + | − | <1 | − | 4 | 0 |
| 17 | + | + | <1 | + | 8 | 1 |
| 18 | + | + | <1 | + | 4 | 0 |
| 19 | + | − | <1 | + | 8 | 0 |
| 20 | + | + | <1 | + | 9 | 2 |
| 21 | + | + | <1 | + | 8 | 1 |
| 22 | + | + | 1 | + | 8 | 1 |
| 23 | + | + | 2 | + | 6 | 1 |
| 24 | + | + | 8 | + | 6 | 0 |
| 25 | + | + | 2 | + | 9 | 3 |
| 26 | + | + | 3 | + | 8 | 1 |
| 27 | ± | + | <1 | − | 8 | 0 |
| 28 | + | + | 4 | + | 4 | 1 |
| 29 | + | − | <1 | + | 6 | 2 |
| 30 | + | + | 4 | + | 6 | 0 |
| 31 | + | + | 1 | + | 9 | 1 |
| 32 | + | + | 2 | + | 5 | 1 |
| 33 | + | + | 1 | + | 11 | 2 |
| 34 | + | + | 4 | + | 8 | 1 |
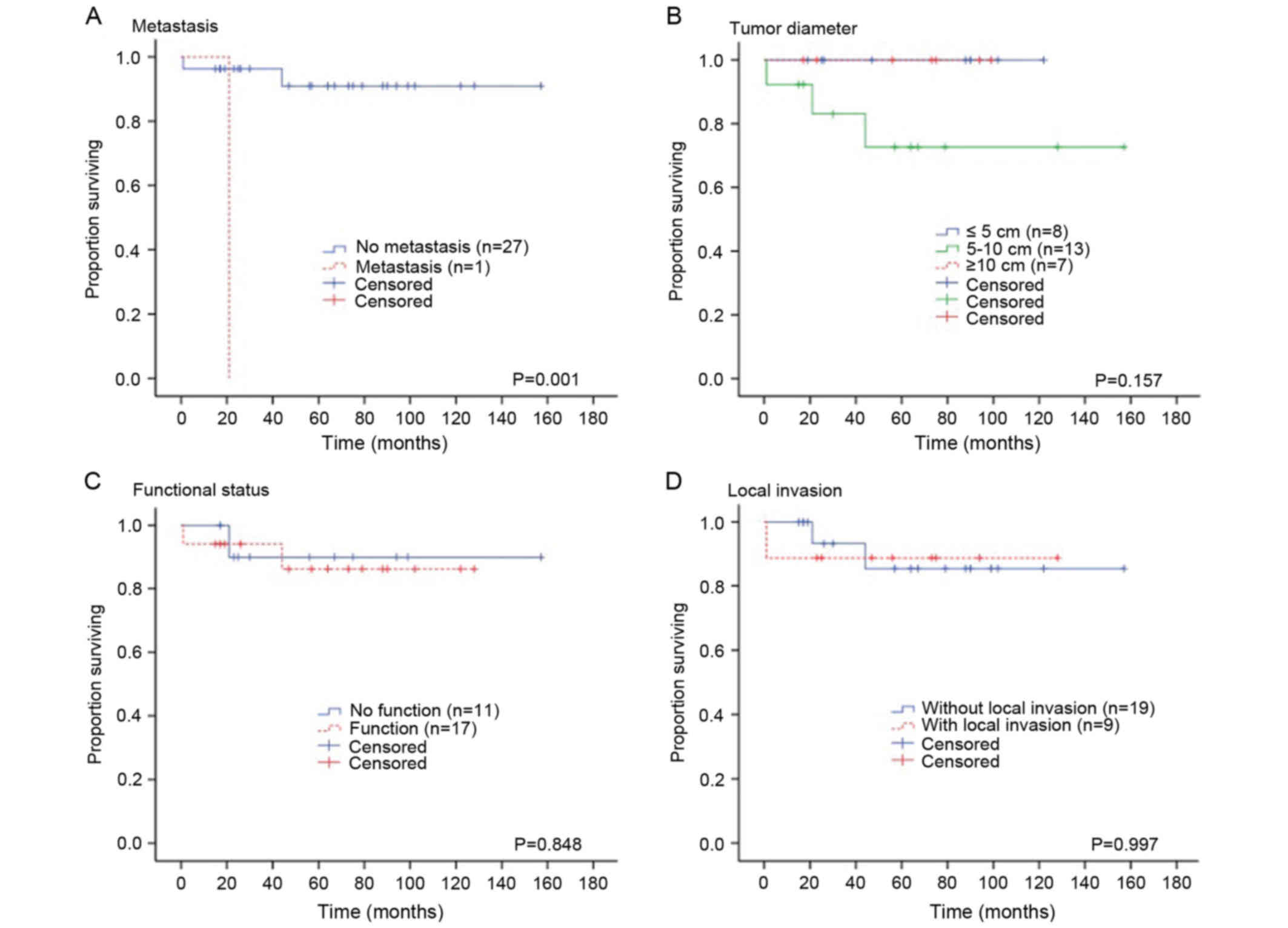
Survival and recurrence
The Kaplan-Meier estimator analysis was used to
evaluate the 5-year survival rate as a group and was stratified by
tumor size (≤5 vs. 5–10 vs. ≥10 cm), tumor functional status
(functional vs. non-functional), local invasion (present vs.
absent) and distant metastasis (present vs. absent). The overall
5-year survival rate was 91%. A significant association was
identified between the survival rate and the tumor malignancy (the
presence of distant metastasis) (P=0.001; Fig. 5A). There was no significant
association identified between the survival rate and tumor size
(P=0.151; Fig. 5B), tumor functional
status (P=0.812; Fig. 5C) and local
invasion (P=0.814; Fig. 5D).
The median follow-up time was 67 months (range,
6–188 months). In addition, patient no. 7 exhibited tumor
recurrence in the abdominal cavity with mesenteric metastasis 10
months after primary surgery, and exhibited lung and liver
metastasis following secondary surgery. Only 1 patient succumbed
due to surgery complications and thus was excluded from recurrence
rate analysis. The recurrence rate for patients with
retroperitoneal paraganglioma was 2.9% (1/34 patients).
Discussion
Retroperitoneal tumors are challenging for surgeons
to treat due to the inaccessible location of the tumor, uncertain
diagnosis and limited effective treatment. The retroperitoneum may
host a variety of pathologies, including a number of rare benign
tumors and malignant neoplasms that may be eitherprimary or
