Introduction
Colorectal cancer (CRC) is a type of cancer that is
most associated with dietary factors, and has the third highest
mortality rate of common malignancies in males and females
(1–3).
Due to the continued urbanization of the population, the morbidity
and mortality rates associated with CRC are increasing gradually
worldwide. In addition to genetic factors, eating habits and
environmental factors markedly influence the relative risk for the
occurrence, and development of colon cancer (4,5). Although
CRC incidence rates have decreased to a certain degree, there is
certain evidence indicating that current therapies are expensive
and induce debilitating side effects; in addition, recurrence rates
of >50% have been reported, primarily due to the development of
acquired chemoresistance to conventional chemotherapeutic regimens
(6,7).
Grape seeds are an effective source of
proanthocyanidins (8). Grape seed
proanthocyanidins (GSPs) are primarily composed of dimers, trimers
and oligomers of monomeric anthocyanidin (9,10). There
are a number of biological functions of GSPs, including
antimutagenic, anti-inflammatory, anti-angiogenic and
anticarcinogenic activities (11–15).
Previous studies have demonstrated that GSPs have cytotoxic effects
in numerous cancer cell lines; however, the mechanisms underlying
these anticancer effects remain uncharacterized (16–18).
Apoptosis, or programmed cell death, serves a key
role in regulating the development and growth of normal cells, and
is often dysregulated in cancer cells (19). p53, the tumor suppressor protein,
serves an essential role in apoptosis and acts as a suppressor of
transformation (20,21), in addition, the expression level of
p53 has been revealed to be induced by DNA damage (22). In turn, p53 orchestrates a global
transcriptional response that counters cell proliferation or
induces apoptosis (23–25). GSPs have been demonstrated to protect
against stress-induced changes in the expression levels of p53 and
the anti-apoptotic protein, B cell lymphoma-2 (Bcl-2), in human
oral epithelial (26), and liver
(27) cells. The Bcl-2 family of
proteins consists of pro- and anti-apoptotic regulators of
apoptosis. The established mode of action of each separate entity
involves protecting or disrupting mitochondrial integrity, thereby
activating, or inhibiting the release of downstream factors,
including cytochrome c from the mitochondrion; these
stimulate apoptosis (28). The
apoptosis regulator Bax (Bax) and Bcl-2 homologous
antagonist/killer (Bak) genes encode apoptosis-promoting members of
the Bcl-2 gene family. The Bcl-2 protein is known to form
heterodimers with the Bax protein in vivo and the molar
ratio of Bcl-2 to Bax determines whether apoptosis is induced or
inhibited in target tissues (29).
The Bax protein, considered to be one of the primary targets of
p53, controls cell death by its participation in the disruption of
mitochondria and the subsequent release of cytochrome c
(30). Bak also regulates the release
of cytochrome c. Cytochrome c release, in turn,
activates caspase-9 and −3 (31).
Cleaved caspase-3 is regarded as a proximate mediator of apoptosis,
whereas caspase-2 triggered Bax-Bak-dependent and -independent cell
death in colon cancer cells treated with resveratrol (32). Caspase family proteins serve a key
role in the downstream events involved in p53-mediated apoptosis
(33). Clustering of the initiator
caspases activates the effector caspases, which trigger apoptosis
(34).
The present study investigated the molecular
mechanisms underlying GSP-induced apoptosis in the HCT-116 colon
cancer cell line. Exposure of these cells to various concentrations
of GSPs resulted in profound changes in morphology, proliferation
and apoptosis. The present study investigated the involvement of
p53, cytochrome c, Bcl-2 family proteins and caspases in
this process, and the results indicated that GSPs may provide a
novel approach to the treatment of CRC.
Materials and methods
Cell lines and culture
The HCT-116 human CRC cell line (Type Culture
Collection of the Chinese Academy of Sciences, Shanghai, China) was
cultured with McCoy's 5A (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% (vol/vol) heat-inactivated fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 100 IU penicillin and 100 µg/ml streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.) in a humidified incubator at 37°C
in 5% CO2. The HF-91 human fibroblast cell line (Type
Culture Collection of the Chinese Academy of Sciences) was cultured
in DEME/H (Sigma-Aldrich; Merck KGaA) supplemented with 10%
(vol/vol) heat-inactivated FBS (Gibco; Thermo Fisher Scientific,
Inc.), 100 IU penicillin and 100 µg/ml streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.) in a humidified incubator at 37°C
in 5% CO2. HCT-116 and HF-91 cells were plated in
96-well cell culture plates at 5×103 cells/well, and
incubated for 24 h. Triplicate wells were then treated with GSPs at
various concentrations (25, 50 or 100 µg/ml), 25 µg/ml
5-fluorouracil (5-Fu) as a positive control or PBS as the negative
solvent control for 48 h prior to the analyses described below.
