Introduction
The incidence of translocations in the mixed-lineage
leukemia (MLL) gene at chromosome band 11q23 is high in
acute lymphoblastic leukemia (ALL) and acute myeloid leukemia in
infants (1,2). MLL forms rearrangements with
>60 translocation partner genes (3,4). The most
common rearrangements are AFF1/AF4,
MLLT1/ENL, and MLLT3/AF9, and the less
common are ELL, myeloid/lymphoid or mixed-lineage leukemia;
translocated To, 10 (MLLT10)/AF10 and
MLLT4/AF6 (3). However,
in a significant fraction of patients with leukemia and MLL
rearrangements, such alterations are absent (3). To address the pathobiology of leukemia
with MLL rearrangements and determine potential involvement
of fusion genes, the authors of the present report previously
performed transcriptome sequencing in an infant with ALL and
MLL rearrangement. The infant was negative for
MLL-AF4, MLL-ENL, MLL-ELL and MLL-AF9
fusion transcripts, and the presence of a
MLL-MLLT10/AF10 fusion transcript was detected
(5).
In the present report, a new case of infantile ALL
with MLL-MLLT10/AF10 and 10;11 rearrangements was
presented. A chromosomal mechanism leading to
MLL-MLLT10/AF10 fusion and alternative splicing of an
MLL-exon-8-MLLT10 fusion genes, resulting in two
different isoforms, was described. In addition, it was determined
that MLLT10/AF10-homeobox protein Mohawk (MKX)
resulted from an inversion (10p12.1;10p12.31). Furthermore,
transcriptome sequencing revealed a separate chromosomal
translocation leading to a previously unreported AT-rich
interaction domain (ARID)5B-MLL-positive 10;11
rearrangement in this patient. ARID5B polymorphisms are
important determinants of childhood ALL susceptibility, and
treatment outcomes and contribute to racial disparities in ALL
(6). Taken together, these results
support the hypothesis of the authors that precise control of
MLL and MLLT10/AF10 fusion transcripts is
crucial in leukemogenesis.
Case report
Patient characteristics
A 2-month-old Japanese male infant was admitted to
Tokyo University Hospital (Tokyo, Japan) in January 2008.
Laboratory tests demonstrated a leukocyte count of
5.44×1010/l (normal range,
4.6×109-18.9×109/l) with 88% blasts,