metastatic lesions. Paraganglioma is a relatively rare
retroperitoneal tumor compared with the majority of common
retroperitoneal tumors, including sarcomas, lymphoproliferative
tumors, epithelial tumors and neurogenic tumors (8,9). If
functional retroperitoneal paragangliomas is misdiagnosed and
improper surgery is performed, hypertensive crisis may happen and
result in serious consequences. Therefore, it is important to
improve the diagnosis and treatment of retroperitoneal
paraganglioma.
The present study, to the best of our knowledge,
included the largest number of cases with retroperitoneal
paragangliomas (n=34) with complete clinical data in the current
literature. None of patients included exhibited a family history of
paraganglioma, which is distinct from previous studies that have
determined the association of paraganglioma with a family history
(10,11). This distinction may be due to the
inclusion of different ethnicities between studies. Although a
previous study identified that retroperitoneal paragangliomas
preferentially occurred in males (2),
the present study revealed no predilection between sexes, which is
similar to the study of Cunningham et al (1). In the present study, the tumor size
ranged between 3 and 25 cm. The decreased tumor sizes were observed
in the functionally active retroperitoneal tumors, which may be a
result of early detection of tumors due to exhibition of endocrine
symptomatology. All the tumors were located in the para-aortic
plexus and primarily concentrated in the mesenteric artery region
(Figs. 2 and 3). The tumors occurred on the left side at
an increased frequency, which may be associated with the left slant
of the abdominal aorta. In 4 cases, the tumor was identified to be
posterior to the inferior vena cava, which may be due to the tumor
originating from the para-aortic plexus. Tumors posterior to the
inferior vena cava possesses the specific characteristics of
retroperitoneal paragangliomas. Furthermore, retroperitoneal
paraganglioma tumor sites are distant from the intervertebral
foramen, which is distinct from other types of retroperitoneal
neurogenic tumor.