Reagents and antibodies
GSPs were purchased from Sigma-Aldrich (Merck KGaA;
20315-25-7). The antibodies used for western blotting were as
follows: Mouse anti-caspase-2 (sc-5292), mouse anti-caspase-3
(sc-7272), mouse anti-caspase-9 (sc-56073), mouse anti-Bcl-2
(sc-7382), mouse anti-Bax (sc-20067), mouse anti-β-actin
(sc-47778), rabbit anti-Bak (sc-7873), rabbit anti-mouse
IgG-horseradish peroxidase (HRP; sc-358914) and mouse anti-rabbit
IgG-HRP (sc-2357). All antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
Cell viability
HCT-116 and HF-91 cells were plated in 96-well cell
culture plates at 5×103 cells/well and incubated for 24
h at 37°C. Triplicate wells were then treated with GSPs at various
concentrations (25, 50 or 100 µg/ml), 25 µg/ml 5-Fu as a positive
control or PBS as the negative solvent control. Cell viability was
determined after 48 h by adding 10 µl (5 mg/ml) cell proliferation
reagent (MTT; Sigma-Aldrich; Merck KGaA) and incubating for 24 h at
37°C until a purple precipitate was visible. The precipitate was
then solubilized by adding dimethyl sulfoxide (Sigma-Aldrich; Merck
KGaA). The absorbance was evaluated at 570 nm using a microplate
reader. The percentage inhibition of cell viability was determined
using the following formula: Percentage inhibition=1-optical
density (OD)treatment group/ODsolvent control
×100%.
Annexin V analysis
Annexin V and fluorescein isothiocyanate (FITC)
staining was performed using a commercial Annexin V-FITC kit (BD
Biosciences, Franklin Lakes, NJ, USA). Briefly, cells were treated
with GSPs (25, 50 or 100 µg/ml), 25 µg/ml 5-Fu as a positive
control or PBS as a solvent control for 48 h. The cells were then
harvested, washed twice with cold PBS and resuspended in binding
buffer at a concentration of 5×105 cells/ml. A 100-µl
aliquot containing 5×104 cells was incubated in the dark
with 5 µl Annexin V-FITC and 5 µl propidium iodide (PI) at room
temperature for 15 min. Subsequently, 400 µl binding buffer was
added and the cells were viewed (5 fields for each group) using a
fluorescence microscope (Olympus Corporation, Tokyo, Japan) at
magnification ×400. Viable cells were stained green and apoptotic
cells were stained red. This experiment was repeated three
times.
JC-1 assay
JC-1 analysis was performed using a commercial
Mitochondrial Membrane Potential Detection JC-1 kit (BD
Biosciences). Briefly, cells were treated with GSPs (25, 50 or 100
µg/ml), 25 µg/ml 5-Fu as a positive control or PBS as a solvent
control for 48 h prior to harvesting, washing twice with cold PBS
and resuspending in binding buffer at a concentration of
1×106 cells/ml. A 100-µl aliquot was incubated with 5 µl
JC-1 and 5 µl PI in the dark at room temperature for 15 min.
Subsequently, 400 µl binding buffer was added and the cells were
analyzed using a flow cytometer (Beckman Coulter, Inc., Brea, CA,
USA). Cells negative for JC-1 and PI were considered to be viable;
JC-1+/PI+ cells were in early apoptosis; JC-1+/PI- cells were
necrotic or in late apoptosis. This experiment was repeated three
times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from treated cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). First-strand cDNA was reverse transcribed using the
PrimeScript™ RT reagent kit (Takara Bio, Inc., Otsu, Japan), in
accordance with the manufacturer's protocols. Relative expression
levels of mRNA were determined by qPCR using the primer sequences
presented in Table I and a
Thermo® PikoReal 96 system (Thermo Fisher Scientific.,
Inc.). GAPDH was used as the endogenous reference gene. cDNA was
subjected to 40 PCR cycles of 94°C for 30 sec, 60°C for 30 sec and
72°C for 45 sec using FastStart Universal SYBR Green Master (Roche
Diagnostics, Basel, Switzerland). Experiments were performed in
triplicate and data were analyzed using Pikoreal software version
2.1. The average cycle threshold (Cq) values of the target genes
were normalized to GAPDH gene expression level as 2−ΔΔCq
(35). Changes in expression levels
were presented either as fold increases or as the ratio of the
target gene expression in the treated cells to its expression level
in the control cells.
 | Table I.Quantitative polymerase chain
reaction primer sequences. |
Table I.
Quantitative polymerase chain
reaction primer sequences.
| Gene | Primer sequence
(5′-3′) |
|---|
| Bcl-2 | Forward:
CATGTGTGTGGAGAGCGTCAA |
|
| Reverse:
GCCGGTTCAGGTACTCAGTCA |
| Bax | Forward:
GATCCAGGATCGAGCAGA |
|
| Reverse:
AAGTAGAAGAGGGCAACCAC |
| Bak | Forward:
AGATAGATAGCAGTAGTGCCTCA |
|
| Reverse:
ATTGCCAGTAGAAGCTCTCATGGTT |
| Caspase-2 | Forward:
CAAGTTCCTGAGCCTGGACTACATT |
|
| Reverse:
GACAGATTGCTTTCCTCCAACATT |
| Caspase-3 | Forward:
CAGAACTGGACTGTGGCATTGAG |
|
| Reverse:
GGATGAACCAGGAGCCATCCT |
| Caspase-9 | Forward:
GCGAACTAACAGGCAAGCAGC |
|
| Reverse:
CGACATCACCAAATCCTCCAGAAC |
| Cytochrome C | Forward:
GGTCAACAAATCATAAAGATATTGG |
|
| Reverse:
TAAACTTCAGGGTGACCAATAAATCA |
| p53 | Forward:
GCGGACTAACAGGCAAGCAAC |
|
| Reverse:
CTCGTTTATACTCCTCCAGAAC |
| GAPDH | Forward:
CGGAGTCAACGGATTTGGTCGTAT |
|
| Reverse:
AGCCTTCTCCATGGTTGGTGAAGAC |
Western blotting
Six proteins involved in apoptosis (caspase-2,
caspase-3, caspase-9, Bcl-2, Bak and Bax) were analyzed by western
blotting. HCT-116 cells were exposed to GSPs (25, 50 or 100 µg/ml)
at a concentration of 5×105 cells/well for 48 h in
6-well plates, harvested and lysed in RIPA buffer (Pierce; Thermo
Fisher Scientific, Inc.). Following a 30-min incubation on ice, the
cell lysate was centrifuged at 10,000 × g at 4°C for 5 min and the
supernatants were stored at −80°C until use. The protein
concentrations were determined using the Bradford assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Proteins (30 µg) were
separated by 12% SDS-PAGE, transferred to polyvinyl difluoride
membranes (EMD Millipore, Billerica, MA, USA) and blocked using 5%
non-fat dry milk in Tris-buffered saline (TBS) for 2 h at room
temperature. The membranes were then incubated with 1:1,000
dilutions of the aforementioned primary antibodies in TBS overnight
at 4°C, washed three times with TBS-Tween-20 and incubated with
1:5,000 dilutions of the appropriate secondary antibodies
conjugated with HRP in TBS for 1 h at room temperature. Membranes
were washed three times in TBS-Tween-20 at room temperature.