hemoglobin of 9.0 g/dl (normal range, 9.5–13.7 g/dl), and platelet
count of 3.9×1010/l (normal range,
25×1010-82×1010/l). Leukemic cells were
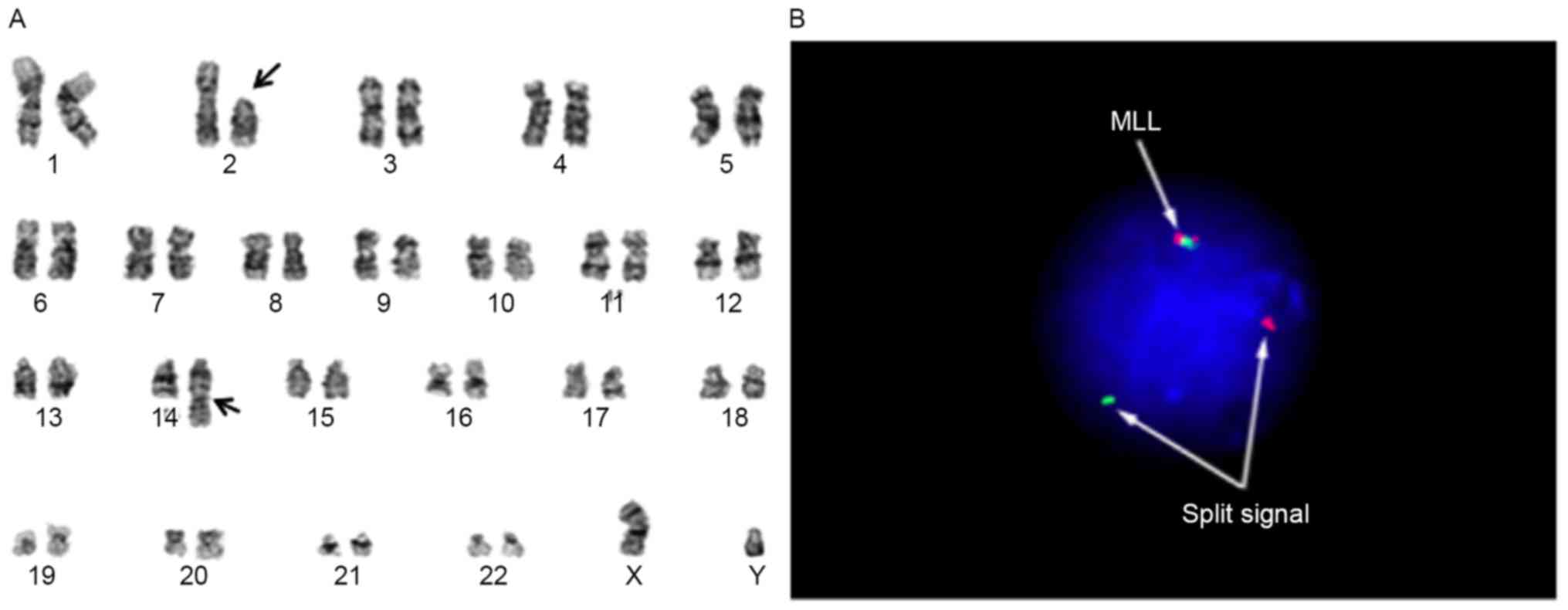
cytogenetically characterized as 46, XY, t(2;14)(p11.2;q32),
add(11)(q23) (Fig. 1A) and were found to express cluster of
differentiation (CD)10 and CD19 by bone marrow biopsy. Analysis
with fluorescence in situ hybridization using the MLL
break-apart probe for the determination of add(11)(q23) revealed the typical split signal
(Fig. 1B). Based on the above data,
the diagnosis was established as infantile B-precursor ALL with
MLL rearrangement. The patient achieved complete remission
with chemotherapy and received stem cell transplantation. Treatment
was well tolerated, and he has been in complete remission for 7
years.
The present study was approved by the Gene Analysis
Research Ethics Committee at the University of Tokyo (Tokyo,
Japan). Informed consent was obtained from the guardian of the
patient.
Paired-end RNA sequencing and
identification of fusion genes
High-quality RNA with an RNA integrity number
>6.0 from the patient was used to prepare RNA sequencing
libraries, according to the TruSeq® RNA (Illumina, San Diego, CA,
USA) protocol, which were then sequenced on an Illumina HiSeq 2000
device. An in-house pipeline, Genomon-fusion, was used to identify
fusion transcripts. All candidate gene fusions that were >2
paired reads were confirmed by reverse transcription-polymerase
chain reaction (RT-PCR) and Sanger sequencing.
RT-PCR and Sanger sequencing
Total RNA (4 µg) was reverse transcribed to cDNA in
a total volume of 33 µl with random primers using the Ready-To-Go
You-Prime First-Strand beads (Pharmacia Biotech; GE Healthcare,
Chicago, IL, USA). RT-PCR and Sanger sequencing were performed as
previously described (4). In brief, 1
µl cDNA was used as a template in RT-PCR and the reaction was
performed for 35 cycles in a GeneAmp PCR System 9700 (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA), with
denaturation at 95°C for 30 sec, annealing at 58°C for 30 sec and
extension at 72°C for 1 min and a final cycle of 72°C for 7 min.
RT-PCR experiment was repeated three times.