For those patients exhibiting functional
paragangliomas, the most common type of presenting symptom was the
classic triad of symptoms associated with catecholamine-secreting
paragangliomas: Episodic headache, diaphoresis and tachycardia.
Episodic hypertension has been identified as a characteristic
feature of catecholamine-secreting paragangliomas and is used for
the differential diagnosis of paragangliomas (6); however, in clinical practice, ~50% of
these patients exhibit true paroxysmal hypertension. The present
study identified that functional tumors occurred in 20/34 (59%)
patients, which is consistent with results of a previous study
(1), and only 12/20 (60%) patients
exhibited hypertension preoperatively. The remaining 8 (40%)
patients with no preoperative hypertension exhibited a fluctuation
in blood pressure during dissection of the tumor intraoperatively.
In addition, for patients with non-functional paragangliomas, the
most common type of presenting symptom was abdominal mass (46%).
Non-functional retroperitoneal paraganglioma, which lacks symptoms
at the early stage, is identified to be markedly larger compared
with the functional tumor. Furthermore, a number of previous
studies have revealed that patients with paraganglioma additionally
exhibit a variety of uncommon non-specific symptoms, including
palpitation, panic attacks and dyspnea (6,12). In the
present study, uncommon symptoms were identified to include
palpitation, umbilical discomfort, emaciation, chest pain and
nausea, and accounted for ~25% of all symptoms.
Radiological techniques, including CT and MRI, are
useful for identifying and locating retroperitoneal paragangliomas.
The present study identified that the imaging characteristics of
retroperitoneal paragangliomas included soft-tissue masses in the
sympathetic chains associated with the abdominal aorta, cystic
degeneration and necrosis inside the masses, and a marked
peripheral enhancement in the arterial phase, which was consistent
with previous studies (1,13–15).
However, these imaging characteristics are not specific for the
diagnosis of retroperitoneal paragangliomas since other
retroperitoneal tumors, particularly neurofibromas, neuromas and
sarcomas, exhibit similar image characteristics. Therefore, the
limited number of unique imaging characteristics of paragangliomas
may explain the decreased rate of correct diagnosis. In our
previous study, conducted between 1999 and 2009, the preoperative
CT misdiagnosis rate was 89% (16).
In addition, the low diagnosis rate of retroperitoneal
paragangliomas using CT and MRI scans may be associated with the
ability of these tumors to invade adjacent tissues, including the
intestine and pancreas, mimicking intestinal or pancreatic tumors
(17–19). In the present study, the tumors were
misdiagnosed as intestinal stromal tumors in 2 cases due to the
marked association of the tumor with the intestine on CT images.
However, intraoperative results revealed that that the tumors did
not adhere to the intestine. On the basis of our previous study
(1999–2009) (16), image
characteristics of retroperitoneal paragangliomas were identified
and summarized, including: Thick tortuous arteries and veins inside
the tumor; tumor location close to the renal arteries and veins
surrounding the abdominal aorta and inferior vena cava; and
location of the tumor behind the inferior vena cava, but not in the
region of the intervertebral foramen. Using these novel features,
the misdiagnosis rate using CT scans between 2000 and 2015 markedly
decreased (14%). Functional imaging techniques, including
123I-meta-iodobenzylguanidine (MIBG) scan and
somatostatin receptor scintigraphy, in combination with CT or MRI
scans may be used to improve the sensitivity and specificity of
diagnosis (20). Although MIBG and
fluorine-8-L-dihydroxyphenylalanine (18F-DOPA) positron
emission tomography (PET) is specific for diagnosis of
paraganglioma, in China, patients refused to undergo these two
techniques due to concerns about the damage of nuclear radiation to
the body. Furthermore, the problem of PET/CT is the limited
availability and high cost, which is currently not reimbursable by
medical insurance for this use. Thus, MIBG and 18F-DOPA
PET remain of limited use to diagnose retroperitoneal
paragangliomas in China. Therefore, for retroperitoneal
paragangliomas with the aforementioned imaging characteristics,
clinical symptoms and measurement of catecholamines may be
considered to confirm the diagnosis. Ultrasound- or CT-guided
percutaneous biopsy of paragangliomas may be used to validate the
diagnosis. However, functional paragangliomas require exclusion
prior to biopsy, since tachycardia and hypertension crisis may
occur due to excessive secretion of catecholamine in this type of
tumor (21,22). In addition, all patients in the
present study exhibited a single paraganglioma without multifocal
disease or family history, although genetic analysis was not
performed to exclude the involvement of a syndrome, including von
Hippel-Lindau disease.