Protein bands were visualized on X-ray film using an enhanced
chemiluminescence (GE Healthcare, Chicago, IL, USA) detection
system. Western blotting bands were quantified using the Odyssey
infrared imaging system version 1.2 (LI-COR Biosciences, Lincoln,
NE, USA).
Statistical analysis
Data are presented as the mean ± standard deviations
and all analyses were performed using Origin8 version 8.1.10.86 SRO
(Origin8 Technologies Ltd., London, UK). Statistical significance
was evaluated using two-way analysis of variance and Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of GSPs on HCT-116 cell
viability and apoptosis
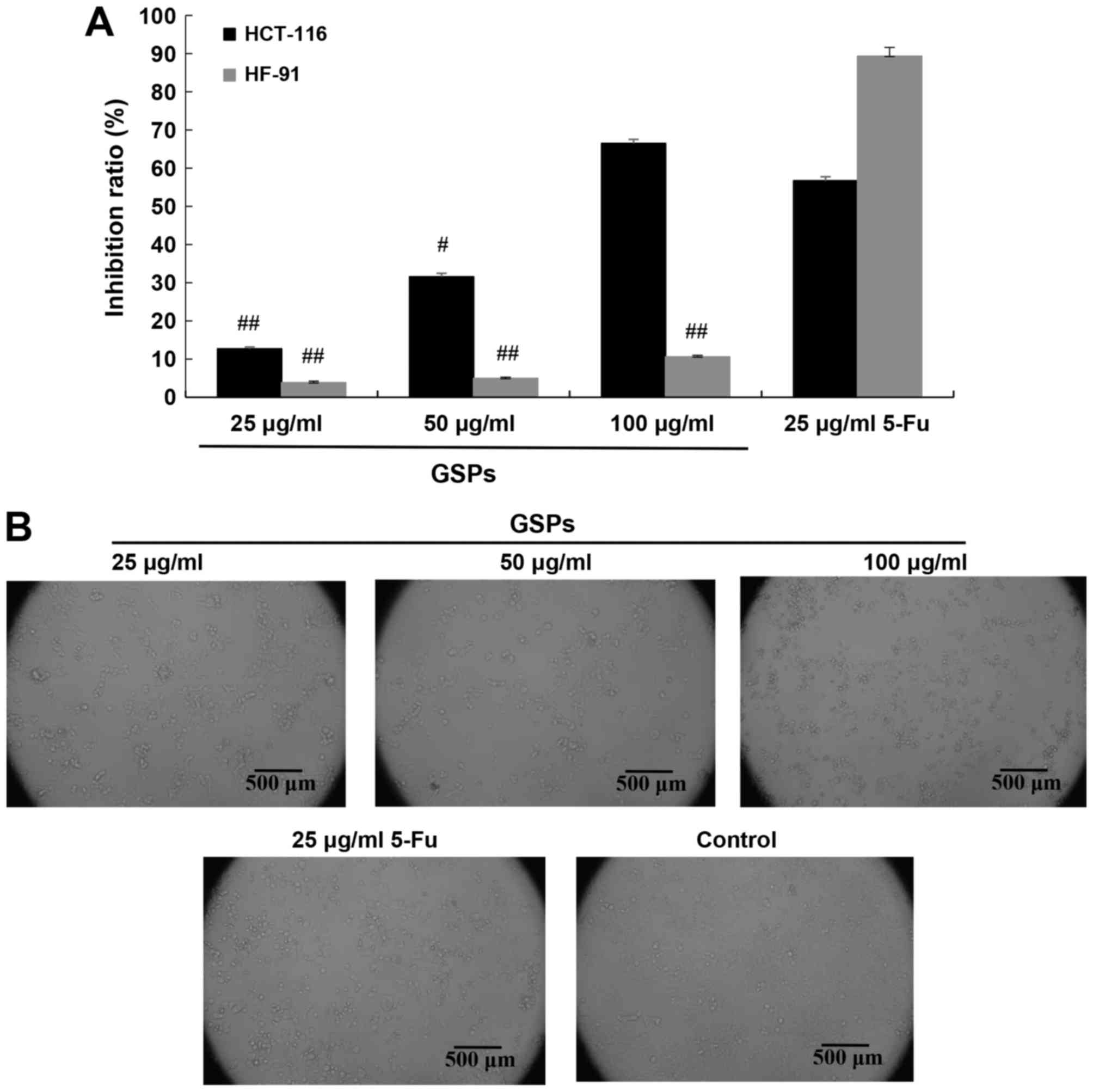
MTT assays of HCT-116 and HF-91 cells treated with
GSPs (25, 50 or 100 µg/ml), 5-Fu (25 µg/ml) or solvent control
indicated that GSPs reduced HCT-116 cell viability in a
concentration-dependent manner, whereas the inhibition of GSPs in
HF-91 cells was significantly lower compared with that in
5-Fu-treated cells (P<0.01; Fig.