MLL-MLLT10/AF10 was amplified using the following
forward (F) and reverse (R) primers: MLL F1,
5′-CCTGAGGACTGTGGTGTTTGTAC-3′ and MLLT10/AF10 R1,
5′-CCTGACTGAGAGAAGATCCAGAT-3′. ARID5B-MLL was amplified
using the following forward and reverse primers: ARID5B F1,
5′-TCGATGCTGAAACGCATCCA-3′ and MLL R1, 5′-CACTGCTCTCTTTGCTGTCT-3′.
MLLT10/AF10-MKX was amplified using the following
forward and reverse primers: MLLT10/AF10 F1,
5′-ATGGAAGTTTACAGAGCCTCAG-3′ and MKX R1,
5′-TTCGTTCATGTGGGTTCTTGG-3′. Nucleotide sequences of PCR products
and, if necessary, subcloned PCR products were analyzed as
described previously (4).
Detection of fusion transcripts
To identify high-confidence fusion transcripts, an
in-house pipeline for RNA sequencing data analysis, Genomon-fusion,
was used for analysis of RNA sequencing data from the patient bone
marrow cells. A total of 49 fusion transcripts supported by
discordant read pairs as well as one perfectly matched
junction-spanning read, with the other end of the read-pair mapping
to either of the fusion gene partners, were identified.
To focus on fusion transcripts identified in
infantile leukemia, from the initial list of 49 fusion transcripts,
two in-frame fusion transcripts (MLL-MLLT10/AF10 and
ARID5B-MLL) and one out-of-frame fusion transcript
(MLLT10/AF10-MKX) that were supported by >10