Paragangliomas are primarily composed of chief cells
and sustentacular cells. Typically, paragangliomas are diagnosed by
identifying neuroendocrine granules with silver staining and
electron microscopy. In recent years, immunohistochemical methods
have been used in the diagnosis of paragangliomas. Neuron-specific
enolase and Cg-A are sensitive markers for chief cells (Fig. 4A), and combined use of these two
markers may identify chief cells in all cases of paraganglioma
(23). The S-100 protein is typically
used for labeling sustentacular cells (Fig. 4B) and it has been identified that
expression levels of S-100 protein are useful for excluding the
malignancy of paragangliomas (24).
However, immunohistochemical results remain unreliable for
diagnosis of malignant paragangliomas. Previous studies have
identified that decreased Ki-67 expression is associated with
benign tumors (25), and that
expression levels of Ki-67 and human telomerase reverse
transcriptase may be used to distinguish between malignant and
benign paragangliomas (26). Boltze
et al (7) revealed that the
expression of HSP-90 was upregulated in malignant pheochromocytoma.
In addition, Feng et al (27)
identified that vimentin was selectively expressed in malignant
paragangliomas. Furthermore, IGF-2 may be used as a marker to
distinguish between malignant and benign paragangliomas (28). Consistent with previous studies
(1,2,23–26), the present study identified that a
patient with malignant paraganglioma (patient no. 7) exhibited an
increased expression level of Ki-67 (20%), HSP-90 (12 points) and
IGF-2 (3 points), and was negative for Cg-A, S-100 and vimentin.
Although, as there was only 1 malignant case in the present study,
statistical analysis was not possible; however, the present study
may enable improved distinction between malignant and benign
retroperitoneal paragangliomas. In addition, the genetic testing
for hereditary syndromes is used to predict malignancy and
recurrence. Patients with identified germline mutations in subunit
B of succinate dehydrogenase exhibit an increased likelihood of
experiencing malignancy, multiple pheochromocytomas and recurrences
(11).
A number of previous studies have identified that
malignant paragangliomas have a tendency to exhibit necrosis inside
the tumor and decreased endocrine granules in the cytoplasm
(29,30). A Pheochromocytoma of the Adrenal Gland
Scaled Score (PASS) has been used to distinguish between malignant
(PASS ≥4) and benign (PASS <4) tumors (30). However, additional previous studies
have revealed that the PASS is not a reliable method for evaluating
the malignancy of pheochromocytomas (31,32). The
presence of distant metastasis is used to diagnose malignant
paragangliomas. It has been previously identified that the
malignancy rate of paragangliomas varies between 0 (33) and 50% (4,34). In the
present study, only 1 case (patient no. 7) exhibited distant
metastasis. However, the low incidence of malignancy (2.9%, 1/34
patients) may not be accurate, since patients were only followed up
for an average of 67 months. Distant metastasis occurs at between 7
and 9 years after the initial discovery of a paraganglioma
(3,4)
and local recurrence occurs ~13 years following surgical removal of
the tumor (35).