1A). HCT-116 cells exposed to GSPs demonstrated cell shrinkage
and membrane blebbing (Fig. 1B).
Annexin V/PI double staining of the treated and untreated cells
revealed GSP concentration-dependent formation of apoptotic bodies
in HCT-116 cells (Fig. 2).
GSP-mediated effects on p53, caspases
and the Bcl-2 family
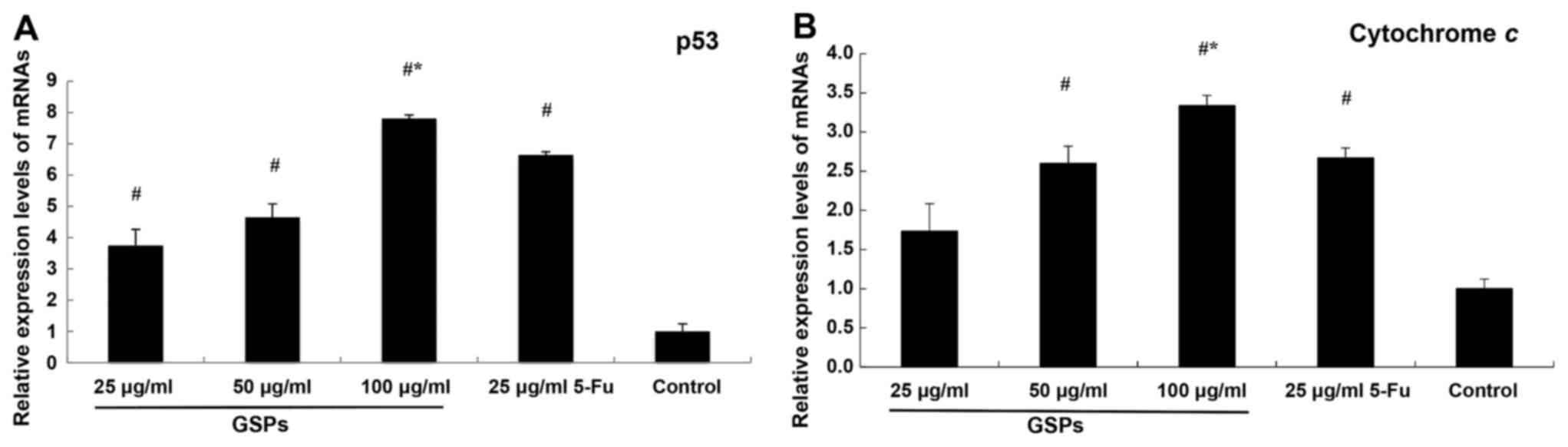
In the present study, the expression levels of p53
and cytochrome c increased in GSP-treated cells. Significant
overexpression was observed in HCT-116 cells exposed to 100 µg/ml
GSPs compared with 5-Fu, but not in those treated with lower
concentrations of GSPs or with PBS (negative control; Fig. 3).
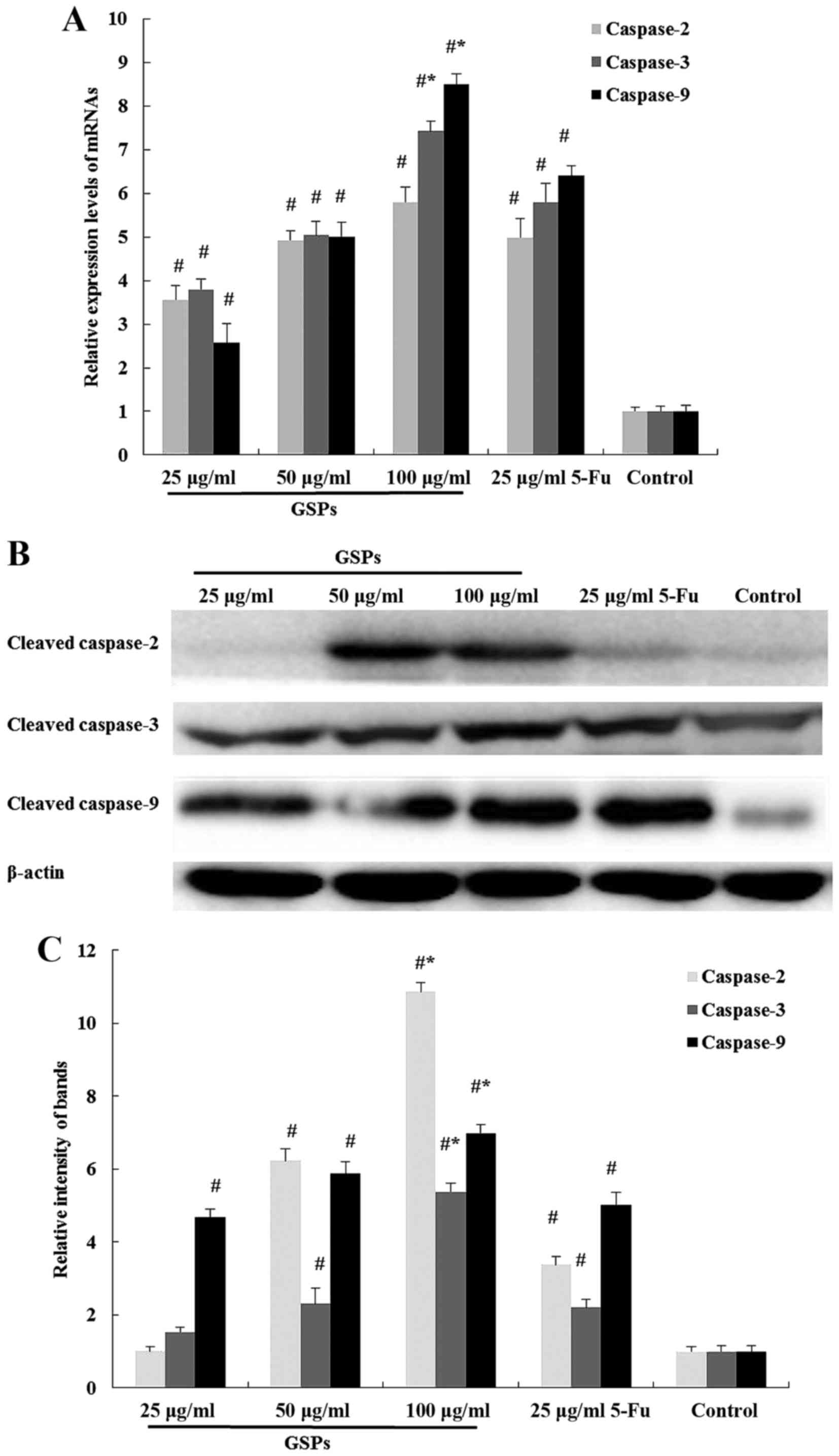
Considering the significance of caspases to
apoptosis, the present study determines the mRNA and protein
expression levels of initiator caspases (caspase-2 and −9) and an