junction-spanning reads were identified. The ARID5B-MLL
fusion transcript has not been previously reported. No fusion
events involving genes on chromosomes 2 and 14 were detected.
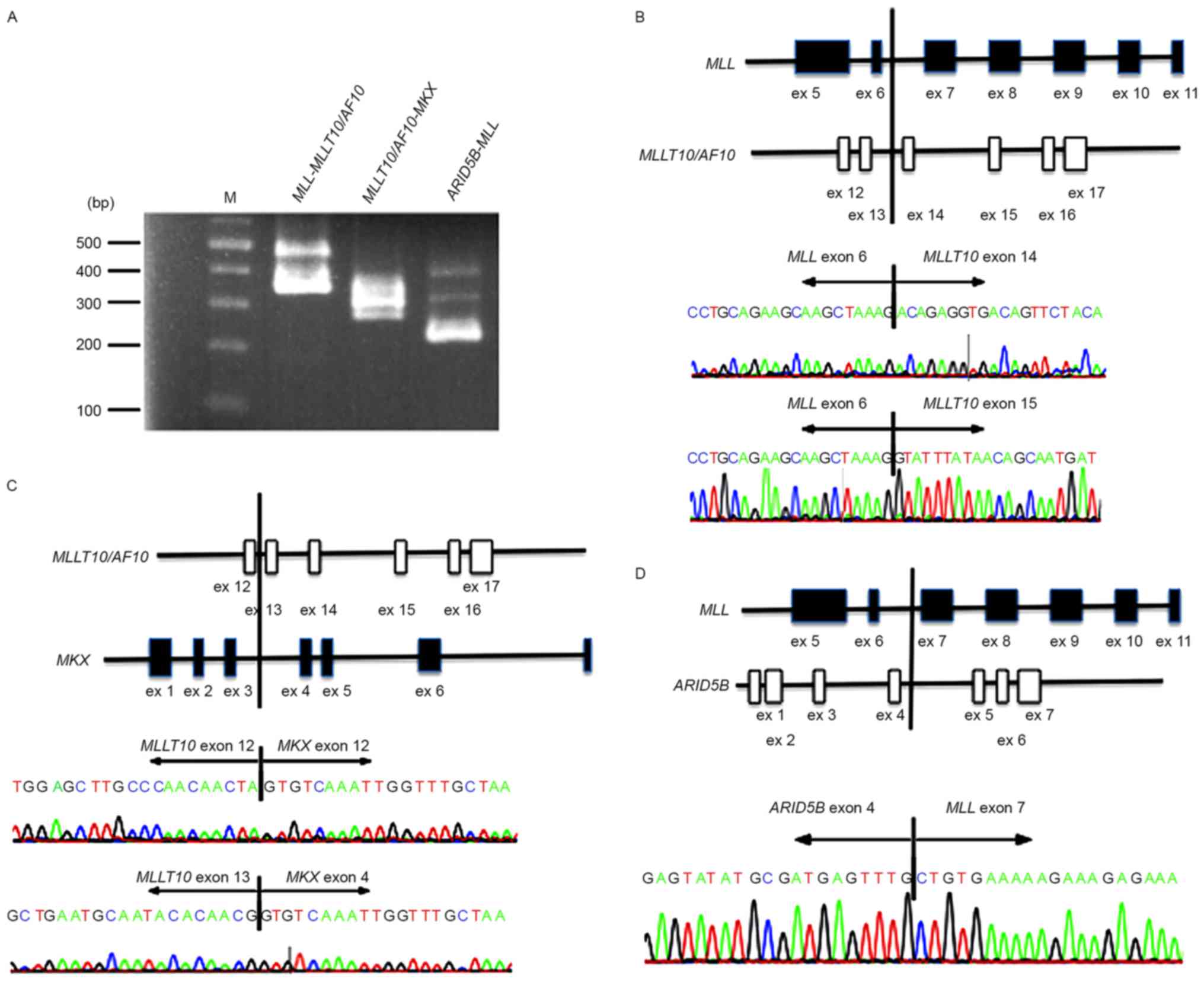
Validation of fusion transcripts
To evaluate the authenticity and validity of the
gene fusion transcripts, Sanger sequencing of RT-PCR amplification
products spanning fusion junction points was performed to validate
all transcripts detected in the patient bone marrow cells. Although
the RT step is able to potentially produce artifactual fusion
transcripts, all three fusion transcripts,
MLL-MLLT10/AF10, ARID5B-MLL, and
MLLT10/AF10-MKX, were detected by Sanger sequencing
(Fig. 2). Specifically, all fusion
transcripts showing >10 junction-spanning reads were
identifiable by Sanger sequencing.
Discussion
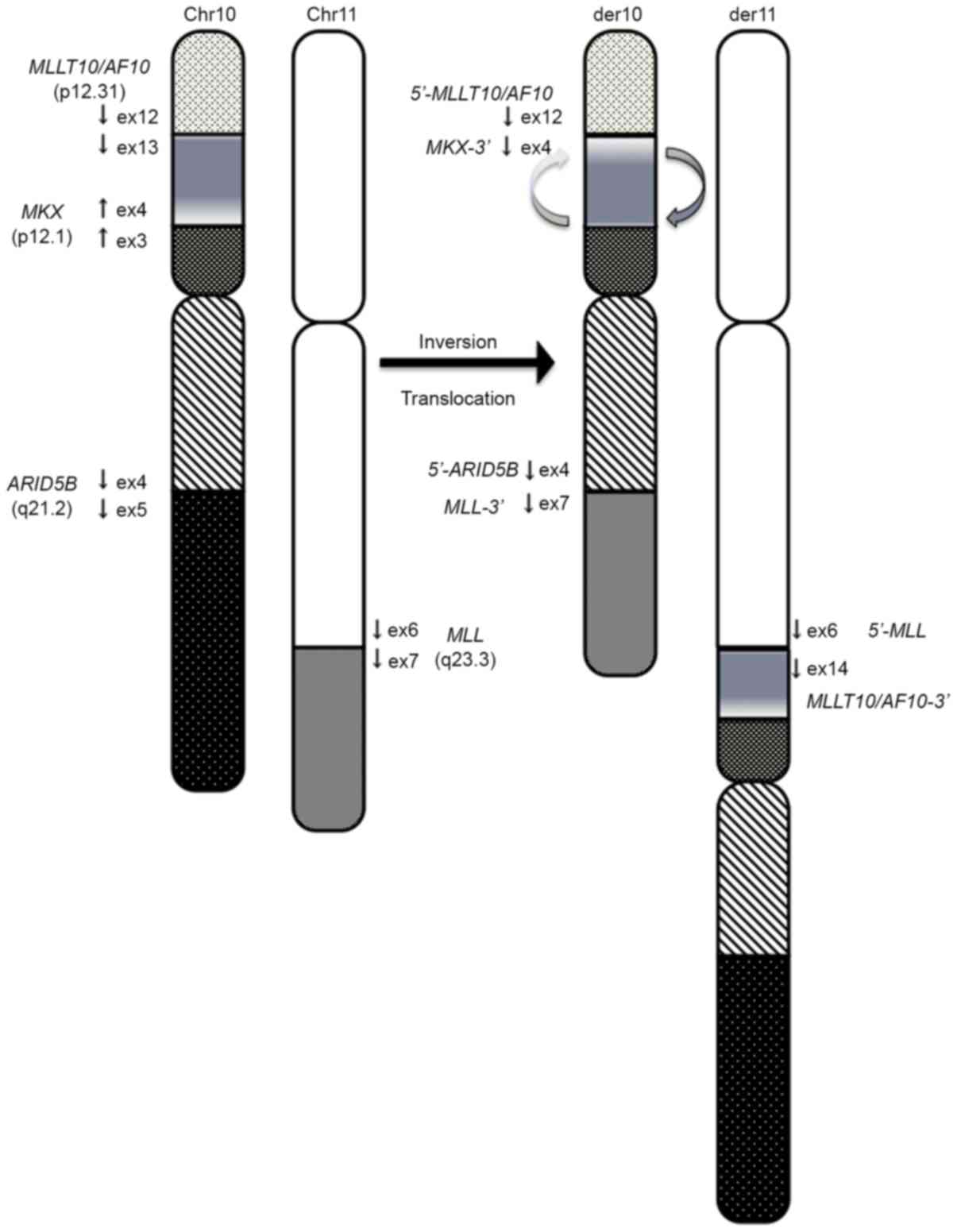
The authors herein presented a new case with
inv(10)(p12.1;p12.31) and
t(10;11)(q21;q23). In this case, the MLL-MLLT10/AF10
fusion resulted from several rearrangements, including inversion
followed by translocation. This patient exhibited complex
rearrangements, which included inversion of 10p12.1-p12.31 followed
by translocation of 11q23 to 10p12 that was associated with the
generation of MLL-MLLT10 (Fig.