To the best of our knowledge, surgical resection is
the only option available to patients with paraganglioma and it is
associated with improved survival rate, even in patients with
distant metastasis (1,36). Sclafani et al (4) demonstrated that the 5-year survival rate
of patients with extra-adrenal retroperitoneal paragangliomas was
19% for patients without resection of the tumor and 75% for
patients following the removal of the tumors. In the present study,
the 5-year survival rate was identified to be 91%. Consistent with
a previous study by Cunningham et al (1), a marked association between the survival
rate and the presence of distant metastasis was identified in the
present study. However, no marked association was identified
between the survival rate and tumor size, tumor functional status
and local invasion, which may be due to the small sample size of
the present study and a limited follow-up period. Additional
studies with larger sample sizes and long-term follow-ups are
required to validate the results of the present study.
Removing retroperitoneal paragangliomas remains
difficult due to the rich blood supply and the proximity of tumors
to major abdominal vessels. In addition, hypertensive crisis and
hypotension typically occur during intraoperative resection of the
tumor. Non-selective drugs, including α- and β-adrenoceptor
antagonists and calcium channel blockers, and/or drugs that inhibit
catecholamine synthesis may be administered preoperatively to
prevent the release of catecholamines (37). Preoperative imaging techniques,
including CT, particularly those which allow the 3D imaging of the
tumor and blood vessels, are important for evaluating the tumor
size, blood supply, invasion to adjacent vessels and tissues, and
for planning surgical procedures to decrease surgical risks.
Preoperative identification of a large tumor with a rich blood
supply or adhesion to blood vessels or adjacent tissues may require
surgical resection and reconstruction of major vessels and tissues.
In the present study, patient no. 10 exhibited large tumors, and
the abdominal aorta and inferior vena cava were damaged during
resection of the tumor. In addition, patient no. 10 lost a large
amount (~7,500 ml) of blood, and 19 units packed red blood cells,
1,500 ml plasma and 2,800 ml autologous blood were transfused.
Furthermore, Lebuffe et al (38) revealed that ~62% of patients
experienced transient hypertension during surgery, including 26%
with systolic blood pressure >200 mmHg for >10 min (38). Similarly, in the present study, 17
patients (50%) exhibited transient hypertension and 9 patients
(26.5%) exhibited systolic blood pressure >200 mmHg. Following
tumor removal, hypotension occurred in 6 (17.6%) patients who
required administration of noradrenaline to maintain blood
pressure. The blood pressure of patient no.1 increased to 230/110
mmHg during the dissection of the tumor, followed by a sudden
decrease in blood pressure and cardiac arrest occurred following
the removal of the tumor. The heart rate of the aforementioned
patient was restored following cardiopulmonary resuscitation for 90
min. All the other tumors in the present study were successfully
removed, which suggests that retroperitoneal paragangliomas, even
if of a large size, may be safely removed if preoperative
preparations are thoroughly conducted.
Retroperitoneal paraganglioma is a rare tumor that
is primarily located close to renal arteries surrounding the
abdominal aorta and inferior vena cava. Large thick blood vessels
inside the tumor represent a characteristic feature of CT imaging.
The accuracy of the preoperative diagnosis may be markedly improved
by attaining the location and functional characteristics of the
tumor, in combination with CT results. Surgical resection of the
tumor requires adequate preoperative preparations and evaluation of
surgical risk. In addition, the combined use of immunohistochemical
markers is useful for the determination of tumor malignancy. The
patient survival rate is associated with tumor metastasis. Lifelong
follow-ups may be performed in all patients with retroperitoneal
paragangliomas.
References
|
1
|
Cunningham SC, Suh HS, Winter JM,
Montgomery E, Schulick RD, Cameron JL and Yeo CJ: Retroperitoneal
paraganglioma: Single-institution experience and review of the
literature. J Gastrointest Surg. 10:1156–1163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lack EE, Cubilla AL, Woodruff JM and
Lieberman PH: Extra-adrenal paragangliomas of the retroperitoneum:
A clinicopathologic study of 12 tumors. Am J Surg Pathol.