effector caspase (caspase-3). Upregulation of the mRNAs encoding
caspase-2, caspase-3 and caspase-9 were observed in HCT-116 cells
exposed to GSPs for 48 h (Fig. 4A).
Furthermore, the expression levels of cleaved caspase-2, caspase-3
and caspase-9 protein were also significantly increased in cells
exposed to 100 µg/ml GSPs compared with 5-Fu for 48 h (Fig. 4B and C).
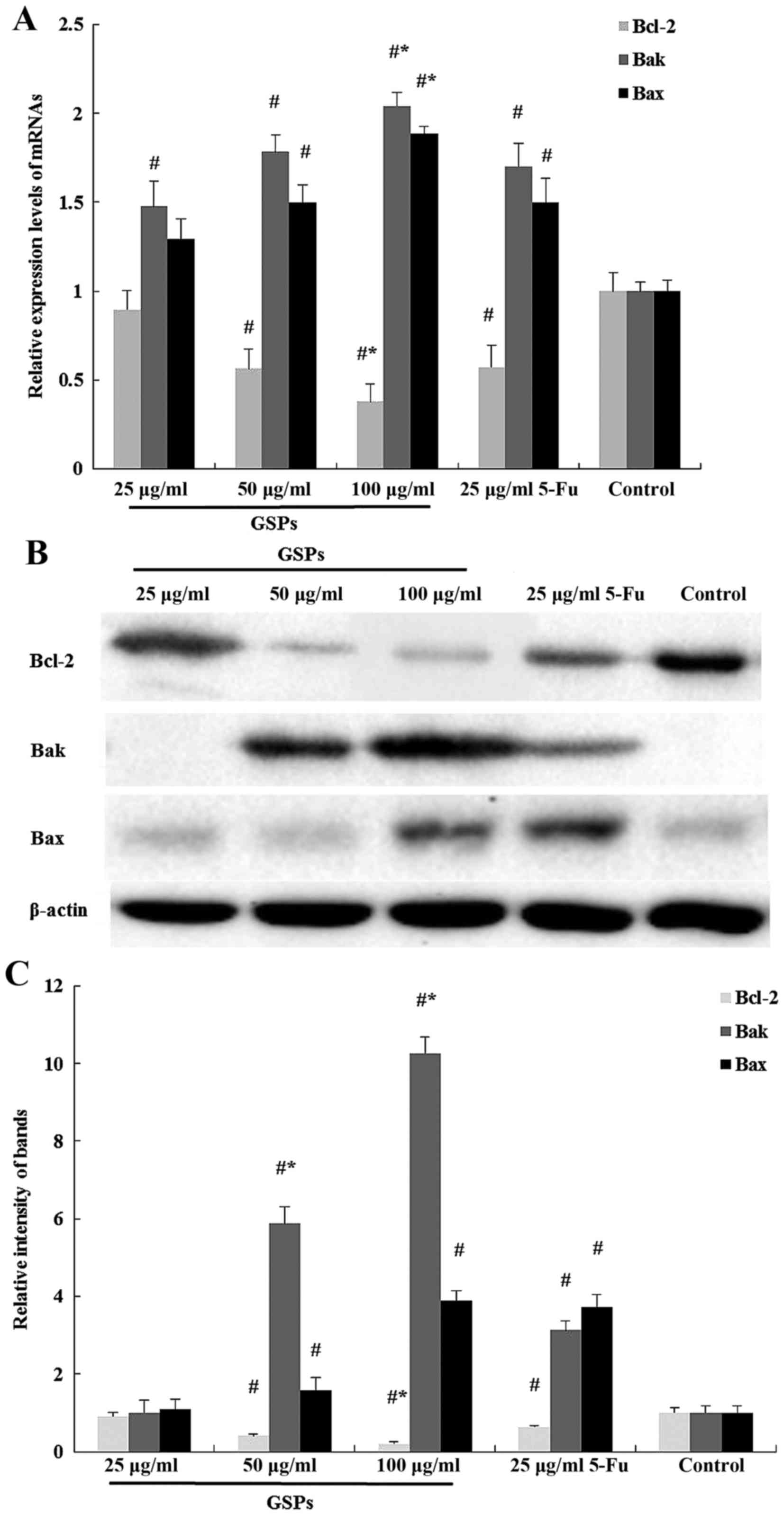
To further investigate the mechanism underlying
GSP-induced apoptosis, the present study evaluated the mRNA and
protein expression levels of Bcl-2, Bax and Bak by qPCR, and
western blotting. The Bcl-2 expression level in 100 µg/ml GSPs
group was significantly reduced compared with 5-Fu, whereas the
expression levels of Bax and Bak were upregulated in HCT-116 cells
exposed to GSPs for 48 h (Fig.