3). The proximal inversion breakpoint at 10p was heterogeneous
and contained a region within 10p12.1-p12.31.
MLLT10/AF10 has been reported to be involved in a
complex rearrangement that resulted in an inversion of either 10p
or 11q followed by translocation of 10p;11q or insertion of the
inverted segment into MLLT10/AF10 or MLL
(7,8).
MLLT10/AF10 was also one of the 20 genes involved in
complex rearrangements, with three- or four-way translocations
resulting in >2 fusion alleles (8). While the 3′ portions of the
translocation partner genes were regularly fused to the 5′-portion
of MLL, the genes involved in complex rearrangements, including
MLLT10/AF10, were fused to the 3′ portion of MLL
(8). CALM-MLLT10/AF10
and reciprocal MLLT10/AF10-CALM transcripts have been
detected in patients with various hematological malignancies
(9). Silliman et al (10) emphasized that truncated MLLT10/AF10
protein in normal cells and truncated MLLT10/AF10-CALM protein in
leukemic cells retain a cysteine-rich motif and leukemia-associated
protein (LAP) (11) and would
function as dominant-negative inhibitors of full-length AF10 or
associated proteins (12). There is a
high likelihood that the rearrangements in the patient in the
present report also involved a complex mechanism, given that the 3′
portion of MKX was fused to the 5′ portion of MLLT10/AF10.