4:109–120. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noda T, Nagano H, Miyamoto A, Wada H,
Murakami M, Kobayashi S, Marubashi S, Takeda Y, Dono K, Umeshita K,
et al: Successful outcome after resection of liver metastasis
arising from an extraadrenal retroperitoneal paraganglioma that
appeared 9 years after surgical excision of the primary lesion. Int
J Clin Oncol. 14:473–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sclafani LM, Woodruff JM and Brennan MF:
Extraadrenal retroperitoneal paragangliomas: Natural history and
response to treatment. Surgery. 108:1124–1130. 1990.PubMed/NCBI
|
|
5
|
Melicow MM: One hundred cases of
pheochromocytoma (107 tumors) at the columbia-presbyterian medical
center, 1926–1976: A clinicopathological analysis. Cancer.
40:1987–2004. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joynt KE, Moslehi JJ and Baughman KL:
Paragangliomas: Etiology, presentation and management. Cardiol Rev.
17:159–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boltze C, Mundschenk J, Unger N,
Schneider-Stock R, Peters B, Mawrin C, Hoang-Vu C, Roessner A and
Lehnert H: Expression profile of the telomeric complex
discriminates between benign and malignant pheochromocytoma. J Clin
Endocrinol Metab. 88:4280–4286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strauss DC, Hayes AJ and Thomas JM:
Retroperitoneal tumours: Review of management. Ann R Coll Surg
Engl. 93:275–280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Roggen JF and Hogendoorn PC: Soft
tissue tumours of the retroperitoneum. Sarcoma. 4:17–26. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Riordain DS, Young WF Jr, Grant CS,
Carney JA and van Heerden JA: Clinical spectrum and outcome of
functional extraadrenal paraganglioma. World J Surg. 20:916–922.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barski D: Management and follow up of
extra-adrenal phaeochromocytoma. Cent European J Urol. 67:156–161.
2014.PubMed/NCBI
|
|
12
|
Manger WM: The vagaries of
pheochromocytomas. Am J Hypertens. 18:1266–1270. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baez JC, Jagannathan JP, Krajewski K,
O'Regan K, Zukotynski K, Kulke M and Ramaiya NH: Pheochromocytoma
and paraganglioma: Imaging characteristics. Cancer Imaging.
12:153–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brink I, Hoegerle S, Klisch J and Bley TA:
Imaging of pheochromocytoma and paraganglioma. Fam Cancer. 4:61–68.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sahdev A, Sohaib A, Monson JP, Grossman
AB, Chew SL and Reznek RH: CT and MR imaging of unusual locations
of extra-adrenal paragangliomas (pheochromocytomas). Eur Radiol.
15:85–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ji XK, Zeng QQ, Wu XL, Huang YP, Zhou MT,
Huang KT, Yu ZP, Han SL and Zhang QY: Surgical treatment and
prognostic analysis of retroperitoneal paragangliomas: A study of
19 cases. Zhonghua Yi Xue Za Zhi. 90:2385–2388. 2010.(In Chinese).
PubMed/NCBI
|
|
17
|
Kimura N, Ishidate T, Kogawa T, Miura Y,
Ishizaka M and Ogita M: A retroperitoneal sympathetic paraganglioma
invading the duodenum and mimicking a submucosal tumor. Endocr
Pathol. 19:128–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inzani F, Rindi G, Tamborrino E, Cobelli R
and Bordi C: Extra-adrenal composite paraganglioma with
ganglioneuroma component presenting as a pancreatic mass. Endocr
Pathol. 20:191–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sangster G, Do D, Previgliano C, Li B,
LaFrance D and Heldmann M: Primary retroperitoneal paraganglioma
simulating a pancreatic mass: A case report and review of the
literature. HPB Surg. 2010:6457282010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gimenez-Roqueplo AP, Caumont-Prim A,
Houzard C, Hignette C, Hernigou A, Halimi P, Niccoli P, Leboulleux
S, Amar L, Borson-Chazot F, et al: Imaging work-up for screening of
paraganglioma and pheochromocytoma in SDHx mutation carriers: A
multicenter prospective study from the PGL.EVA investigators. J
Clin Endocrinol Metab. 98:E162–E173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dalal T, Maher MM, Kalra MK and Mueller
PR: Extraadrenal pheochromocytoma: A rare cause of tachycardia and
hypertension during percutaneous biopsy. AJR Am J Roentgenol.