5A-C).
GSPs induce apoptosis via the
mitochondrial pathway
Caspase-9 is involved in the mitochondrial apoptosis
pathway and an increased expression level of cleaved caspase-9
therefore implied mitochondrial involvement in GSPs-induced
apoptosis. JC-1 staining revealed that the percentage of cells with
a loss of mitochondrial membrane potential increased from 18.7% in
the negative control cells to 80.1% in the HCT-116 cells exposed to
100 µg/ml GSPs (Fig. 6A). The
percentage of apoptotic cells observed in Fig. 6A is summarized in Fig. 6B. This loss of mitochondrial membrane
potential, coupled with the observed increase in cleaved caspase-9,
suggested that GSPs induced HCT-116 cell death via the
mitochondrial apoptosis pathway.
Discussion
GSPs are observed in dietary botanical supplements
and these compounds have been revealed to have anticarcinogenic
properties (12–18,36).
However, the molecular mechanisms underlying their effects in CRC
are not clearly understood.
The cell proliferation inhibitory results in the
present study regarding GSP treatment on the CRC cell line
(HCT-116) are in agreement with the concentration-dependent effects
reported for cyanidin on human colon cancer cell lines HCT-15 and
HT-29 (37,38). However, GSPs revealed more active
inhibition compared with cyanidin on colon cancer cells. This was
accompanied by early and late apoptosis, and necrosis, observed
using fluorescence microscopy and flow cytometry (37,38). A
series of experimental evidence suggested that GSPs are associated
with the p53-induced mitochondrial apoptosis pathway, and involved
with the Bcl-2 and caspase families (39). The Bcl-2 family consists of pro- and
anti-apoptotic members that are associated with opposing effects on
mitochondria. Bax and Bak regulate the release of cytochrome
c, and the enhancement of cytochrome c activates
caspase-2, −3 and −9 (31,32). Caspase family proteins serve a key
role in the regulation of p53-mediated apoptosis (33). Bax is a p53 target and a pro-apoptotic
member of the Bcl-2 family of proteins (40,41).
Repression of anti-apoptotic members, including Bcl-2 and Bcl-xL,
which are transcriptionally suppressed by p53 (42), preserves the integrity of the
mitochondria. The present study demonstrated that GSPs upregulated
the pro-apoptotic proteins (Bax and Bak) and caspase family
proteins (caspase-2, −3 and −9), and downregulated the
anti-apoptotic protein Bcl-2 in HCT-116 cells. It was observed that
the increased expression level of Bcl-2 induced the decrease of
mitochondrial potential and eventually lead to apoptosis (43). The results of the present study
revealed that GSPs mediated the reduction of the mitochondrial
membrane potential in a concentration-dependent manner. Based on
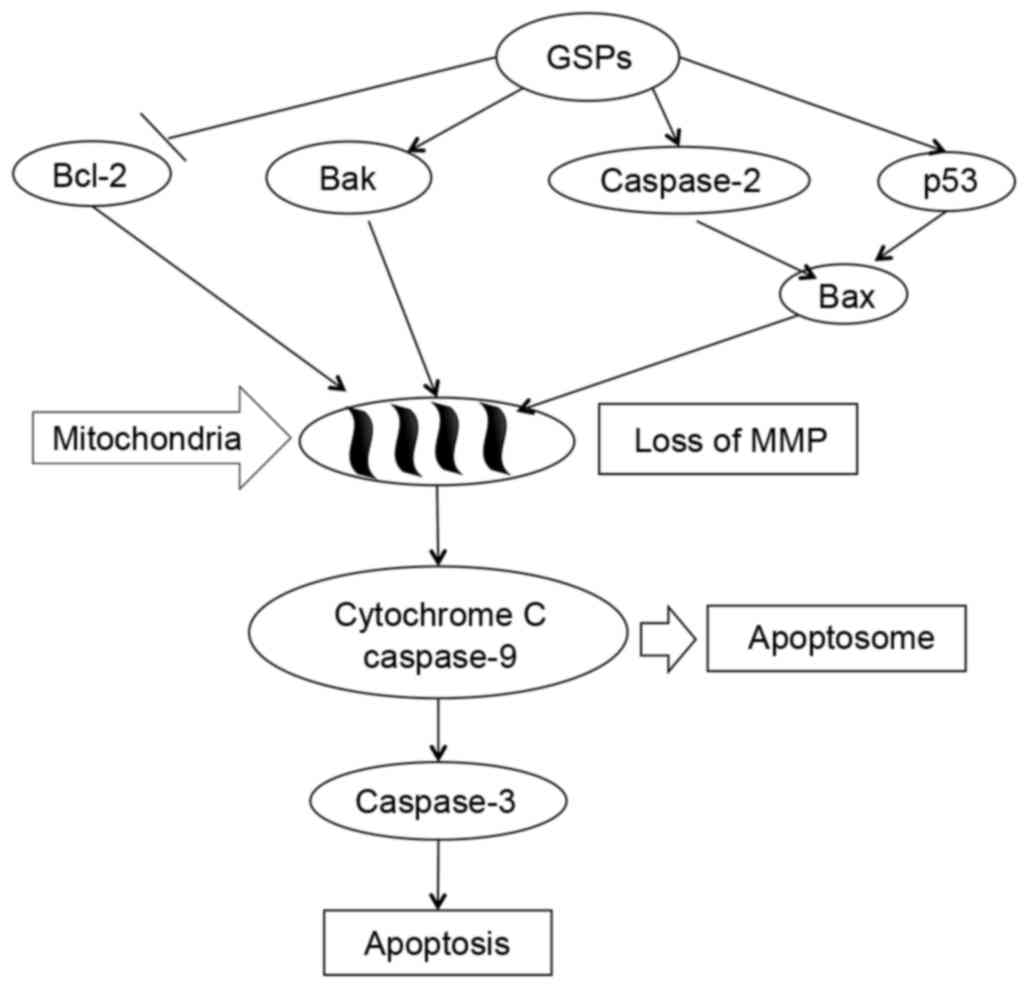
these results, Fig. 7 presents a
schematic of the proposed molecular mechanisms underlying these
effects of GSPs. Exposure to GSPs activated cleavage of caspase-2,
caspase-3 and caspase-9 in HCT-116 cells, induced p53-mediated
mitochondrial apoptosis signaling pathway with a
concentration-dependent decrease in the expression level of the
survival protein Bcl-2, and increased the expression level of the
pro-apoptotic proteins, Bax and Bak.
In conclusion, GSPs modulated the expression level
and activation of numerous genes and proteins involved in the
mitochondrial apoptotic pathway. These results proposed that GSPs
prompted colon cancer cell apoptosis via the mitochondrial pathway