MLLT10/AF10-MKX proteins in leukemic cells retain a cysteine-rich
motif and LAP. MKX is a member of the three-amino acid loop
extension superclass of homeobox genes and is associated with
cryptorchidism (13). The DNA
recognition motif for MKX is largely dependent on a core set of
amino acids in helix III, and residues at the N terminus of the
homeodomain that make direct contact with the DNA (14,15).
However residues at the N terminus were not maintained in the
MLLT10/AF10-MKX fusion transcript due to the presence
of an out-of frame fusion transcript, and MKX may be biologically
inactivated. These results suggested that MLLT10/AF10-MKX may have
a similar role in leukemogenesis as MLLT10/AF10-CALM.
In the present study, ARID5B-MLL fusion was
caused by the presence of a translocation between derivative
chromosomes 10q21 and 11q23. ARID5B gene variants were
consistently shown to increase the risk of childhood ALL in various
populations (16–18). The ARID5B gene encodes a member
of the AT-rich interaction domain (ARID) family of DNA-binding
proteins. The encoded protein forms a histone H3K9me2 demethylase
complex together with PHD finger protein 2 to regulate the
transcription of target genes involved in adipogenesis and liver
development (19). Although the
function of ARID5B in lympho-hematopoiesis has not been well
studied, it may be involved in epigenetic regulation of gene
expression in hematopoietic stem cells and early lymphoid
progenitors, similar to other AT-rich DNA-binding proteins
(20–23). Interestingly, a single nucleotide
polymorphism in ARID5B (RefSNP: rs10821936) was demonstrated
to increase the risk of MLL-MLLT3/AF9 (17). This finding suggests that genetic
susceptibility may be associated with the differences regarding
MLL breakpoints and partner genes. Although the role of
truncated isoforms of ARID5B in tumorigenesis remains unknown,
epigenetic dysregulation may occur in early B progenitor cells in
ALL with ARID5B-MLL fusion. Two recent studies clearly
demonstrated that reciprocal MLL fusion proteins may have an
important role in cancer development (24,25).
During follow-up analyses, a large collection of reciprocal
MLL fusions was identified, and ~15% of these were in-frame
fusions that may be readily expressed as reciprocal fusion proteins
(8). All other characterized
reciprocal MLL alleles represented out-of-frame fusions with
either a chromosomal locus or a reciprocal translocation partner
gene (8). However, even these events
allowed the transcription and expression of a 5′-truncated MLL
protein, termed MLL* (26). This
truncated version of MLL has no ability to bind Menin1, lens
epithelium-derived growth factor or MYB, but it is able to carry
out all enzymatic functions necessary to execute H4K16 acetylation
events by associating with MOF or H3K4 methylation via the SET
domain complex (27). Together with
these results, the findings of the present report suggested that
reciprocal MLL fusion proteins, including ARID5B-MLL, may inhibit
early B-cell development and have oncogenic functions. Additional
functional studies are required to understand the role of these
fusion genes in the development of MLL-associated infantile
leukemia.
Acknowledgements
The authors of the present report would like to
thank Ms. Masayo Matsumura and Ms. Yin Yi, Department of
Pediatrics, University of Tokyo (Tokyo, Japan), or special
technical assistance. The present study was supported by KAKENHI of
Japan Society of Promotion of Science (grant nos. 25253095 and
26293242), Research on Measures for Intractable Diseases, Health
and Labor Sciences Research Grants, the Ministry of Health, Labor
and Welfare, Research on Health Sciences Focusing on Drug
Innovation, the Japan Health Sciences Foundation, Core Research for
Evolutional Science and Technology, Japan Science and Technology
Agency and the Project for Development of Innovative Research on
Cancer Therapeutics (P-DIRECT) (grant no. 886695).