185:554–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sood SK, Balasubramanian SP and Harrison
BJ: Percutaneous biopsy of adrenal and extra-adrenal
retroperitoneal lesions: Beware of catecholamine secreting tumours!
Surgeon. 5:1–281. 2007. View Article : Google Scholar
|
|
23
|
Kliewer KE, Wen DR, Cancilla PA and
Cochran AJ: Paragangliomas: Assessment of prognosis by histologic,
immunohistochemical, and ultrastructural techniques. Hum Pathol.
20:29–39. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Achilles E, Padberg BC, Holl K, Klöppel G
and Schröder S: Immunocytochemistry of paragangliomas-value of
staining for S-100 protein and glial fibrillary acid protein in
diagnosis and prognosis. Histopathology. 18:453–458. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pávai Z, Orosz Z, Horváth E, Seres-Sturm L
and Jung J: Immunohistochemical features of paragangliomas. J Cell
Mol Med. 5:311–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Elder EE, Xu D, Höög A, Enberg U, Hou M,
Pisa P, Gruber A, Larsson C and Bäckdahl M: KI-67 and hTERT
expression can aid in the distinction between malignant and benign
pheochromocytoma and paraganglioma. Mod Pathol. 16:246–255. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng N, Zhang WY and Wu XT:
Clinicopathological analysis of paraganglioma with literature
review. World J Gastroenterol. 15:3003–3008. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Korevaar TI and Grossman AB:
Pheochromocytomas and paragangliomas: Assessment of malignant
potential. Endocrine. 40:354–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Varma K, Jain S and Mandal S:
Cytomorphologic spectrum in paraganglioma. Acta Cytol. 52:549–556.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thompson LD: Pheochromocytoma of the
adrenal gland scaled score (PASS) to separate benign from malignant
neoplasms: A clinicopathologic and immunophenotypic study of 100
cases. Am J Surg Pathol. 26:551–566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Agarwal A, Mehrotra PK, Jain M, Gupta SK,
Mishra A, Chand G, Agarwal G, Verma AK, Mishra SK and Singh U: Size
of the tumor and pheochromocytoma of the adrenal gland scaled score
(PASS): Can they predict malignancy? World J Surg. 34:3022–3028.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu D, Tischler AS, Lloyd RV, DeLellis RA,
de Krijger R, van Nederveen F and Nosé V: Observer variation in the
application of the pheochromocytoma of the adrenal gland scaled
score. Am J Surg Pathol. 33:599–608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Somasundar P, Krouse R, Hostetter R,
Vaughan R and Covey T: Paragangliomas- a decade of clinical
experience. J Surg Oncol. 74:286–290. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Altergott R, Barbato A, Lawrence A,
Paloyan E, Freeark RJ and Prinz RA: Spectrum of
catecholamine-secreting tumors of the organ of Zuckerkandl.
Surgery. 98:1121–1126. 1985.PubMed/NCBI
|
|
35
|
van Heerden JA, Roland CF, Carney JA,
Sheps SG and Grant CS: Long-term evaluation following resection of
apparently benign pheochromocytoma(s)/paraganglioma(s). World J
Surg. 14:325–329. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsui H, Ikeuchi S, Onoda N and Tsutsumi
Y: Malignant paraganglioma of the retroperitoneum with lung
metastases: A 13-year survivor after radical surgery. Asian J Surg.
30:75–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pacak K: Preoperative management of the
pheochromocytoma patient. J Clin Endocrinol Metab. 92:4069–4079.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lebuffe G, Dosseh ED, Tek G, Tytgat H,
Moreno S, Tavernier B, Vallet B and Proye CA: The effect of calcium
channel blockers on outcome following the surgical treatment of
phaeochromocytomas and paragangliomas. Anaesthesia. 60:439–444.
2005. View Article : Google Scholar : PubMed/NCBI
|