and provided evidence demonstrating that GSPs may postulate
potential chemotherapeutic agents for colorectal cancer.
Acknowledgements
The present study was supported by the Heilongjiang
Province Science Foundation, China (grant no. H201380).
References
|
1
|
Jemal A, Center MM, Ward E and Thun MJ:
Cancer occurrence. Methods Mol Biol. 471:3–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Markowitz SD and Bertagnolli MM: Molecular
origins of cancer: Molecular basis of colorectal cancer. N Engl J
Med. 361:2449–2460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Lu N, Yang Q, Gong D, Lin C, Zhang
S, Xi M, Gao Y, Wei L, Guo Q and You Q: Studies on chemical
modification and biology of a natural product, gambogic acid (III):
Determination of the essential pharmacophore for biological
activity. Eur J Med Chem. 46:1280–1290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang N, Yin Y, Xu SJ and Chen WS:
5-Fluorouracil: Mechanisms of resistance and reversal strategies.
Molecules. 13:1551–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang WQ, Fu FF, Li YX, Wang WB, Wang HH,
Jiang HP and Teng LS: Molecular biomarkers of colorectal cancer:
Prognostic and predictive tools for clinical practice. J Zhejiang
Univ Sci B. 13:663–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mantena SK, Baliga MS and Katiyar SK:
Grape seed proanthocyanidins induce apoptosis and inhibit
metastasis of highly metastatic breast carcinoma cells.
Carcinogenesis. 27:1682–1691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Silva RC, Cheynier V and Chemina A:
Procyanidin dimers and trimers from grape seeds. Phytochemistry.
30:1259–1264. 1991. View Article : Google Scholar
|
|
10
|
Prieur C, Rigaud J, Cheynier V and
Moutounet M: Oligomeric and polymeric procyanidins from grape
seeds. Phytochemistry. 36:781–789. 1994. View Article : Google Scholar
|
|
11
|
Vayalil PK, Mittal A and Katiyar SK:
Proanthocyanidins from grape seeds inhibit expression of matrix
metalloproteinases in human prostate carcinoma cells, which is
associated with the inhibition of activation of MAPK and NF kappa
B. Carcinogenesis. 25:987–995. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh RP, Tyagi AK, Dhanalakshmi S,
Agarwal R and Agarwal C: Grape seed extract inhibits advanced human
prostate tumor growth and angiogenesis and upregulates insulin-like
growth factor binding protein-3. Int J Cancer. 108:733–740. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agarwal C, Singh RP, Dhanalakshmi S and
Agarwal R: Anti-angiogenic efficacy of grape seed extract in
endothelial cells. Oncol Rep. 11:681–685. 2004.PubMed/NCBI
|
|
14
|
Mittal A, Elmets CA and Katiyar SK:
Dietary feeding of proanthocyanidins from grape seeds prevents
photocarcinogenesis in SKH-1 hairless mice: Relationship to
decreased fat and lipid peroxidation. Carcinogenesis. 24:1379–1388.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi J, Yu J, Pohorly J and Kakuda Y:
Polyphenolics in grape seeds-biochemistry and functionality. J Med
Food. 6:291–299. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bagchi D, Bagchi M, Stohs S, Ray SD, Sen
CK and Preuss HG: Cellular protection with proanthocyanidins
derived from grape seeds. Ann N Y Acad Sci. 957:260–270. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tyagi A, Agarwal R and Agarwal C: Grape
seed extract inhibits EGF-induced and constitutively active
mitogenic signaling but activates JNK in human prostate carcinoma
DU145 cells: Possible role in anti-proliferation and apoptosis.