Glossary
Abbreviations
Abbreviations:
|
ALL
|
acute lymphoblastic leukemia
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
FISH
|
fluorescence in situ
hybridization
|
References
|
1
|
Felix CA: Secondary leukemias induced by
topoisomerase targeted drugs. Biochim Biophys Acta. 1400:233–255.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Chen A, Yan XM and Huang G:
Disordered epigenetic regulation in MLL-related leukemia. Int J
Hematol. 96:428–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyer C, Kowarz E, Hofmann J, Renneville
A, Zuna J, Trka J, Ben Abdelali R, Macintyre E, De Braekeleer E, De
Braekeleer M, et al: New insights to the MLL recombinome of acute
leukemias. Leukemia. 23:1490–1499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hiwatari M, Taki T, Taketani T, Taniwaki
M, Sugita K, Okuya M, Eguchi M, Ida K and Hayashi Y: Fusion of an
AF4-related gene, LAF4, to MLL in childhood acute lymphoblastic
leukemia with t(2;11)(q11;q23). Oncogene. 22:2851–2855. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chaplin T, Ayton P, Bernard OA, Saha V,
Valle V Della, Hillion J, Gregorini A, Lillington D, Berger R and
Young BD: A novel class of zinc finger/leucine zipper genes
identified from the molecular cloning of the t(10;11) translocation
in acute leukemia. Blood. 85:1435–1441. 1995.PubMed/NCBI
|
|
6
|
Xu H, Cheng C, Devidas M, Pei D, Fan Y,
Yang W, Neale G, Scheet P, Burchard EG, Torgerson DG, et al: ARID5B
genetic polymorphisms contribute to racial disparities in the
incidence and treatment outcome of childhood acute lymphoblastic
leukemia. J Clin Oncol. 30:751–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Limbergen H, Poppe B, Janssens A, De
Bock R, De Paepe A, Noens L and Speleman F: Molecular cytogenetic
analysis of 10;11 rearrangements in acute myeloid leukemia.
Leukemia. 16:344–351. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyer C, Hofmann J, Burmeister T, Gröger
D, Park TS, Emerenciano M, de Oliveira M Pombo, Renneville A,
Villarese P, Macintyre E, et al: The MLL recombinome of acute
leukemias in 2013. Leukemia. 27:2165–2176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ashihara E, Nakamura S, Inaba T, Taki T,
Hayashi Y and Shimazaki C: A novel AF10-CALM fusion transcript in
gamma/delta-T cell type lymphoblastic lymphoma. Am J Hematol.
82:859–860. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Silliman CC, McGavran L, Wei Q, Miller LA,
Li S and Hunger SP: Alternative splicing in wild-type AF10 and CALM
cDNAs and in AF10-CALM and CALM-AF10 fusion cDNAs produced by the
t(10;11)(p13-14;q14-q21) suggests a potential role for truncated
AF10 polypeptides. Leukemia. 12:1404–1410. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saha V, Chaplin T, Gregorini A, Ayton P
and Young BD: The leukemia-associated-protein (LAP) domain, a
cysteine-rich motif, is present in a wide range of proteins,
including MLL, AF10, and MLLT6 proteins. Proc Natl Acad Sci USA.
92:pp. 9737–9741. 1995; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumon K, Kobayashi H, Maseki N, Sakashita
A, Sakurai M, Tanizawa A, Imashuku S and Kaneko Y: Mixed-lineage
leukemia with t(10;11)(p13;q21): An analysis of AF10-CALM and
CALM-AF10 fusion mRNAs and clinical features. Genes Chromosomes
Cancer. 25:33–39. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mroczkowski HJ, Arnold G, Schneck FX,
Rajkovic A and Yatsenko SA: Interstitial 10p11.23-p12.1
microdeletions associated with developmental delay, craniofacial
abnormalities, and cryptorchidism. Am J Med Genet A 164A. 1–2626.
2014.
|
|
14
|
Anderson DM, George R, Noyes MB, Rowton M,
Liu W, Jiang R, Wolfe SA, Wilson-Rawls J and Rawls A:
Characterization of the DNA-binding properties of the Mohawk
homeobox transcription factor. J Biol Chem. 287:35351–35359. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noyes MB, Christensen RG, Wakabayashi A,
Stormo GD, Brodsky MH and Wolfe SA: Analysis of homeodomain
specificities allows the family-wide prediction of preferred
recognition sites. Cell. 133:1277–1289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Papaemmanuil E, Hosking FJ, Vijayakrishnan
J, Price A, Olver B, Sheridan E, Kinsey SE, Lightfoot T, Roman E,
Irving JA, et al: Loci on 7p12.2, 10q21.2 and 14q11.2 are
associated with risk of childhood acute lymphoblastic leukemia. Nat
Genet. 41:1006–1010. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Emerenciano M, Barbosa TC, Lopes BA,
Blunck CB, Faro A, Andrade C, Meyer C, Marschalek R and
Pombo-de-Oliveira MS; Brazilian Collaborative Study Group of Infant
Acute Leukemia, : ARID5B polymorphism confers an increased risk to
acquire specific MLL rearrangements in early childhood leukemia.
BMC Cancer. 14:1272014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prasad RB, Hosking FJ, Vijayakrishnan J,
Papaemmanuil E, Koehler R, Greaves M, Sheridan E, Gast A, Kinsey
SE, Lightfoot T, et al: Verification of the susceptibility loci on
7p12.2, 10q21.2, and 14q11.2 in precursor B-cell acute
lymphoblastic leukemia of childhood. Blood. 115:1765–1767. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patsialou A, Wilsker D and Moran E:
DNA-binding properties of ARID family proteins. Nucleic Acids Res.
33:66–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Webb CF, Bryant J, Popowski M, Allred L,
Kim D, Harriss J, Schmidt C, Miner CA, Rose K, Cheng HL, et al: The
ARID family transcription factor bright is required for both
hematopoietic stem cell and B lineage development. Mol Cell Biol.
31:1041–1053. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Will B, Vogler TO, Bartholdy B,
Garrett-Bakelman F, Mayer J, Barreyro L, Pandolfi A, Todorova TI,
Okoye-Okafor UC, Stanley RF, et al: Satb1 regulates the
self-renewal of hematopoietic stem cells by promoting quiescence
and repressing differentiation commitment. Nat Immunol. 14:437–445.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Satoh Y, Yokota T, Sudo T, Kondo M, Lai A,
Kincade PW, Kouro T, Iida R, Kokame K, Miyata T, et al: The Satb1
protein directs hematopoietic stem cell differentiation toward
lymphoid lineages. Immunity. 38:1105–1115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yokota T and Kanakura Y: Role of
tissue-specific AT-rich DNA sequence-binding proteins in lymphocyte
differentiation. Int J Hematol. 100:238–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bursen A, Schwabe K, Rüster B, Henschler
R, Ruthardt M, Dingermann T and Marschalek R: The AF4.MLL fusion
protein is capable of inducing ALL in mice without requirement of
MLL.AF4. Blood. 115:3570–3579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Emerenciano M, Kowarz E, Karl K, de
Almeida Lopes B, Scholz B, Bracharz S, Meyer C, Pombo-de-Oliveira
MS and Marschalek R: Functional analysis of the two reciprocal
fusion genes MLL-NEBL and NEBL-MLL reveal their oncogenic
potential. Cancer Lett. 332:30–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kowarz E, Burmeister T, Lo Nigro L, Jansen
MW, Delabesse E, Klingebiel T, Dingermann T, Meyer C and Marschalek
R: Complex MLL rearrangements in t(4;11) leukemia patients with
absent AF4.MLL fusion allele. Leukemia. 21:1232–1238. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scharf S, Zech J, Bursen A, Schraets D,
Oliver PL, Kliem S, Pfitzner E, Gillert E, Dingermann T and
Marschalek R: Transcription linked to recombination: A
gene-internal promoter coincides with the recombination hot spot II
of the human MLL gene. Oncogene. 26:1361–1371. 2007. View Article : Google Scholar : PubMed/NCBI
|