Oncogene. 22:1302–1316. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma G, Tyagi AK, Singh RP, Chan DC and
Agarwal R: Synergistic anti-cancer effects of grape seed extract
and conventional cytotoxic agent doxorubicin against human breast
carcinoma cells. Breast Cancer Res Treat. 85:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang LS and Stoner GD: Anthocyanins and
their role in cancer prevention. Cancer Lett. 269:281–290. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Finlay CA, Hinds PW and Levine AJ: The p53
proto-oncogene can act as a suppressor of transformation. Cell.
57:1083–1093. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karpinich NO, Tafani M, Rothman RJ, Russo
MA and Farber JL: The course of etoposide induced apoptosis from
damage to DNA and p53 activation to mitochondrial release of
cytochrome c. J Biol Chem. 277:16547–16552. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kastan MB, Onyekwere O, Sidransky D,
Vogelstein B and Craig RW: Participation of p53 protein in the
cellular response to DNA damage. Cancer Res. 51:6304–6311.
1991.PubMed/NCBI
|
|
23
|
Sherr CJ: Principles of tumor suppression.
Cell. 116:235–246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mercer WE, Shields MT, Amin M, Sauve GJ,
Appella E, Romano JW and Ullrich SJ: Negative growth regulation in
a glioblastoma tumor cell line that conditionally expresses human
wild-type p53. Proc Natl Acad Sci USA. 87:6166–6170. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baker SJ, Markowitz S, Fearon ER, Willson
JK and Vogelstein B: Suppression of human colorectal carcinoma cell
growth by wild-type p53. Science. 249:912–915. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bagchi M, Kuszynski CA, Balmoori J, Joshi
SS, Stohs SJ and Bagchi D: Protective effects of antioxidants
against smokeless tobacco-induced oxidative stress and modulation
of Bcl-2 and p53 genes in human oral keratinocytes. Free Radic Res.
35:181–194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joshi SS, Kuszynski CA and Bagchi D: The
cellular and molecular basis of health benefits of grape seed
proanthocyanidin extract. Curr Pharm Biotechnol. 2:187–200. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia G, Wang Q, Wang R, Deng D, Xue L, Shao
N, Zhang Y, Xia X, Zhi F and Yang Y: Tubeimoside-1 induces glioma
apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome
C/Caspase-3 pathway. Onco Targets Ther. 8:303–311. 2015.PubMed/NCBI
|
|
29
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marzo I, Brenner C, Zamzami N,
Jürgensmeier JM, Susin SA, Vieira HL, Prévost MC, Xie Z, Matsuyama
S, Reed JC and Kroemer G: Bax and adenine nucleotide translocator
cooperate in the mitochondrial control of apoptosis. Science.
281:2027–2031. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
32
|
Mohan J, Gandhi AA, Bhavya BC, Rashmi R,
Karunagaran D, Indu R and Santhoshkumar TR: Caspase-2 triggers
Bax-Bak-dependent and -independent cell death in colon cancer cells
treated with resveratrol. J Biol Chem. 281:17559–17611. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian Z, Shen J, Moseman AP, Yang Q, Yang
J, Xiao P, Wu E and Kohane IS: Dulxanthone A induces cell cycle
arrest and apoptosis via up-regulation of p53 through mitochondrial
pathway in HepG2 cells. Int J Cancer. 122:31–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao J, Wang J, Chen Y and Agarwal R:
Anti-tumor-promoting activity of a polyphenolic fraction isolated
from grape seeds in the mouse skin two-stage initiation-promotion
protocol and identification of procyanidin B5-3′-gallate as the
most effective antioxidant constituent. Carcinogenesis.
20:1737–1745. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kamei H, Hashimoto Y, Koide T, Kojima T
and Hasegawa M: Anti-tumor effect of methanol extracts from red and
white wines. Cancer Biother Radiopharm. 13:447–452. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang SY, Seeram NP, Nair MG and Bourquin
LD: Tart cherry anthocyanins inhibit tumor development in ApcMin
mice and reduce proliferation of human colon cancer cells. Cancer
Letts. 194:13–19. 2003. View Article : Google Scholar
|
|
39
|
Roy AM, Baliga MS, Elmets CA and Katiyar
SK: Grape seed proanthocyanidins induce apoptosis through p53, Bax,
and caspase 3 pathways. Neoplasia. 7:24–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McCurrach ME, Connor TM, Knudson CM,
Korsmeyer SJ and Lowe SW: Bax-deficiency promotes drug resistance
and oncogenic transformation by attenuating p53-dependent
apoptosis. Proc Natl Acad. 94:2345–2349. 1997. View Article : Google Scholar
|
|
41
|
Yin C, Knudson CM, Korsmeyer SJ and Van
Dyke T: Bax suppresses tumorigenesis and stimulates apoptosis in
vivo. Nature. 385:637–640. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ryan KM, Phillips AC and Vousden KH:
Regulation and function of the p53 tumor suppressor protein. Curr
Opin Cell Biol. 13:332–337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shih PH, Yeh CT and Yen GC: Effects of
anthocyanidin on the inhibition of proliferation and induction of
apoptosis in human gastric adenocarcinoma cells. Food Chem Toxicol.
43:1557–1566. 2005. View Article : Google Scholar : PubMed/NCBI
|